Abstract—
One of the methods for obtaining 123I is the bombardment of gaseous 124Xe with protons, in which nuclear reactions of production and decay of 123Xe and 123I isotopes occur. After irradiation, the gas phase is condensed from the target into a special “decay container,” in which the target isotope 123I is produced and accumulated during 123Xe decay. The amount of 123I produced in the target and deposited on its walls during the irradiation is comparable to the amount of 123I obtained in the decay container. A laboratory setup has been created and a process technology for extracting 123I from the walls of the target has been developed to increase the total yield of 123I. Organic solvents (acetone and diethyl ether) are used for this purpose. The proportion of the 123I extracted by washing off from the walls of the aluminum target is at least 84%. The loss during subsequent vacuum distillation of solvents does not exceed 5%. After vacuum distillation, the extracted 123I is dissolved in NaOH. At this stage, the efficiency of 123I washing-off with a 0.01 M NaOH solution is at least 95%. Nevertheless, even taking into account these losses, the proposed method makes it possible to additionally extract the 123I radionuclide from the target in an amount equal to or greater than the activity of the 123I produced using the existing technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Radioactive isotope 123I is widely used for diagnostics of many diseases. There are several methods today for producing 123I radionuclide for medical applications using accelerators. Extraction of 123I accumulated in 124Xe gas targets under irradiation is mainly carried out by washing off with water. Depending on the target material, weak solutions of NaOH, NaCl, and NaHSO3 are also used in addition to water, and extraction is followed by concentration and purification by applying various radiochemical methods [1–3].
The Kurchatov Institute National Research Center has developed and implemented a method for obtaining 123I radionuclide [4, 5], in which the 123I radioisotope is produced by proton bombardment of 124Xe isotope according to the reactions
The 124Xe and 123Xe gases are cryogenically condensed after irradiation into a “decay container,” where they are kept for the decay of 123Xe and the accumulation of 123I, after which 124Xe is recondensed into a storage and reuse tank. The efficiency of different technologies for collecting 123I after irradiation was analyzed in [4, 5]. The best (in terms of the minimum losses) is the technology in which 123I is extracted both from the target (immediately after irradiation) and from the decay container (at the moment when the 123I activity in it reaches a maximum).
The method currently being implemented by the Kurchatov Institute National Research Center does not allow the extraction of 123I produced in the target during irradiation. Extraction is carried out only from the decay container, which makes it possible to obtain high-purity 123I radionuclide; however, a significant part of it is lost. Although the use of the method in which 123I is extracted only from the decay container leads to large 123I losses, this method has been selected for the study since it is easier and faster to implement. The use of this method with frequent short exposures has made it possible to meet the initial needs of obtaining 123I in an amount of ~1 Ci and to move on to a higher-efficiency extraction technique based on this method.
2 KINETICS OF 123I ACCUMULATION
Let us estimate the level of unused 123I remaining inside the target after standard 6-h-long irradiation and 123Xe condensation into the decay container.
The relative amounts of 123Xe and 123I isotopes that are produced and decay during and after the irradiation are represented by simple kinetic equations using only the 123Xe and 123I decay constants.
2.1 123Xe and 123I in the Target
The change in the amounts of 123Xe and 123I isotopes during irradiation is
where λXe = 0.333 h–1 and λI = 0.052 h–1 are the decay constants of 123Xe and 123I, n1Xe(t) and N1I(t) are the amounts of 123Xe and 123I in the target during irradiation, V [h–1] is the cumulative rate of the nuclear reaction, and t [h] is the irradiation time.
Within 6 h after the irradiation and condensation of the gas phase into the decay container, the accumulated 123I remains on the walls of the target body and is subject to decay.
2.2 123Xe and 123I in the Decay Container
Provided that all the 123Xe, the potential source of the next portion of 123I, is completely transferred to the decay container, the change in their amounts is
where n2Xe(t) and N2I(t) are the amounts of 123Xe and 123I isotopes in the decay container during decay.
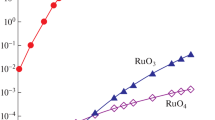
Figure 1 shows graphs of the change in the 123Xe and 123I activities in relative units. The 123I activity accumulated in the target body for 6 h, which is А1I(6) = λIN1I(6), is referred to unity. After the end of irradiation, the 123I activity begins to decrease due to decay (Fig. 1a). The iodine activity А2I(t) = λIN2I(t) in the decay container reaches its maximum max(А2I) = 0.606 rel. units after the exposure of 6.2–7.0 h (Fig. 1b). This time is determined only by the 123Xe and 123I decay constants, is independent of the irradiation parameters, and is the most advantageous for the 123I extraction from the decay container. The amount of 123I accumulated in the target by the end of the 6-h-long irradiation is 1.65 times larger than the maximum amount of 123I extracted from the decay container. The collection of 123I from the decay container at the time of its maximum activity has already been worked out well, but the technology for extracting the 123I remaining in the target has not yet been developed. Table 1 presents the values of ratio q of the 123I activity inside the target to the maximum activity in the decay container as a function of the extraction time:
where Δt is the time from the end of irradiation to the moment of 123I extraction from the target.
The data presented in Table 1 allow one to estimate the achieved technological yield of 123I upon timely extraction of 123I from the target body. In order to reduce the time from the end of irradiation to the extraction of 123I from the target, it is necessary that the technology, design, and computer control of this process be developed.
3 SETUP DESIGN
Judging by the available literature, no significant attention has been paid to the search for alternative solvent types for the extraction of the 123I remaining in the target. The 123I isotope was obtained by proton bombardment of xenon enriched in the 124Xe isotope to 20 and 40% [6]. The target bodies made of fused silica and cobalt alloy (Havar) were washed sequentially with 0.1 M NaOH and 0.1 M HCl solutions. A work is known in which highly enriched 124Xe (99.9%) was used for irradiation and the target body was made of stainless steel. The target was washed with a weak water solution of NaI and NaHSO3, and 75 ± 5% of iodine was extracted [7]. The KIPROS system in which the target is made of aluminum with an internal nickel coating has become widespread [8]. The target is washed with water, and iodine is then concentrated by ion exchange chromatography and subsequent dissolution in 0.02M NaOH.
The method that we developed is based on the use of organic solvents that provide high solubility of iodine and do not interact with the material of the target walls. The use of organic solvents offers the following advantages:
1. an increase in the 123I extraction efficiency;
2. the absence of contact between the inner surface of the target and water;
3. the applicability of simple methods for purifying the extracted 123I of radionuclide impurities using the vacuum distillation methods.
Acetone and diethyl ether were selected for the experiments since they are low-toxicity (Class 3) and high-volatility solvents and are completely removed during vacuum distillation. The use of “wet” chemistry in the 123I extraction procedure has required complications in the target design.
Figure 2 shows the diagram of the laboratory setup for 123I radionuclide production by bombarding 124Xe with protons, collecting gaseous products into a decay container, and extracting 123I from the target walls using organic solvents. The setup consists of two units: a gas-vacuum unit (the metal tubes of this unit are indicated with bold lines in the diagram) and an extraction unit (polymer tubes of the extraction unit are indicated with thin lines). Both units are connected to the target. A 3D-model and a photo of the target are shown in Fig. 3.
Diagram of the laboratory setup: (1, 10) AIR-20/M2-DA pressure sensors, (2) adsorption trap with activated carbon, (3) container with the source 124Xe, (4) measuring (auxiliary) trap for 124Xe, (5) trap for collecting irradiated 124Xe and 123Xe, (6) target, (7) syringe dispenser with acetone, (8) syringe dispenser with diethyl ether, (9) syringe dispenser with 0.01 M NaOH, (11) backing vacuum pump, (12) fused silica flask for collecting washed-off samples, (13) pumped trap, (d1–d6) Dewar vessels, and (v1–v15) valves.
Design of the target 6 in Fig. 2.
The target body is made of AD1 grade aluminum. The working chamber is a cylinder 15 mm in diameter and 50 mm in length. The chamber volume is 8.8 cm3. The entrance membrane made of aluminum with a thickness of 200 μm is sealed with Viton gaskets.
4 DEVICES AND REAGENTS
The sample activity was measured at successive technological stages of processing by detecting γ rays from 123I with energy Eγ = 159 keV using a GEM 35P4 γ-ray spectrometer from ORTEC (United States) with an HPGe detector. The weight balance during operations was controlled gravimetrically using Mettler PM6100 analytical scales; the gas pressure was monitored by AIR-20/M2-DA sensors from ELEMER (Moscow, Zelenograd, Russia). Acetone (ultrapure), diethyl ether (analytically pure), sodium hydroxide (ultrapure), and deionized water were used in the study. After working out the main technological operations, we proceeded to work with samples of 124Xe isotope enriched to 99.9% by AO PO Electrochemical Plant (Zelenogorsk, Krasnoyarsk krai, Russia) and 123I radionuclide produced at the U-150 cyclotron of the Kurchatov Institute National Research Center.
5 TECHNOLOGICAL OPERATIONS
5.1 Filling the Target with 124Xe
The preparation and the target filling were carried out as follows (Fig. 2). Before the 124Xe was fed into the working communication chamber, measuring trap 4 and target 6 were evacuated using adsorption trap 2 to a residual pressure of 1 Pa. For 124Xe gas puffing into the target to a required pressure, xenon from the vessel 3 was condensed into the measuring trap 4 with a volume of 1 cm3. The measuring trap was then slowly warmed up, and 124Xe was fed into the target to a pressure of 300 kPa. The residual 124Xe was condensed back into vessel 3.
5.2 Cyclotron Irradiation
The target with valve v6 was disconnected from the setup and was transferred for irradiation to the U-150 cyclotron. The standard irradiation for testing the technology for extracting 123I from the target using organic solvents was carried out with 30-MeV protons at a current of 1 µA for 10 min. After the irradiation, the target waited for 24 h, the 123I activity was measured by the γ-ray spectrometer, and the target was then connected to the setup.
5.3 Removal of Irradiated 124Xe
The setup was evacuated, and 124Xe condensed from the target into receiving trap 5 to a residual pressure of 1 Pa. Next, the target with valve v6 was disconnected from the setup and the remaining 123I activity was measured in it.
5.4 Filling the Target with Solvent and Draining the Eluate
The target was connected to the setup to perform 123I extraction. For this purpose, with the help of the backing vacuum pump 11, the pipelines limited by valves v5–v11 and the fused silica flask 12 were evacuated. Valve v11 on the fused silica flask and valve v10 were then blocked. Valve v6 on the target was then opened, and the target was filled with acetone, diethyl ether, or a mixture of them from syringe dispensers 7 and 8 via valves v7 and v8. After the solvent filled the target, valves v6, v7, and v8 were blocked and the pipelines were evacuated, while solvent residues were captured in pumped trap 13, which was cooled with liquid nitrogen. To carry out the extraction, the solvent was kept in the target for 15 min. The solvent was then drained from the target into the evacuated fused-silica receiving flask 12, which was cooled with liquid nitrogen. To do this, valves v11, and v6 were sequentially opened. After the solvent with the extracted 123I radionuclide was drained, valves v6 and v11 were closed and the target with valve v6 and the receiving fused-silica flask with valve v11 were disconnected from the setup in order to measure the 123I activity in them.
5.5 Vacuum Distillation of Solvent
After measuring the activity, the target and the fused silica flask were connected to the setup and the solvent was vacuum-distilled from the fused silica flask at room temperature. The solvent was captured in pumped trap 13, which was cooled with liquid nitrogen. The duration of vacuum distillation of the solvent was 20–30 min. The distillation was carried out to a residual pressure of 5 Pa in the flask. After the vacuum distillation was completed, the receiving fused-silica flask 12 together with valve v11 was disconnected from the setup to measure the 123I activity. After measuring the activity, the fused silica flask was connected to the setup and the pipelines were evacuated.
5.6 Washing 123I off with 0.01 M NaOH Solution
By sequentially opening valves v11 and v9, 0.01 M NaOH solution with a volume of 5 cm3 was fed into the flask to wash off the 123I radionuclide from the walls of the flask. Valves v11 and v9 were then blocked, and the pipelines were washed with a small amount of acetone from syringe dispenser 7 and evacuated through pumped trap 13. The receiving fused-silica flask 12 was disconnected from the setup, and 0.01 M of NaOH solution was manually sampled using a syringe with a capillary tube. After the sampling, the activity in the fused silica flask and the activity of the solution in the syringe were measured to estimate the washing-off efficiency.
6 RESULTS AND DISCUSSION
Experiments on the extraction of accumulated 123I from the target were carried out using acetone, diethyl ether, and a mixture of these solvents with a composition of 50 : 50 vol %.
The target was preliminarily evacuated before filling with solvents. When the valve was opened, the solvents were drawn into it due to the pressure drop. The results of the model experiments have shown that the degree of filling depends on both the solvent type and the filling conditions. The temperatures of the target device, pipelines, and solvent during filling were the same. The filling capacity of the inner volume of the target with acetone was at least 95% under the vacuum condition in the target and a pressure of 1 ata over the liquid acetone. In the case of filling with diethyl ether, the filling efficiency decreased to ~85%; at the same time, it was necessary to create an excess pressure of approximately 1 ata above the liquid ether during filling. Apparently, this was caused by the formation of a vapor plug in the target due to the high elasticity of diethyl ether vapor. The solvent-filled target device was kept at room temperature for 15 min.
The proportion of the solvent extracted from the target changed depending on the conditions of the process. When the solvent was drained into the evacuated fused-silica flask 12 having room temperature, it was possible to collect 85% of ether and 90% of acetone. When the flask was cooled with liquid nitrogen, the collection efficiency approached 100%. Cooling the receiving fused-silica flask 12 was also necessary to make the distillation process smoother and eliminate boiling of the solvent at the initial stage.
The next stage, i.e., the vacuum distillation of solvents from the flush condensed at a temperature of ‒196°C, was carried out when the trap was heated in an air atmosphere at room temperature. For diethyl ether, the duration of vacuum distillation under the same conditions was reduced by approximately one-third relative to the acetone; it was ~20 min for diethyl ether and ~30 min for acetone.
The obtained 123I radionuclide was washed off from the fused silica flask after vacuum distillation of organic solvents with a 0.01 M NaOH solution. As a result of this operation, the washing-off efficiency, which was determined by the ratio of the 123I activity in the flask before and after washing-off, was at least 95%.
The efficiency of 123I extraction from the target and the level of losses during solvent distillation were evaluated by measuring the 123I activities in the target and the flask before and after the operations. The measurement results are presented in Table 2.
Comparison of the results presented in Table 2 shows that the efficiency of 123I extraction with acetone is twice as high as the efficiency of 123I extraction with diethyl ether. At the same time, the 123I loss during vacuum distillation of acetone is more than five times higher than the loss during distillation of diethyl ether. These results show the possibility of further optimizing the procedure by changing the composition of the solvent mixture. This has been demonstrated by using a mixture of acetone and diethyl ether with a composition 50 : 50 vol % as an extractant, which provided the highest percentage of 123I extraction.
7 CONCLUSIONS
The laboratory setup to work out the technological process of 123I extraction from the target by washing off with organic solvents has been developed, manufactured, tested, and used. It has been demonstrated that the developed method allows extraction of at least 80% of the 123I remaining on the inner walls of the target during the 124Xe irradiation.
We believe that the developed design of the laboratory setup and technology allow computer control of the process, which will help to reduce the time from the end of irradiation to the 123I extraction from the target and to additionally extract the 123I radionuclide in an amount equal to or greater than the accumulated 123I activity by using the existing technology without increasing the cyclotron irradiation time.
REFERENCES
Levin, V.I., Popovich, V.B., Malinin, A.B., and Kurenkov, N.V., USSR Patent SU671194A1, 1980.
Flerov, G.N., Oganesyan, Yu.G., Belov, A.G., and Starodub, G.Ya., Preprint of Joint Institute for Nuclear Research, Dubna, 1985, no. 18-85-750.
Alekseev, E.G., Gusel’nikov, V.S., and Zaitsev, V.M., USSR Patent SU1709399A1, 1989.
Venikov, N.I., Vorob’ev, O.A., Novikov, V.I., Sebyakin, A.A., Sokolov, A.Yu., Fomichev, D.I., and Shabrov, V.A., Preprint of Kurchatov Institute of Atomic Energy, Moscow, 1989, no. 4934/14.
Venikov, N.I. and Sebyakin, A.A., USSR Patent SU1661842, Byull. Izobret., 1991, no. 25.
Firouzbakht, M.L., Teng, R.R., Schlyer, D.J., and Wolf, A.P., Radiochim. Acta, 1987, vol. 41, p. 1. https://doi.org/10.1524/ract.1987.41.1.1
Tarkanyi, F., Quaim, S.M., Stöcklin, G., Sajjad, M., Lambrecht, R.M., and Schweickert, H., Int. J. Radiat. Appl. Instrum., Part A, 1991, vol. 42, p. 221. https://doi.org/10.1016/0883-2889(91)90080-K
Oberdorfer, F., Meissner, M., Tiede, A., and Schweickert, H., Proc. Research Coordination Meeting on Improved High Current Liquid and Gas Targets for Cyclotron Produced Radioisotopes, Vienna, 2009, Report no. IAEA-RC-1025.3.
Funding
This work was supported by the Kurchatov Institute National Research Center, order no. 98 of January 20, 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Goryacheva
Rights and permissions
About this article
Cite this article
Artyukhov, A.A., Zagryadskiy, V.A., Kravets, I.M. et al. A Laboratory Setup for Increasing the Technological Yield of 123I from a 124Xe Target under Proton Bombardment. Instrum Exp Tech 66, 1003–1008 (2023). https://doi.org/10.1134/S0020441223060015
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020441223060015