Abstract—
The cumulative yield of the 103Ru radioisotope was measured using the activation method when a 100MoO3 target was irradiated by 4He nuclei with an energy of 60.3 MeV at the U-150 cyclotron of the Kurchatov Institute National Research Center. The measured 103Ru yield was (4.93 ± 0.84) × 104 Bq/(µA h). An experimental technique has been developed for prompt gas thermal separation of 103Ru radioisotope from an irradiated target. An experimental setup has been created to implement the 103Ru extraction technique. The design of the setup and the principle of its operation are presented. It is shown that the technique ensures the extraction of at least 97% 103Ru from the target material and the return of at least 96% 100MoO3 for reuse. The developed technique can find practical application in the production of the 103Ru radioisotope by irradiating of 100MoO3 cyclotron targets with 4He nuclei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
A new approach to cancer therapy is currently discussed in a number of publications. This approach is based on the use of modular nanotransporters (MNT) [1]. MNT are synthesized on the basis of a polypeptide platform and are capable of delivering therapeutic “short-range” radionuclides to a cancer cell nucleus. Loading MNTs with “short-range” radionuclides can effectively destroy both individual cancer cells and micrometastases that are not detected by modern diagnostic methods. At the same time, they hardly have a negative cytotoxic effect on healthy cells and tissues. This technique can be used both after surgery and after chemotherapy as a finishing therapeutic procedure aimed at eliminating the cancer recurrence.
It is proposed to use Auger electron emitters in tandem with MNT as therapeutic “short-range” radionuclides [2]. Auger electrons have a short range and a high specific linear energy loss. They are capable of damaging cells within a few tens of nanometers without having a cytotoxic effect at long distances. 103mRh isotope is considered to be one of the most promising Auger electron emitters for immunotherapy [3]. It has the smallest ratio of the number of emitted γ rays to the number of electrons and can be produced using the generator method. The 103Ru radionuclide (T1/2 = 39.274 days) is the 103mRh (T1/2 = 56.1 min) precursor in the generator. One of the ways to obtain 103Ru at the U-150 cyclotron of the Kurchatov Institute National Research Center can be the 100Mo(4He, n + p)103Ru reaction. Experimental data on the 103Ru cumulative yield in the 100Mo(4He, n + p)103Ru reaction are not available in the literature. In order to clarify the efficiency of 103Ru production in this reaction, the cumulative yield of 103Ru was measured upon irradiating a 100MoO3 target by 4He nuclei with an energy of 60.3 MeV.
The developed gas-thermal technique was used to extract 103Ru from the irradiated 100MoO3 target. An experimental setup was manufactured to implement the technique. The structural elements used in the setup and the temperature conditions were selected to ensure the target gasification and almost complete 103Ru separation from the 100MoO3 matrix.
MEASUREMENT OF THE 103Ru CUMULATIVE YIELD IN THE 100Mo(4He, n + p)103Ru REACTION
The yield of the 103Ru radioisotope was determined using an activation technique. A target in the form of a disk with a diameter of 8 mm and a thickness of 3 mm was made of MoO3 powder with molybdenum enriched to 99.7% in the 100Mo isotope. It was packed into a target device that had a 100-µm-thick aluminum foil window at the beam inlet. The target device was placed in the chamber of the U-150 cyclotron of the Kurchatov Institute National Research Center and was irradiated with 4He nuclei at a current of ≈0.15 µA until a total charge of ≈0.3 µA h was attained. The target had a bulk density of 2.0 g/cm3, which was determined by weighing. The energy of the 4He nuclei at the entrance to the 100MoO3 target was 60.3 MeV. The range of 4He nuclei, calculated using the SRIM program [4], fit into the target thickness. The total charge of nuclei incident on the target during irradiation was measured using a special integrating device. The energy of the accelerated nuclei was determined by the cyclotron parameters.
After the target was irradiated and then held for 3 days, the 103Ru activity was determined by the full-energy peak of γ rays with energy Eγ = 497.085 keV (Kγ = 91%) [5]. The measurements were made using an ORTEC GEM 35P4 γ spectrometer (United States) with a high-purity germanium detector with a volume of ~100 cm3. The targets were placed at a distance of 40 cm from the end surface of the detector during the measurements. The dead time of the spectrometer did not exceed 5% in the activity measurements. The energy dependence of the γ-ray detection efficiency was determined experimentally using reference spectrometric γ-ray sources from an OSGI kit. The acquisition time of γ spectra was 1 h. The target activity was measured several times during the 103Ru half-life.
The 103Ru yield was determined using the formula
where V [Bq/(µA h)] is the 103Ru yield; A [Bq] is the 103Ru activity in the target, referred to the end of irradiation; Z1 [rel. units] is the current-integrator reading that corresponds to a charge of 1 µA h; λ [s–1] is the decay constant of 103Ru; T1 [s] is the irradiation time equal to 1 h; Z2 [rel. units] is the current-integrator reading over the irradiation time; and T2 [s] is the the actual time of the target irradiation.
The 103Ru cumulative yield obtained in the experiment was (4.93 ± 0.84) × 104 Bq/(µA h). The measurement error of the yield was 17% with a confidence probability of 68%. The following error components were taken into account: the error in determining the detector efficiency, the error in determining the full-energy peak area in the measured γ-ray spectrum, and the error of the branching fraction.
TECHNIQUE FOR GAS THERMAL SEPARATION OF 103Ru FROM A 100MoO3 TARGET
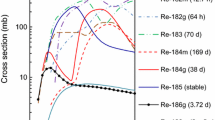
The developed technique for gas thermal separation of 103Ru from a 100MoO3 target irradiated at the cyclotron is based on a significant difference in the volatility of MoO3 and Ru oxides. Figure 1 presents comparative data on the temperature dependence of the MoO3 vapor pressure [6] and the partial pressures of RuO3 and RuO4 [7, 8] over RuO2 in an oxygen atmosphere. According to Fig. 1, the partial pressures of MoO3 vapors and Ru oxides in comparable temperature ranges differ by several orders of magnitude.
A setup has been created to implement the technique for separating 100MoO3 and Ru oxides; its diagram is shown in Fig. 2. The setup was evacuated by forevacuum pump 10 and was filled with oxygen from cylinder 1 using reducer 16. The central elements of the setup were two coaxial fused-silica ampoules 4 and 5, which were connected to a gas stand, and crucible 7 with irradiated 100MoO3 powder. The external fused-silica ampoule 4 was connected to the gas stand using a vacuum seal through a fitting made of D16 aluminum alloy. One end of the fitting was glued to the ampoule by ED-6 epoxy resin, and the other was connected to the flanges of the stand using gaskets. Such a device simplifies the sealing process, since it allows less stringent requirements for the quality of the fused-silica ampoule (the degree of its ellipsis and the surface roughness) and reduces the risk of ampoule splitting during the sealing. The vacuum or the required O2 pressure in ampoule 4 was maintained at various temperature conditions provided by thermostat−heater 6.
Block diagram of the experimental setup for separating 103Ru from the cyclotron 100MoO3 target: (1) oxygen cylinder, (2) AIR-20-M2-DA pressure gauge, (3) 1.9-L measuring flask, (4) external fused-silica ampoule (Dout = 35.5 mm, din = 31 mm, l = 400 mm), (5) two replaceable internal fused-silica ampoules (Dout = 27 mm, din = 22.5 mm, l = 250 mm), (6) thermostat−heater, (7) fused-silica crucible (Dout = 15 mm, din = 12 mm, l = 17 mm) with irradiated 100MoO3 powder containing 103Ru, (8) chromel–alumel thermocouple, (9) IRT/M2 temperature meter, (10) NVR-5D forevacuum pump, (11–15) valves, and (16) GSE gas reducer.
While testing the technique, we noticed that a black deposit of 100MoO2 appeared on the walls of the crucible after vacuum sublimation of 100MoO3 at 700°C, and its weight was ≈1% of the initial 100MoO3 weight.
According to [9, 10], the appearance of MoO2 was caused by the content of a certain proportion of lower oxides (Mo2O5, Mo4O11, Mo5O14) in MoO3. When MoO3 sublimates in vacuum, they disproportionate according to the schemes Mo2O5(solid) ↔ MoO3(gas) + MoO2(solid) and Mo4O11(solid) ↔ (MoO3)3(gas) + MoO2(solid), thus leaving MoO2 in a solid state under these conditions.
Therefore, after removing the bulk of 100MoO3, we needed an additional technological operation to gasify the residual 100MoO2.
In view of the above, the developed 103Ru separation technique consisted of a sequence of three technological operations implemented in three stages. Figure 3 illustrates the results obtained at each of the three stages.
At the first stage, 100MoO3 was distilled in vacuum at a temperature of 700°C. To do this, irradiated 100MoO3 powder with a mass of 113 mg was loaded into crucible 7. After that, the crucible was inserted into inner ampoule 5, which in turn was placed in outer ampoule 4. Ampoule 4 was sealed and then connected to the vacuum stand upon immersing in thermostat−heater 6 to a depth of 10 cm. Evacuation to a pressure of ≈2 × 10−2 mm Hg was carried out using forevacuum pump 10. A voltage was then applied to the thermostat−heater, the temperature was raised to 700°C for 45 min, and this temperature was maintained for 20 min. the 100MoO3 sample was sublimated and deposited on the walls of ampoule 5. At the end of the sublimation process, the thermostat-heater was removed, the system was cooled, and the inner ampoule and crucible were withdrawn. The crucible and ampoule 5 were weighed to balance the masses. Monitoring of the 103Ru activity in the crucible before and after the technological operation was carried out by the relative intensity of the 497.08-keV γ-ray peak of 103Ru. It was stated that at least 99% of the 100MoO3 mass were removed from the crucible at the first stage, but the 103Ru activity in the crucible did not change. However, a smoky black coating of 100MoO2 remained on the crucible walls (see Fig. 3, stage 1).
The 100MoO2 remaining in the crucible was oxidized to 100MoO3 at the second stage. To do this, the crucible was placed in a clean internal ampoule, and the preparatory operations described above were performed. The entire system (with open valves 12−15) was pumped down to a pressure of ≈2 × 10−2 mm Hg. Valve 15 was then closed, and the entire system was filled with oxygen from cylinder 1 using reducer 16 to a pressure of 75 kPa, which was monitored by pressure gauge 2. Next, valve 12 was closed, a voltage was applied to the thermostat−heater, the temperature was raised to 475°C, and this value was maintained for 20 min. After ampoule 4 was cooled, the crucible was removed, the intensity of the 497.08-keV γ-ray peak of 103Ru was measured by the area of the full-energy peak, the crucible was weighed, and the mass of molybdenum oxides contained in it was determined. As a result of oxidation (see Fig. 3, stage 2), the smoky black coating of 100MoO2 on the walls of the crucible acquired a light gray color that is characteristic of 100MoO3. (The mass change during this operation was within the measurement error.) It was found that the relative decrease in the activity of 103Ru in the crucible after the oxidation procedure, which was determined by the intensity of the 497.08-keV γ-ray peak of 103Ru, did not exceed 3%.
At the third stage that was similar to stage 1, 100MoO3 residues (i.e., the oxidized 100MoO2) were sublimated from the crucible in a vacuum at a temperature of 700°C. The 103Ru activity in the crucible did not change, and the walls of the fused-silica crucible became transparent (see Fig. 3, stage 3).
To close the circulation cycle of the 100Mo raw isotope, the 100MoO3 deposit on the walls of ampoule 5 was washed off with a 10% solution of ammonium hydroxide (NH4OH). As a result of the 100MoO3 interaction with ammonium hydroxide, ammonium para-molybdate (NH4)6100Mo7O24 was formed in the solution. By evaporating the solution and calcining the ammonium paramolybdate deposit, 100MoO3 was obtained from the reaction (NH4)6100Mo7O24 = 6NH3↑ + 7100MoO3 + 3H2O↑ according to the technique [11, 12]. The 100MoO3 loss during ammonium paramolybdate calcination did not exceed 4%.
CONCLUSIONS
The cumulative yield of the 103Ru radioisotope was measured for the first time using the activation method. The 100MoO3 target was irradiated by 4He nuclei with an energy of 60.3 MeV. The value of the measured 103Ru yield was (4.93 ± 0.84) × 104 Bq/(µA h). The experimental technique was developed for express gas thermal separation of 103Ru radioisotope from the irradiated 100MoO3 cyclotron target. The technique ensured the extraction of at least 97% of 103Ru from the target material and the return of at least 96% 100MoO3 for reuse.
REFERENCES
Sobolev, A.S., Front. Pharmacol., 2018, vol. 9, no. 952, p. 1. https://doi.org/10.3389/FPHAR.2018.00952
Filosofov, D., Kurakina, E., and Radchenko, E., Nucl. Med. Biol., 2021, no. 94, p. 1. https://doi.org/10.1016/J.NUCMEDBIO.2020.12.001
Bernhardt, P., Forssell-Aronsson, E., Jacobsson, L., and Skarnemark, G., Acta Oncol., 2001, vol. 40, no. 5, p. 602.
Ziegler, J.F., Ziegler, M.D., and Biersack, J.P., Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, vol. 268, nos. 11–12, p. 1818. https://doi.org/10.1016/j.nimb.2010.02.091
Frenne, D., Nucl. Data Sheets, 2009, vol. 110, no. 10, p. 2081. https://doi.org/10.1016/j.nds.2009.08.002
Efimov, A.I., Belokurova, L.P., Vasil’kova, I.P., and Chechev, V.P., Svoistva neorganicheskikh soedinenii. Spravochnik (Properties of Inorganic Compounds. Handbook), Leningrad: Khimiya, 1983.
Bell, W.E. and Tagami, M., J. Phys. Chem., 1963, vol. 67, p. 2432. https://doi.org/10.1021/j100805a042
Garisto, F., Thermodynamic Behavior of Ruthenium at High Temperatures, Report AECL-9552, Atomic Energy of Canada Ltd., 1988.
Kazenas, E.K. and Tsvetkov, Yu.V., Termodinamika ispareniya okislov (Thermodynamics of Oxides’ Evaporation), Moscow: LKI, 2015.
Blackburn, P.E., Hoch, M., Herrick, L., and Johnston, H.L., J. Phys. Chem., 1958, vol. 62, no. 7, p. 769. https://doi.org/10.1021/j150565a001
Lidin, R.A., Molochko, V.A., and Andreeva, L.L., Khimicheskie svoistva neorganicheskikh veshchestv (Chemical Properties of Inorganic Substances), Lidin, R.A., Ed., Moscow: Khimiya, 2000.
Zhoulan, Y. and Xinhai, L., Trans. Nonferrous Met. Soc. China, 1994, vol. 4, no. 3, p. 46.
Funding
This work was supported by the Kurchatov Institute National Research Center, order no. 2751 dated October 28, 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Goryacheva
Rights and permissions
About this article
Cite this article
Zagryadskii, V.A., Kravets, Y.M., Malamut, T.Y. et al. Measurements of the Cumulative Yield of the 103Ru Radioisotope through the 100Mo(4He, n + p)103Ru Reaction and a Technique for Gas Thermal Separation of 103Ru from a 100MoO3 Target. Instrum Exp Tech 65, 891–895 (2022). https://doi.org/10.1134/S0020441222060203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020441222060203