Abstract—
Due to the primary localization of both epiphytic and endophytic plant growth-promoting rhizobacteria on the surface of the plant root system, biofilm formation is an adaptive trait for these microorganisms. Under conditions of nitrogen limitation in liquid media, nitrogen-fixing Azospirillum brasilense strains switch mainly to the biofilm mode of growth. Overall ultrastructural similarities of the cells within A. brasilense biofilms were revealed, and their resistance to desiccation and oxidative stress was characterized. In strains Sp7, Cd, and Sp245, several types of single and undivided cells were revealed, as well as cystlike cells with pronounced morphological diversity. Resistance to desiccation and to oxidative stress was higher in the biofilm populations of these strains than in planktonic cultures. Dormant forms remained viable in dry biofilms of strains Sp245, Sp7, and Cd formed in a nitrogen-free medium after storage for 120 days. Viability of the biofilms of the same strains formed in the presence of nitrogen was retained for 120, 90, and 60 days, respectively. The minimal inhibitory concentration of H2O2 for biofilms was 1.0% for strains Sp7 and Cd and 0.1% for strain Sp245. Both the dormant forms and biofilms of strain Sp245 were more sensitive to H2O2 than those of strains Sp7 and Cd. Peroxidase activity was not previously reported in Azospirillum biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Azospirillum brasilense is a soil bacterium involved in productive associative interrelations with diverse cultivated plants (Bashan and de-Bashan, 2010). Azospirilla are able to provide plants with bound nitrogen, synthesize phytohormones, improve mineral nutrition of plants, increase their resistance to heavy metals, control plant pathogens, and neutralize toxic compounds (Bashan and de-Bashan, 2010; Fibach-Paldi et al., 2012). These features of azospirilla make them attractive for inclusion into biofertilizers the use of which in agricultural biotechnology is promising. The ability of azospirilla to form biofilms on the surface of the underground organs of the host plant may be of certain importance for successful functioning of the plant-microbial association. The latter contributes to the adaptation of microorganisms to the existence in a dynamic soil environment and provides a benefit to plants (Fibach-Paldi et al., 2012; Bogino et al., 2013). Bacterial biofilms are spatially and metabolically structured communities of matrix-embedded microorganisms (Flemming and Wingender, 2010). Apart from acting as a structural framework, the biofilm matrix is also a reservoir of growth factors, enzymes, and nutrients; it also plays a protective role. The matrix is a highly hydrated structure, which dries slowly and protects microorganisms from water potential changes. A mixture of exopolysaccharides, proteins, and nucleic acids is the structural basis of the extracellular biofilm matrix. Bacterial flagella and pili are also integrated into the matrix and contribute to its architecture. Thus, the preservation of the polar flagellum in A. brasilense Sp245 cells integrated into the mature biofilm contributes to the integrity of the latter under the conditions of hydrodynamic shift (Shelud’ko et al., 2019). The variety of the structural components of the biofilm matrix is comparable to the number of bacterial species forming biofilms (Flemming and Wingender, 2010; Bogino et al., 2013). Biofilm bacterial cells are also characterized by morphological and functional diversity (Hunter and Beveridge, 2005). The composition, structure, and functions of the main biopolymers of the Azospirillum biofilm matrix, which are involved in stabilization of mature films on a solid surface and fulfill the framework function, were studied. These features contribute to the development of the conditions favorable for nitrogen fixation in biofilms (Ramírez-Mata et al., 2016; Wang et al., 2017; Shelud’ko et al., 2018, 2019). The morphological and functional polymorphism of cell forms in Azospirillum biofilms is poorly characterized, although it is typical of the vegetative cells of these bacteria grown in liquid or on solid media (Döbereiner and Day, 1976; Eskew et al., 1977; Tarrand et al., 1978; Sadasivan and Neyra, 1987). Dormant forms increase the resistance of bacterial populations to deleterious factors, such as heating and desiccation, oxidative stress, etc. in azospirilla, in particular (Pope and Wyss, 1970; Eskew et al., 1977; Sadasivan and Neyra, 1987). Soil microorganisms that infect or colonize the root system are subject to oxidative stress caused by reactive oxygen species (H2O2, in particular) produced by plants (Vasil’eva et al., 2007; Pradedova et al., 2011). Thus, characterization of the cell forms in biofilms is required to understand the mechanisms of formation and functioning of bacterial biofilms and their resistance to external factors, as well as to select the methods to control these processes for environmental and biotechnological purposes. Analysis of the resistance of biofilms to the negative effects of hydrogen peroxide is also of interest for understanding their role in the adaptation of azospirilla to the dynamic soil environment, as well as in association with plants.
Strains Sp7, Cd, and Sp245 were the subjects of our study. They are used as model strains in the studies of the modes and mechanisms of adaptation of A. brasilense to various conditions, including association with plants (Eskew et al., 1977; Tarrand et al., 1978; Baldani et al., 1983; Schloter et al., 1998; Schelud’ko et al., 2009; Bashan and de-Bashan, 2010; Petrova et al., 2010; Shelud’ko et al., 2010, 2015, 2019; Fibach-Paldi et al., 2012; Tugarova et al., 2017; Wang et al., 2017). All the strains under study fix atmospheric nitrogen (in the absence of its bound forms in the incubation medium), synthesize phytohormones, and form biofilms on plant roots, abiotic surfaces, and at the liquid‒air interface (Petrova et al., 2010; Shelud’ko et al., 2010, 2015; Tugarova et al., 2017; Wang et al., 2017; Shelud’ko et al., 2019). Strain Sp245 is able to penetrate the roots and colonize the intercellular spaces of the vascular system and root hairs of wheat (Schloter et al., 1998). Isolation of strain Cd from the sterilized surfaces of the Bermuda grass (Cynodon dactylon) roots (Eskew et al., 1977) also suggests the ability of this bacterium to penetrate the root system of the partner plant.
The goal of the present work was to study the polymorphism of cell forms in A. brasilense Sp7, Cd, and Sp245 biofilms, as well as to characterize the dormant forms. The objectives of the study included analysis of the ultrastructure of biofilms formed on nitrogen-containing and nitrogen-free media (conditions promoting fixation of atmospheric nitrogen by azospirilla), as well as characterization of the resistance of biofilm populations to desiccation and oxidative stress.
MATERIALS AND METHODS
Bacterial strains and culture media. We used the following strains of A. brasilense: Sp7 isolated from the rhizosphere of the pangola grass (Digitaria decumbens) in Brazil (Tarrand et al., 1978), Cd isolated from the roots of the Bermuda grass (Cynodon dactylon) in the United States after inoculation with Sp7 (Eskew et al., 1977), and Sp245 isolated from wheat (Triticum aestivum) roots in Brazil (Baldani et al., 1983). Bacteria were cultured on malate‒salt medium (MSM) (Döbereiner and Day, 1976) containing NH4Cl (1 g/L) at 30°C. Agar was added to the medium (to the final amount of 1.8 or 0.3%) or NH4Cl was excluded from the medium if required.
Determination of the growth rates of planktonic cultures, bacterial motility, and aggregation of bacteria under aeration conditions. Overnight (18 h) bacterial cultures were diluted to OD590 = 0.05–0.10 (l = 0.5 cm) in sterile MSM (100 mL) supplemented with NH4Cl in 250-mL conical flasks. The conditions of intense aeration were developed by placing the flasks on a horizontal platform of an Excella E24 shaker incubator (New Brunswick Scientific, United States); incubation was carried out at 30°С (140 rpm). Every 2 h, optical density of the bacterial culture was measured at OD590 (l = 0.5 cm).
During cultivation, planktonic cultures were collected and used to determine the level of cell aggregation (Madi and Henis, 1989). Liquid cultures were left to precipitate for 30 min. The supernatant was carefully decanted, and a suspension of precipitated cell aggregates in 50 mM phosphate buffer (PB, pH 7.0) was prepared. After this, the suspension was left to precipitate for 2 h, and OD590 of the supernatant was measured. Bacteria were dispersed for 2 min on a magnetic stirrer, and optical density of the suspension was determined once again. To assess the percentage of cell aggregation, the following formula was used: A = {[(OD590)2 – (OD590)1]/(OD590)1]} × 100%, where (OD590)1 was the optical density of the supernatant after aggregate precipitation and (OD590)2 was the optical density of the suspension obtained after dispersion of the precipitated bacterial aggregates.
To study bacterial motility, 24-h planktonic cultures were diluted to OD590 = 0.2–0.3 (l = 0.5 cm) and a hanging drop was prepared. The slides were examined under a JENAVAL transmission microscope (Carl Zeiss, Jena, Germany) (phase contrast microscopy) using a lens with a wide field of view. The observations were recorded using a DCR-TRV900E video camera (SONY, Japan). Motility of all cells in the field of view of the microscope was assessed. Video analysis was performed using the software developed at the Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences (Schelud’ko et al., 2009).
Analysis of the static growth of planktonic cultures, the process of formation of Azospirillum biofilms, and their microstructure. The 24-h cultures of azospirilla grown in the aerated liquid MSM containing nitrogen were washed with 50 mM PB (pH 7.0), diluted with fresh nitrogen-containing or nitrogen-free MSM to OD590 = 0.05–0.10 (l = 0.5 cm), and dispensed into glass tubes (2 mL of each culture) and polystyrene petri dishes (3 mL each), which contained glass coverslips for microscopy at the bottom. The incubation was carried out for six days at 30°C under stationary conditions. By the sixth day of incubation, the relative amount of Azospirillum biofilm biomass steadied, and the process of biofilm formation on the surface of the coverslips (mature biofilms) placed in liquid medium was completed (Shelud’ko et al., 2015). Coverslips with formed biofilms were used to analyze the microstructure of biofilms by light microscopy.
Mature biofilms were stained with crystal violet in order to assess the relative amount of their biomass (O’Toole and Kolter, 1998). Prior to biofilm staining, planktonic bacteria were collected from the test tubes and used to determine the optical density (OD590; l = 0.5 cm) of the liquid cultures surrounding the biofilms. Aqueous solution of crystal violet (1%; 2 mL) was added to the biofilms carefully washed with distilled water (the residues of the planktonic culture and fragments of biofilms formed at the interface of the liquid MSM and air by azospirilla and unattached to a coverslip surface were removed during washing), incubated at room temperature for 10 min, and washed with water once again after removal of the solution. The dye bound to the biofilm biomass was dissolved in ethanol (2 mL), and optical density of this solution was measured at a wavelength of 590 nm using a KFK-2 photocolorimeter (Zagorsk Optical and Mechanical Plant, Russia). Test tubes containing sterile medium were incubated for six days, stained, and used as a control. OD590 (l = 0.5 cm) of the desorbed dye in the control was less than 0.04 U. The control values of OD590 (l = 0.5 cm) were subtracted from the results of the biofilm biomass staining.
In some experiments, the biofilms were washed, suspended in 50 mM PB (pH 7.0), and applied onto formvar-coated grids by flotation (planktonic cells were applied in a similar way). The grids with applied preparations were dried and analyzed using transmission electron microscopy.
Phase contrast, fluorescence, and transmission electron microscopy of biofilms, suspensions of washed-off biofilms, and individual cells of planktonic cultures was performed at the Simbioz Center for the Collective Use of Research Equipment of the Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences (Saratov, Russia) using the following equipment: Leica DM6000 B (Leica-Microsystems, Germany) and Libra 120 (Carl Zeiss, Germany).
Preparation of ultrathin sections. Mature biofilms were washed and carefully removed (washed off by aspiration with 50 mM PB, pH 7.0). The biofilm fragments in the wash-out fluid were precipitated by centrifugation (6000 rpm, 15 min). Planktonic cultures grown for 24 h under aeration conditions were also precipitated from the nitrogen-containing liquid MSM. The pellets of biofilms or planktonic cells were fixed in a 2.5% glutaraldehyde solution in 0.1 M cacodylate buffer (CB; pH 7.2) for 12 h at 4°С, washed three times with 0.1 M CB (pH 7.2), and fixed with a 1% solution of OsO4 in the same buffer for 4 h at 20°С. Dehydration was sequentially carried out in alcohols (from 30 to 100%), acetone, and propylene oxide. The dehydrated material was embedded in Epon 812. The sections were obtained using an ultratome, transferred to grids, and contrasted with a 3% uranyl acetate solution in 70% alcohol if required. Ultrathin sections were examined under a Libra 120 transmission electron microscope (Carl Zeiss, Germany) at an accelerating voltage of 120 kV.
Determination of the abundance of viable forms in biofilms and their resistance to desiccation. After planktonic cultures were separated from mature biofilms, the latter were carefully washed with 50 mM PB (pH 7.0). Washed tubes were used to determine the number of colony-forming units (CFU) in native (unexposed to any effects) and dry biofilms. In the latter case, washed biofilms were dried at 30°C and stored at 37°C (Sadasivan et al., 1987; Malinich and Bauer, 2018). To determine the CFU, 50 mM PB (pH 7.0) (2 mL) was added to the test tubes containing biofilms and incubated for 1 h. After that, the biomass was washed off, resuspended, and dispersed on a Vortex shaker for 1 min. A series of 100-μL dilutions were plated from the obtained suspension onto solid MSM containing nitrogen. Taking into account the dilution, the results were recalculated for 2 mL, which corresponded to one biofilm wash-out. To control the efficiency of the biofilm mass washing off, the tubes were stained with crystal violet after removal of the biofilms (see above) and compared to the results of staining of the tubes that were incubated with a sterile medium for six days (control). After the biofilms were washed off, the results of staining of the surface of coverslips did not differ from the control values and did not exceed 0.04 U of OD590 (l = 0.5 cm).
To determine the duration of viability retention by the dormant forms, every 15 days test tubes with dry biofilms stored for more than seven days were filled with semisolid MSM (with 0.3% agar) containing no source of bound nitrogen and incubated. These cultivation conditions favor initiation of the growth of cyst-like forms of azospirilla (Sadasivan et al., 1987). After 24–48 h of incubation in a semisolid medium, viable cells divided and, moving in the agar, formed a band concentrated below the surface of the medium. Tubes with no growth were incubated for up to seven days. The semisolid medium from the tubes with no visible growth was plated onto the solid media. The lack of growth in this case as well indicated death of the population.
Assays of catalase and peroxidase activity of the biofilm biomass and analysis of the effect of hydrogen peroxide on the viability of azospirilla. After planktonic cells were removed, mature biofilms were carefully washed with 50 mM PB (pH 7.0). The buffer was discarded. To evaluate peroxidase activity, 0.03% o-phenylenediamine solution (1 mL) and 0.02% hydrogen peroxide in 0.1 M sodium citrate buffer (pH 4.5) were added to the tubes, incubated for 10 min, and 1 N H2SO4 (2 mL) was added to stop the reaction. In the control samples, o-phenylenediamine without hydrogen peroxide was added to the biofilms. Optical density (OD490) of the studied samples was measured using a Multiskan Ascent photometer (ThermoLabsystems, Finland) at the Simbioz Center for the Collective Use of Research Equipment of the Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences (Saratov, Russia). OD490 of the control samples (biofilms + o-phenylenediamine) was subtracted from OD490 of the samples containing hydrogen peroxide (biofilms + o-phenylenediamine + H2O2). The catalase activity characteristic of azospirilla was judged by the formation of foam after addition of 1 or 2% H2O2 solution to native biofilms (Tarrand et al., 1978; Manual of Methods …, 1983).
To determine the effect of H2O2 on the viability of azospirilla, 24-h planktonic cultures grown under stirring or mature biofilms were incubated with H2O2 solutions (0.001, 0.01, 0.1, and 1%) in 50 mM PB (pH 7.0) for 18 h. The tubes with biofilms were preliminarily carefully washed with the same buffer, and the H2O2 solution (2.5 mL) was added after the buffer was discarded. In the case of planktonic bacteria, liquid cultures (2 mL) diluted to OD590 = 0.3–0.4 (l = 0.5 cm) (containing (5.5 ± 0.5) × 109/mL of viable cells) were precipitated and resuspended in 2.5 mL of an H2O2 solution. After 18 h of incubation, H2O2 was removed from the tubes containing biofilms; cells of planktonic cultures were collected by centrifugation. Semisolid MSM (2.5 mL) (0.3% agar) without nitrogen was added to the test tubes containing biofilms. The pellet of planktonic cells was added to sterile tubes containing similar culture medium. After 24–48 h of incubation, growth under the agar surface was recorded. The lack of growth indicated death of the population.
Statistical processing of results. In all variants of quantitative measurements, at least three independent experiments with at least two replicates were carried out. The biofilm biomass of each strain was assessed at least six times. Each time, biofilms formed in five glass tubes were stained. The results were processed using the Microsoft Office Excel 2010 software; confidence intervals were determined at a 95% significance level. One-factor analysis of variance (ANOVA) at a significance level of 0.05 was used to determine statistically significant differences between the means.
RESULTS AND DISCUSSION
The study of biofilm formation. Comparison of the growth of A. brasilense strains Sp7, Cd, and Sp245 in liquid media indicated that their planktonic cultures reached the stationary growth phase after 24 h of incubation with vigorous stirring. Cells from these cultures have a single long polar flagellum, which provides for swimming in a straight-line manner with a random change in the direction of movement. In the case of the Sp7, Cd, or Sp245 culture, respectively, (85.5 ± 2.2), (87.3 ± 2.6), or (85.8 ± 3.8)% of bacteria were motile. The level of aggregation of cells of the Sp245 planktonic cultures reached (42.4 ± 7.2)%, which was higher than those noted for Sp7 and Cd: (21.6 ± 3.5) and (17.7 ± 3.2)%, respectively. Thus, bacteria of the planktonic cultures of strains Sp7, Cd, and Sp245 significantly differed in cell surface characteristics that mediated cell aggregation.
The 24-h planktonic cultures described above were inoculated in the nitrogen-containing or nitrogen-free MSM and incubated in glass tubes under static conditions. The steps of biofilm formation by the studied strains at the interface between the liquid (MSM) and solid (hydrophilic glass surface) phases were: cell adsorption and adhesion (within 2 or 3 days), an increase in biofilm biomass, and its stabilization (starting from days 5 and 6; mature biofilms). During the first day of incubation, bacteria formed thin biofilms, microscopy of which revealed scattered cell aggregates (microcolonies) that were easily washed off by aspiration with water. On the third day, microcolonies merged into a biofilm with a more even surface. It is worth mentioning that starting from the second day of incubation, azospirilla not only colonized the solid surface but also formed a biofilm at the air‒liquid interface, which might subsequently precipitate under the liquid layer (to the bottom of the test tube).
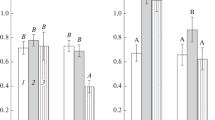
The biomass of the mature Sp7 and Sp245 biofilms did not differ on a nitrogen-containing medium; however, the biofilm biomass of strain Cd was higher than that of other strains (Table 1, columns b). The absence of nitrogen in the cultivation medium stimulated accumulation of Sp7 and Cd biomass but did not affect the biomass of Sp245 biofilms (Table 1, columns b). Mature Sp7, Sp245, and Cd biofilms contained approximately the same numbers of viable bacteria. In wash-out fluids from the test tubes containing biofilms, the CFU numbers varied within the order of magnitude of 1010 (CFU in the biofilm formed in one test tube) independently of the biofilm biomass and/or the presence of a source of bound nitrogen in the medium (Table 1, columns b, c).
For all the studied strains, the optical density of planktonic cultures grown simultaneously with biofilms in a medium containing no source of bound nitrogen was lower than that of the cultures grown in a nitrogen-containing medium (Table 1, columns a). The biofilm biomass either increased (in Sp7 and Cd) or remained the same (in Sp245). Thus, the observed patterns in the effect of nitrogen on the parameters characterizing the growth rate of planktonic cultures and the formation of biofilms indicated that all the studied strains that were in contact with the hydrophilic surface switched predominantly to the biofilm mode of growth under conditions of nitrogen limitation (Table 1, columns a, b).
Analysis of the cell ultrastructure in biofilms. Analysis of the ultrastructure of planktonic cells of strains Sp7, Cd, and Sp245 from 24-h liquid cultures grown in the nitrogen-containing MSM under stirring showed that vegetative cells (V cells) were vibrios (Figs. 1a, 1b, 3a, 4a) with a minimal number of inclusions suggested to be polyhydroxybutyrate (PHB) granules (Berg et al., 1980). The thin layer of the capsule was closely attached to the lipopolysaccharide of the outer membrane of the cells (Figs. 1a, 4a). The cells that lacked a pronounced capsule (Figs. 1b, 3a), as well as dividing and long cells (L cells), were also observed. The presence of L cells was most noticeable in the population of Sp245 planktonic cultures (Fig. 4b).
Transmission electron microscopy of ultrathin sections of A. brasilense Sp7 cells from planktonic 24-h cultures (a, b) and from fragments of mature biofilms (c–l). Bacteria and biofilms were cultured in liquid MSM supplemented with NH4Cl (a, b, h–l) or without bound nitrogen (d–g). Scale bar is 1 μm. Designations: V, V cells; L, L cells; CL, CL forms; LV, large vesicles; (cl), capsule layer; (IM) and (OM): inner and outer membranes; (PHB), polyhydroxybutyrate.
Transmission electron microscopy of ultrathin sections of cyst-like forms of A. brasilense Sp7 from the fragments of mature biofilms. Biofilms were cultured in the liquid MSM containing no NH4Cl. Scale bar is 1 μm. Designations: (c), central body (cI (blocks a, b), cII (blocks c, d), cIII (block e), and cIV (block f) variants); (ol), outer layer; (PHB), polyhydroxybutyrate.
Transmission electron microscopy of ultrathin sections of A. brasilense Cd cells from 24-h planktonic cultures (a) and fragments of mature biofilms (b–f). Bacteria and biofilms were cultured in the liquid MSM containing NH4Cl (a, d–f) or without NH4Cl (b, c). Scale bar is 1 μm. For designations, see Fig. 1.
Transmission electron microscopy of ultrathin sections of A. brasilense Sp245 cells from 24-h planktonic cultures (a, b) and fragments of mature biofilms (c–h). Bacteria and biofilms were cultured in the liquid MSM containing NH4Cl (a, b, e–h) or without NH4Cl (c, d). Scale bar is 1 μm. For designations, see Fig. 1.
The study of suspensions of the biomass of mature Sp7, Cd, and Sp245 biofilms (washed off the glass surface) using transmission electron microscopy showed that the cells with a polar flagellum were present among native cells. The preservation of the polar flagellum in bacteria could help to maintain the structural integrity of biofilms. Thus, the polar flagellum of strain Sp245 is one of the matrix components, which increases the resistance of biofilms formed by this strain to hydrodynamic shift (Shelud’ko et al., 2015, 2019). Analysis of ultrathin sections of biofilms indicated that all studied strains showed a pronounced polymorphism of size and shape (Figs. 1–4). According to the shape, they can be classified as V cells (Figs. 1c, 1e, 1h, 3d, 4e, 4g), characteristic of the vegetative cells of planktonic cultures, long and/or undivided cells (L cells) (Figs. 1f–1h, 1j, 1k, 3b, 3d–3f, 4b, 4d, 4g), thickened cells (Figs. 1i, 2b, 2c), and ovoid forms (Figs. 1c, 1e, 1j, 1l, 2d–2f, 3b–3f, 4c, 4d, 4f) of the medium and large dimension (cyst-like (CL) forms). CL forms were described in old cultures of azospirilla (Berg et al., 1980), in particular, in the medium containing no source of bound nitrogen. Some L cells were covered with a pronounced outer layer (Fig. 3e).
Lysed cells, large (Fig. 1l) and small vesicles, as well as electron-dense granules (e.g., Fig. 1), were also detected in the biofilms. Cell sheaths without a clear stratigraphy of the distribution of intact and lysed cells were observed (Figs. 1c, 1h, 1j, 1l). The presence of cells with signs of destruction in the biofilms can partially explain the absence of the interrelation between the amount of biofilm biomass and the number of CFU (Table 1) plated from them (it should also be taken into account that the biofilm matrix contributed to the amount of biomass). Apart from the cells with obvious signs of destruction, sheaths with intact cell walls containing electron-dense granules in the central part (Fig. 4h) were found. Nevertheless, it was unclear whether these were dead cells.
Figure 2 shows the sections of CL forms of A. brasilense Sp7 from biofilms formed in the liquid medium containing no source of bound nitrogen. In these cell forms, the central body with an uneven cytoplasmic texture contained large electron-transparent PHB granules and small dark electron-dense granules. The central body (cI variant) was surrounded by a thick poorly differentiated outer layer. CL forms with the complex organization had more than one body: cII (Figs. 2c, 2d); cIII (Fig. 2e); and cIV variants (Fig. 2f) contained two, three, and more bodies, respectively. These bodies were covered by a thick layer, which contained the following zones: an external zone (similar to exine) and less electron-dense intine (Figs. 2a, 2e, 2f).
The number of bodies increased after their division, which occurred directly under the thick outer layer covering the CL form (Fig. 2). Some forms contained the central body surrounded by a thin outer layer; as a rule, these were cI variants (Figs. 1e, 1j, 3d, 3f, 4f). The outer layer varying in thickness could be surrounded by small black electron-dense granules that were probably responsible for pigmentation. Ovoid cells of Azospirillum have been compared to Azotobacter cysts (Pope et al., 1970; Berg et al., 1980). Nevertheless, the lack of a clear differentiation of the outer layer into exine and intine is a visible difference between the Azospirillum CL forms and Azotobacter cysts (Berg et al., 1980). However, the formation of CL forms with a clearly differentiated outer layer by strain Sp245 occurred after the cultures grown in the media with minimal nitrogen content were subject to the conditions of complete starvation (Mulyukin et al., 2009). The formation of cyst-like forms of azospirilla is facilitated by an increase in the duration of culturing (aging cultures), intense aeration, replacement of carbon (for instance, malate, which is easily accessible for metabolism, is replaced with fructose) or nitrogen (ammonium is replaced with nitrate) sources, transfer of the bacterial culture to the conditions of nitrogen or phosphorus limitation, and starvation (Berg et al., 1980; Sadasivan et al., 1987; Mulyukin et al., 2009).
We compared cell morphotypes of three strains in the biofilms formed in the standard synthetic malate-containing medium with or without nitrogen. The greatest variety of forms was shown in the Sp7 biofilms from the nitrogen-free medium. These forms were V and L cells, as well as CL forms, which differed in the number of central bodies (variants from cI to cIV). Almost all CL forms from this medium were coated with a thick outer layer (Figs. 1 and 2). Forms with a thin outer layer were less common and mainly observed in cI variants. The morphotypes of the Sp7 cells composing biofilms from the nitrogen-containing medium were less diverse and characterized by the absence of cIII and cIV variants, while most cI variants had a thin outer layer (Fig. 1). The Cd biofilms consisted of V and L cells; cI and cII variants of CL forms were also present (Fig. 3). The absence of nitrogen promoted exclusive formation of CL forms covered with a thick outer layer in the biofilms of this strain (the variants covered with a thin outer layer prevailed on the medium containing nitrogen).
Independently of the presence of nitrogen in the culture medium, biofilms of strain Sp245 contained V cells, numerous L cells, and cI variants of CL forms with a thin or thick outer layer (Fig. 4). The Azospirillum biofilm biomass from MSM supplemented with NH4Cl (1 g/L) contained PHB, a considerable accumulation of which in planktonic cultures was primarily induced by nitrogen deficiency (the results of Fourier-transform infrared spectroscopy; Kamnev et al., 2012; Tugarova et al., 2017). According to our observations, the Sp245 L cells and CP forms contained large electron-transparent granules (Fig. 4) characteristic of PHB (Berg et al., 1980); small granules were mainly present in CL forms of strains Sp7 and Cd.
The CL forms identified in the biofilms were morphologically similar to the cyst-like forms previously described for azospirilla; these forms were resistant to starvation, oxidative stress, and desiccation (Sadasivan et al., 1987; Mulyukin et al., 2009; Malinich and Bauer, 2018). Obviously, only cyst-like forms remained viable in dry biofilms after the death of vegetative cells (dried cultures are stored for at least one week to achieve the maximal effect; Sadasivan et al., 1987; Malinich and Bauer, 2018). Determination of CFU in such samples will make it possible to quantitatively assess the number of dormant forms and to understand the effect of nitrogen in the culture medium on this parameter.
Analysis of the effect of bound nitrogen in the culture medium on the number of viable forms in biofilms and their resistance to desiccation. Biofilms grown in media with and without bound nitrogen were dried at 30°C and stored at 37°C (i.e., at optimal temperature for A. brasilense cultivation; Tarrand et al., 1978). After seven days of storage, colony-forming dormant variants were revealed in the biofilms of all three strains. The maximal abundance of 104 CFU persisted in the biofilms grown in the medium containing no source of bound nitrogen (Table 2). In the medium supplemented with NH4Cl, the number of CFU decreased by one order of magnitude (Sp245) and more (Table 2). It should be noted that dormant biofilm forms that were resistant to desiccation remained viable for a long time (during the studied period). In Sp245, Sp7, and Cd biofilms, viable forms persisted after four months of storage of dry samples from a nitrogen-free medium and after four, three, and two months of storage of the dry biofilms from a nitrogen-containing medium, respectively to the strain (Table 2). To determine the viability duration of the dormant forms, test tubes with dry biofilms were filled with semisolid MSM (0.3% agar) containing no source of bound nitrogen and incubated. These cultivation conditions triggered the growth of cyst-like forms of these bacteria (Sadasivan et al., 1987). Dividing cells formed a band concentrated under the agar surface 24–48 h after the introduction of the semi-solid medium into the test tubes containing dry biofilms. This band is characteristic of azospirilla that are microaerophiles (Wasim et al., 2009; Bashan and de-Bashan, 2010). Bacteria plated from this zone onto a solid media formed colonies typical for the vegetative cells of azospirilla.
Therefore, the presence of nitrogen in the cultivation medium did not affect the formation of dormant cells, which preserved their viability for a long period after drying, in the biofilms of three strains (Table 2). In contrast to biofilm forms, dormant forms of azospirilla from liquid nitrogen-containing media (planktonic cultures) lost their viability more rapidly after desiccation (death was observed by the fourth day) than the cyst-like forms obtained during incubation without bound nitrogen (Sadasivan et al., 1987).
Analysis of resistance of biofilms to oxidative stress. We compared the resistance of the studied strains to hydrogen peroxide (an agent of oxidative stress) evaluating the effect of various concentrations of H2O2 on the viability of bacteria from the stationary-phase planktonic cultures or from mature biofilms. During the treatment with H2O2, the volume of planktonic cultures was selected in such a way that the total CFU number was 1010 (in 2 mL of suspension), which corresponded to the CFU number in one biofilm (Table 1). Table 2 (column a) shows the CFU values in dry biofilms.
Analysis of susceptibility to H2O2-induced oxidative stress indicated that planktonic cultures of strains Sp7, Cd, and Sp245 had similar resistance and remained viable in the presence of 0.001% H2O2 (for instance, at this concentration of H2O2, approximately 10% of the cells of strain Sp245 remained viable when cultured in the minimal liquid synthetic medium; Wasim et al., 2009). H2O2 MIC for all three strains was 0.01% (Table 3, column a).
In native mature biofilms, the resistance of azospirilla to oxidative stress increased. Thus, the MIC value of 1.0% H2O2 was characteristic of bacteria from the Sp7 and Cd biofilms, regardless of the presence of nitrogen in the medium (Table 3, columns b). The composition of the medium had no effect on the MIC for strain Sp245 either, although the MIC for it decreased to 0.1% H2O2. It should be noted that after H2O2 was added to native biofilms, foam production indicating a pronounced catalase activity characteristic of azospirilla was observed (Tarrand et al., 1978). Foaming became intense 5–10 min after the addition of H2O2. Undoubtedly, the enzymes that helped the cells to withstand the negative effects of oxidants made a certain contribution to the bacterial defense against the H2O2-induced oxidative stress. In addition to catalase activity, azospirilla possess superoxide dismutase activity; alkyl hydroperoxide reductase also contributes to the resistance of these bacteria to oxidative stress (Nur et al., 1982; Clara and Knowles, 1984; Wasim et al., 2009). Native Sp7, Cd, and Sp245 biofilms possessed enzymatic activity characteristic of peroxidase as well (Table 3, columns d). In each strain, peroxidase activity was independent of the biofilm biomass amount (parameter affected by the presence of bound nitrogen in the medium in the case of Sp7 and Cd; Table 1). In native Sp245 biofilms, enzymatic activity was lower than that of Sp7 and Cd, which is characteristic of extracellular peroxidases of the phenol oxidase complex of these strains (Nikitina et al., 2010). The role of these enzymes in biofilms remains to be elucidated. Apart from the oxidation of toxic phenol compounds, these enzymes are probably involved in the comprehensive response of bacteria to H2O2.
Dormant forms of strain Sp245 in dry biofilms remained viable after incubation with 0.001% H2O2, and the MIC of H2O2 for them (similarly to the planktonic cultures) was 0.01% (Table 3, columns a, c). The MIC of H2O2 for dormant forms of strains Sp7 and Cd was 0.1%, which was higher than the value characterizing the resistance of planktonic cells of these strains, but lower than the concentration of H2O2, which causes the death of bacteria in biofilms (Table 3). Thus, in comparison to planktonic cultures, the biofilm population of A. brasilense possessed a higher resistance potential against oxidative stress. Dormant forms are involved in the mechanisms that contribute to the survival of the population under stress conditions. Compared to Sp7 and Cd, dormant forms of strain Sp245, as well as its biofilms, proved to be more susceptible to the negative effect of H2O2. This may result from the specific adaptation of this strain, which is able to penetrate plant roots and colonize the intercellular spaces of the vascular system, to this particular stress factor (Schloter et al., 1998). Tissues of wheat roots have an active system, which regulates the level of H2O2 (Pradedova et al., 2011).
Thus, it was possible to identify the common features in the ultrastructure of Azospirillum brasilense biofilms using strains Sp7 and Cd, as well as strain Sp245, which colonizes the root hairs and intercellular spaces of the root vascular system of plants. The biofilms of this bacterial species are characterized by the presence of several structural types of single cells (V and L), undivided L cells, long cells coated with a thick outer layer, and CL forms with a pronounced morphological diversity (variants with differences in the number of bodies and thickness of the outer layer isolating them from the environment). The greatest morphological diversity of cyst-like forms was revealed in strain Sp7 in the biofilms formed under the conditions of nitrogen limitation. Cysts are nonmotile bacterial forms that show no metabolic activity and are often surrounded by a thick capsule layer. They are characterized by long-term viability in the absence of division, as well as by resistance to heat, desiccation, starvation, and oxidative stress (Sadoff, 1975). A bacterial cyst is usually formed by a single bacterial cell. We revealed dividing cells under a thick outer layer covering cyst-like forms of azospirilla. Long Azospirillum cells coated with a thick outer layer can potentially be the cell forms that are resistant to external factors (Mulyukin et al., 2009).
It should be noted that the morphotype of cyst-like forms and the conversion of vegetative actively dividing cells of azospirilla into cyst-like forms are often the result of a simultaneous change in several parameters of the medium composition and cultivation conditions (Sadasivan et al., 1987; Mulyukin et al., 2009). Azospirillum biofilms from the standard minimal culture medium used for cultivation of these bacteria (Döbereiner and Day, 1976) contained cyst-like forms, the morphology of which was diverse, which was probably a part of the biofilm formation program as one of the ways of adaptation of the population to changing conditions and extreme factors. The absence of nitrogen in the medium contributed to an increase in the abundance of morphotypes of cyst-like variants, which may be explained by the necessity of adaptation to these conditions. For instance, dormant forms of azospirilla are characterized by some metabolic activity (detected by transcriptome analysis) (Malinich and Bauer, 2018). The cells morphologically similar to the cyst-like ones are suggested to fix air nitrogen in biofilms (Wang et al., 2017). We showed that dormant cyst-like forms were involved in the mechanisms contributing to survival of the biofilm population exposed to oxidative stress. Bacteria resistant to desiccation even when the abundance of three A. brasilense strains in the biofilms was low (in the presence of nitrogen in the medium) remained viable for a long time (during the studied period of four months), which undoubtedly contributed to the stability of the population under this kind of stress. During long-term storage after drying, the dormant forms of azospirilla from liquid media with nitrogen lost their viability faster than the cells formed under nitrogen-free conditions (Sadasivan et al., 1987).
In conclusion, it should be noted that cyst-like forms of azospirilla were found on the roots of plants colonized by these bacteria (Bashan et al., 1991; Assmus et al., 1995). Our data on the biofilm/liquid/glass model agree with the information about the morphology of azospirilla living on the root surface, as well as provide new insights into the biofilm ultrastructure and resistance to extreme factors; at the same time, biofilm formation is a part of the plant colonization process, which involves endophytic strains as well (Eskew et al., 1977; Schloter et al., 1998; Petrova et al., 2010; Shelud’ko et al., 2010).
REFERENCES
Assmus, B., Hutzler, P., Kirchhof, G., Amann, R., Lawrence, J.R., and Hartmann, A., In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy, Appl. Environ. Microbiol., 1995, vol. 61, pp. 1013–1019.
Baldani, V.L.D., Baldani, J.I., and Döbereiner, J., Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat, Can. J. Microbiol., 1983, vol. 29, pp. 924–929.
Bashan, Y. and de-Bashan, L.E., How the plant growth-promoting bacterium Azospirillum promotes plant growth–a critical assessment, Adv. Agron., 2010, vol. 108, pp. 77–136.
Bashan, Y., Levanony, H., and Whitmoyer, R.E., Root surface colonization of non-cereal crop plants by pleomorphic Azospirillum brasilense Cd, J. Gen. Microbiol., 1991, vol. 137, pp. 187–196.
Berg, R.H., Tyler, M.E., Novick, N.J., Vasil, V., and Vasil, I.K., Biology of Azospirillum–sugarcane association: enhancement of nitrogenase activity, Appl. Environ. Microbiol., 1980, vol. 39, pp. 642–649.
Bogino, P.C., Oliva, M.M., Sorroche, F.G., and Giordano, W., The role of bacterial biofilms and surface components in plant-bacterial associations, Int. J. Mol. Sci., 2013, vol. 14, pp. 15838–15859.
Döbereiner, J. and Day, J.M., Associative symbiosis in tropical grass: Characterization of microorganisms and dinitrogen fixing sites, Symposium on Nitrogen Fixation, Newton, W.E. and Nijmans, Eds., C.J. Pullman: Washington State Univ. Press, 1976, pp. 518–538.
Eskew, D.L, Focht, D.D., and Ting, L.P., Nitrogen fixation, denitrification and pleomorphic growth in a highly pigmented Spirillum lipoferum,Appl. Environ. Microbiol., 1977, vol. 34, pp. 582–585.
Fibach-Paldi, S., Burdman, S., and Okon, Y., Key physiological properties contributing to rhizosphere adaptation and plant growth promoting abilities of Azospirillum brasilense,FEMS Microbiol. Lett., 2012, vol. 326, pp. 99–108.
Flemming, H.-C. and Wingender, J., The biofilm matrix, Nat. Rev. Microbiol., 2010, vol. 8, pp. 623–633.
Hunter, R.C. and Beveridge, T.J., High-resolution visualization of Pseudomonas aeruginosa PAO1 biofilms by freeze-substitution transmission electron microscopy, J. Bacteriol., 2005, vol. 187, pp. 7619–7630.
Madi, L. and Henis, Y., Aggregation in Azospirillum brasilense Cd: conditions and factors involved in cell-to-cell adhesion, Plant Soil, 1989, vol. 115, pp. 89–98.
Malinich, E.A. and Bauer, C.E., Transcriptome analysis of Azospirillum brasilense vegetative and cyst states reveals large-scale alterations in metabolic and replicative gene expression, Microb. Genom., 2018, vol. 4, p. e000200.
Manual of Methods for General Bacteriology, Gerhardt, P., Murray, R.G.E., Costilow, R.N., Nester, E.W., Wood, W.A., Krieg, N.R., and Phillips, G.B., Eds., Washington: Amer. Soc. Microbiol., 1981.
Muliukin, A.L., Suzina, N.E., Pogorelova, A., Antoniuk, L.P., Duda, V.I., and El’-Registan, G.I., Diverse morphological types of dormant cells and conditions for their formation in Azospirillum brasilense,Microbiology (Moscow), 2009, vol. 78, pp. 33–41.
Nikitina, V.E., Vetchinkina, E.P., Ponomareva, E.G., and Gogoleva, Y.V., Phenol oxidase activity in bacteria of the genus Azospirillum,Microbiology (Moscow), 2010, vol. 79, pp. 327–333.
Nur, I., Okon, Y., and Henis, Y., Effect of dissolved oxygen tension on production of carotenoids, poly-β-hydroxybutyrate, succinate oxidase and superoxide dismutase by Azospirillum brasilense grown in continuous culture, J. Gen. Microbiol., 1982, vol. 128, pp. 2937–2943.
O’Toole, G.A. and Kolter, R., Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis, Mol. Microbiol., 1998, vol. 28, pp. 449–461.
Petrova, L.P., Shelud’ko, A.V., and Katsy, E.I., Plasmid rearrangements and alterations in Azospirillum brasilense biofilm formation, Microbiology (Moscow), 2010, vol. 79, pp. 121–124.
Pope, L.M. and Wyss, O., Outer layers of the Azotobacter vinelandii cyst, J. Bacteriol., 1970, vol. 102, pp. 234–239.
Pradedova, E.V., Isheeva, O.D., and Salyaev, R.K., Classification of the antioxidant defense system as the ground for reasonable organization of experimental studies of the oxidative stress in plants, Russ. J. Plant Physiol., 2011, vol. 58, pp. 210–217.
Ramírez-Mata, A., López-Lara, L.I. Xiqui-Vázquez, L., Jijón-Moreno, S., Romero-Osorio, A., and Baca, B.E., The cyclic-di-GMP diguanylate cyclase CdgA has a role in biofilm formation and exopolysaccharide production in Azospirillum brasilense, Res. Microbiol., 2016, vol. 167, pp. 190–201.
Sadasivan, L. and Neyra, C.A., Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145, J. Bacteriol., 1987, vol. 169, pp. 1670–1677.
Sadoff H.L., Encystment and germination in Azotobacter vinelandii,Bacteriol. Rev., 1975, vol. 39, pp. 516–539.
Schelud’ko, A.V., Makrushin, K.V., Tugarova, A.V., Krestinenko, V.A., Panasenko, V.I., Antonyuk, L.P., and Katsy, E.I., Changes in motility of the rhizobacterium Azospirillum brasilense in the presence of plant lectins, Microbiol. Res., 2009, vol. 164, pp. 149–156.
Schloter, M. and Hartmann, A., Endophytic and surface colonization of wheat roots (Triticum aestivum) by different Azospirillum brasilense strains studied with strain-specific monoclonal antibodies, Symbiosis, 1998, vol. 25, pp. 159–179.
Shelud’ko, A.V., Filip’echeva, Y.A., Shumilova, E.M., Kh-lebtsov, B.N., Burov, A.M., Petrova, L.P., and Katsy, E.I., Changes in biofilm formation in the nonflagellated flhB1 mutant of Azospirillum brasilense Sp245, Microbiology (Moscow), 2015, vol. 84, pp. 144–151.
Shelud’ko, A.V., Filip’echeva, Y.A., Telesheva, E.M., Burov, A.M., Evstigneeva, S.S., Burygin, G.L., and Petrova, L.P., Characterization of carbohydrate-containing components of Azospirillum brasilense Sp245 biofilms, Microbiology (Moscow), 2018, vol. 87, pp. 610–620.
Shelud’ko, A.V., Filip’echeva, Y.A., Telesheva, E.M., Yevstigneeva, S.S., Petrova, L.P., and Katsy, E.I., Polar flagellum of the alphaproteobacterium Azospirillum brasilense Sp245 plays a role in biofilm biomass accumulation and in biofilm maintenance under stationary and dynamic conditions, World J. Microbiol. Biotechnol., 2019, vol. 35, no. 2, p. 19.
Shelud’ko, A.V., Shirokov, A.A., Sokolova, M.K., Sokolov, O.I., Petrova, L.P., Matora, L.Yu., and Katsy, E.I., Wheat root colonization by Azospirillum brasilense strains with different motility, Microbiology (Moscow), 2010, vol. 79, pp. 688–695.
Tarrand, J.J, Krieg, N.R., and Döbereiner, J., A taxonomic study of the Spirillum lipoferum group with description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum braslense sp. nov., Can. J. Microbiol., 1978, vol. 24, pp. 967–980.
Tugarova, A.V., Shelud’ko, A.V., Dyatlova, Yu.A., Filip’echeva, Yu.A., and Kamnev, A.A., FTIR spectroscopic study of biofilms formed by the rhizobacterium Azospirillum brasilense Sp245 and its mutant Azospirillum brasilense Sp245.1610, J. Mol. Struct., 2017, vol. 1140, pp. 142–147.
Vasil’eva, G.G., Glyan’ko, A.G., Mironova, N.V., Putilina, T.E., and Luzova, G.B., Active oxygen species in pea seedlings during the interactions with symbiotic and pathogenic microorganisms, Appl. Biochem. Microbiol., 2007, vol. 43, pp. 217–221.
Wang, D., Xu, A., Elmerich, C., and Ma, L.Z., Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions, ISME J., 2017, vol. 11, pp. 1602–1613.
Wasim, M., Bible, A.N., Xie, Z., and Alexandre, G., Alkyl hydroperoxide reductase has a role in oxidative stress resistance and in modulating changes in cell-surface properties in Azospirillum brasilense Sp245, Microbiology (SGM), 2009, vol. 155, pp. 1192–1202.
Funding
The study was partially supported by the Russian Foundation for Basic Research (project nos. 18-34-00089 and 17-08-01696).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest. This article does not contain any studies involving animals or human participants performed by the authors.
Additional information
Translated by A. Panyushkina
Rights and permissions
About this article
Cite this article
Shelud’ko, A.V., Mokeev, D.I., Evstigneeva, S.S. et al. Cell Ultrastructure in Azospirillum brasilense Biofilms. Microbiology 89, 50–63 (2020). https://doi.org/10.1134/S0026261720010142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261720010142