Abstract—
We have studied the effect of low-melting-point salt additives on the morphology and phase composition of silicon nitride prepared by combustion synthesis. The additives have been shown to influence the structure formation mechanism. Based on equilibrium compositions calculated for particular synthesis conditions, we have proposed mechanisms underlying the influence of the additives on the structure formation process. The synthesis temperature and additives have been shown to influence the phase composition of the synthesis products. Conditions have been found for the preparation of alpha-silicon nitride powders consisting of equiaxed particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Silicon nitride ceramic materials are widely used in the production of structural and functional ceramics intended for use at extremely high temperatures and mechanical loads. The strength of ceramics depends significantly on characteristics of starting silicon nitride powders. Depending on the process for the preparation of ceramic materials, use is made of powders differing in chemical composition and morphology. The particle size and shape are important parameters influencing properties of ceramics. Powders for the fabrication of ceramics by hot pressing have a large specific surface area (10–15 m2/g) and consist of equiaxed particles. In producing Si3N4 bodies by slip casting, it is preferable to use coarse powders, with a specific surface area from 5 to 7 m2/g, in order to increase the fill factor of the slip. To make allowance for the shrinkage of ceramics in three planes during sintering, it is also necessary to use powders consisting of equiaxed particles. At the same time, in the production of long bodies by slip casting, it is desirable to use Si3N4 powder consisting of elongated or filamentary particles. When a slip containing such particles is injected into a mold, they align preferentially. Subsequent sintering produces microstructure made up of preferentially aligned β-Si3N4 grains. Such structure of ceramics ensures enhanced bending strength. Thus, the shape of particles is as important as their chemical and phase compositions.
Silicon nitride powders are typically produced by furnace synthesis [1–3] or through silicon diimide synthesis and decomposition [4]. The synthesis process is controllable and allows one to obtain silicon nitride powder with tailored chemical and phase compositions. At the same time, to obtain equiaxed particles, synthesis products should be ground and classified into size ranges.
To ensure low-temperature combustion synthesis of α-Si3N4, use is made of gasifiable additives, such as NH4Cl or NH4F. Silicon nitride particles thus prepared have the form of filamentary crystals and have a fine fibrous microstructure. Such microstructure is due to the gas-phase structure formation mechanism, which involves gaseous products of NH4Cl and NH4F decomposition [5]. The preparation of α-Si3N4 with the use of submicron silicon powder, without NH4Cl or NH4F additions, also involves gas-phase structure formation, with the participation of silicon oxide and silicon suboxide. The process yields elongated particles [6]. The preparation of silicon nitride powders consisting of equiaxed particles also requires prolonged grinding and size classification. Since elongated silicon nitride particles result from gas-phase reactions, to obtain equiaxed particles one should ensure synthesis conditions preventing gas-phase structure formation. The effect of film-forming (nongasifiable) salt additives on the structure formation process during the self-propagating high-temperature synthesis (SHS) of Si3N4 has not yet been studied. In reports concerned with the azide SHS of silicon nitride, a process in which NaF or NaCl is formed as a by-product, the effect of sodium halides on the phase composition or microstructure of silicon nitride was not described [7].
In this paper, we examine the effect of low-melting-point salt additives on the microstructure and phase composition of silicon nitride.
EXPERIMENTAL
In our experiments, a Si + Si3N4 mixture was used as a basic composition. As additives, we used oxygen-free, water-soluble, low-melting-point salts. Table 1 presents characteristics of the starting mixture components.
The silicon content of the starting mixture was varied from 23 to 28 wt % in different experiments. The salt content of the starting mixture was 1 or 3 wt %. The components of the starting mixture were mixed by grinding in a ball mill for 1 h. The initial nitrogen pressure was 6.0 MPa and the weight of the starting mixture was 3.0 kg. Our experiments were carried out in a 30-L industrial SHS reactor.
The synthesized silicon nitride samples prepared using sodium fluoride were ground with ceramic balls in water for 1 h to break agglomerates and remove the sodium fluoride. The silicon nitride particles were removed from the NaF solution by vacuum filtration and washed with distilled water. After drying, the silicon nitride was deagglomerated in a jet mill.
The morphology of the synthesis products was examined by scanning electron microscopy on a LEO 1450 (Carl Zeiss SMT AG Company). The particle size of the powders was determined with a MicroSizer 201 laser analyzer. The phase composition of the synthesis products was determined by X-ray diffraction on a DRON 3M diffractometer. The weight percent of the α-phase was determined as described elsewhere [8]. The combustion temperature was measured using a W–5% Re/W–20% Re thermocouple.
RESULTS AND DISCUSSION
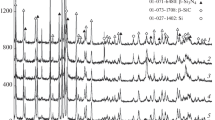
Effect of sodium fluoride. Our results on the effect of sodium fluoride on the microstructure of the synthesized Si3N4 and process parameters demonstrate that the addition of 1 wt % NaF to the starting mixture leads to the formation of agglomerates consisting of equiaxed particles ranging in size from 0.5 to 3 μm (Fig. 1). Reducing the silicon content of the starting mixture from 28 to 24 wt % lowers the combustion temperature from 1849 to 1713°C. The percentage of small particles rises, and the particles ranges in size from 0.3 to 3 μm (Fig. 2). Despite the particle size reduction, the specific surface area of all the samples was 3.4 m2/g, presumably because sodium fluoride caused consolidation of the Si3N4 particles. The α-Si3N4 content decreased from 97 to 96 wt % at 28 wt % Si and increased to 98 wt % at 24 wt % Si. At a salt additive content of 3 wt %, the combustion temperature was 1773°C. The α-phase content of the synthesis product decreased markedly: from 97 to 91 wt %, presumably because of the increase in the amount of NaF in the starting mixture. The synthesis product consisted of agglomerates made up largely of equiaxed particles 0.5 to 3 μm in size. The particles more than 1 μm in size prevailed. Also present were elongated particles, which had faceting characteristic of β-Si3N4 (Fig. 3). The specific surface area of the powder was 2.6 m2/g. No NaF was detected in the synthesis product by X-ray diffraction: the main diffraction peaks of NaF coincide with those of α-Si3N4 and cannot be distinguished. At the same time, elemental analysis on an energy dispersive analyzer indicated that the Si3N4 samples contained 0.7–1.3 wt % fluorine and sodium atoms. The presence of NaF was detected both at the point where the temperature was measured, at a depth of 40 mm, and in the surface layer of the sinter cake, where the temperature was lower. In the central part of the sinter cake, 60 mm from its surface, the microstructure was formed by needle-like particles (Fig. 4). Since there was a temperature gradient across the sinter cake during the SHS of silicon nitride, the combustion temperature in its central part exceeded 1773°C, which led to the formation of needle-like β-Si3N4 particles. The α-Si3N4 content was 51 wt %. No NaF was detected. It seems likely, that sodium fluoride gasification facilitates the α → β transition.
Calculation of the equilibrium composition with the participation of sodium fluoride indicated the formation of Si3N4, Si(l), Na(g), SiF, and liquid Na2SiO3. It seems likely that sodium silicate was formed as a film on the surface of silicon particles as a result of reaction between NaF and the oxide film on the silicon: Si + SiO2 + NaF = Na2SiO3 + SiF. Since sodium silicate has a higher melting point (1089°C) and boiling point than sodium fluoride, the Na2SiO3 film prevents the realization of the gas-phase synthesis mechanism.
The main parameters of the synthesis and characteristics of its products are summarized in Table 2.
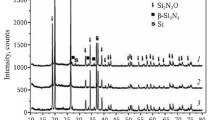
Effect of sodium hexafluorosilicate. Unlike sodium fluoride, which melts at a temperature of 992°C, sodium hexafluorosilicate decomposes at a temperature of 570°C to give sodium fluoride and SiF4 gas. As a result, after decomposition of 1 wt % Na2SiF6, 0.45 wt % NaF remained in the reaction mixture. In the case of a starting mixture containing 26 wt % silicon, the synthesis temperature was 1808°C, and the α‑Si3N4 content decreased slightly in comparison with the starting mixture: from 97 to 93 wt %. Microstructural examination showed that the synthesis product consisted predominantly of equiaxed particles 0.5 to 3 μm in size. Also present were needle-like particles, characteristic of β-Si3N4 (Fig. 5). When the silicon content of the starting mixture was reduced to 23 wt %, the combustion temperature was 1634°C. The α-Si3N4 content increased from 97 to 98 wt %. The Si3N4 agglomerates consisted of equiaxed particles 0.3 to 3 μm in size (Fig. 6). The specific surface area increased from 3.2 to 3.6 m2/g. The microstructure of the silicon nitride prepared using a starting mixture containing 26 wt % silicon and 3 wt % Na2SiF6 was formed largely by equiaxed particles 0.5 to 3 μm in size. The particles more than 1 μm in size prevailed, and there were also elongated particles (Fig. 7). The partial formation of β-Si3N4 was due to the high combustion temperature (1750°C) and the relatively high salt additive content. X-ray diffraction data indicated a decrease in the percentage of the α-phase in comparison with the starting mixture: from 97 to 95 wt %. The specific surface area decreased to 2.3 m2/g. Elemental analysis with an energy dispersive analyzer indicated the presence of 0.66 wt % Na. The main parameters of the synthesis and characteristics of its products are summarized in Table 3.
Effect of sodium chloride. On the addition of 1 wt % sodium chloride to the starting mixture, the combustion temperature was 1677 and 1615°C at 26 and 24 wt % Si, respectively. The microstructure was formed by equiaxed and elongated particles (Fig. 8). The decrease in combustion temperature was accompanied by an increase in the percentage of elongated particles (Fig. 9). The content of the α-phase increased by 2 wt % relative to the starting mixture. On the addition of 3 wt % NaCl to the starting mixture, the combustion temperature was 1802°C at 26 wt % Si. The percentage of the α-phase was the same as in the starting mixture. The microstructure was also formed by equiaxed and elongated particles (Fig. 10). The specific surface area of Si3N4 in all of the samples containing 1–3 wt % sodium chloride, with combustion temperatures in the range 1615–1802°C, ranged from 3.7 to 4.2 m2/g. The particle size and shape remained unchanged. X-ray diffraction data indicated the presence of NaCl in all of the Si3N4 samples. NaCl was shown to be present only in a surface layer of the sinter cake. It seems likely that, during the combustion of the starting mixture, NaCl vaporized in the central, hotter part of the combustion wave and condensed in a near-surface, colder layer of the sinter cake.
The formation of filamentary crystals points to a gas-phase structure formation mechanism [9]. Calculation of the equilibrium composition with the participation of sodium chloride indicated the presence of silicon nitride, Na(g), SiCl, SiO, and Si(l), which probably facilitated partial realization of a gas-phase structure formation mechanism. Because of the low boiling point of NaCl, the reaction NaCl(g) + Si(l) = SiCl + Na(g) is possible, ensuring gas-phase synthesis of needle-like Si3N4 particles according to the reaction scheme SiCl + Na(g) + N2 = Si3N4 + NaCl. In this process, NaCl condenses in a surface layer of the sinter cake. The main parameters of the synthesis and characteristics of its products are presented in Table 4.
By grinding and deagglomerating the silicon nitride samples prepared with the participation of sodium fluoride, we obtained silicon nitride powder consisting of equiaxed particles with a polydisperse distribution (Figs. 11, 12). The average particle diameter of the powder was 1.2–1.5 μm, and its specific surface area was 5–6 m2/g.
CONCLUSIONS
The use of NaF and Na2SiF6 salt additives ensures the formation of equiaxed Si3N4 particles. Increasing the percentage of the salts in the starting mixture leads to a reduction in α-Si3N4 content. The particle size of Si3N4 decreases with decreasing synthesis temperature. Increasing the salt content from 1 to 3 wt % increases the percentage of particles more than 1 μm in size. The smallest silicon nitride particles have been obtained at 1 wt % salt in the starting mixture. The particle size of Si3N4 synthesized in the presence of low-melting-point salt additives is comparable to that of the starting silicon powder. The formation of equiaxed particles is due to the low melting point and high boiling point of NaF, as well as to the formation of liquid sodium silicate on the surface of the silicon particles. The liquid NaF or Na2SiO3 film on the surface of a silicon droplet prevents gas-phase synthesis, and the nitridation process is then diffusion-controlled and occurs inside the liquid silicon droplet.
The use of NaCl as an additive leads to the formation of equiaxed and needle-like Si3N4 particles. As the combustion temperature decreases, the percentage of needle-like particles rises. It seems likely that the formation of needle-like particles is favored by the low boiling point of NaCl, which stimulates a gas-phase structure formation mechanism according to the reaction schemes NaCl(g) + Si(l) = SiCl + Na(g) and SiCl + Na(g) + N2 = Si3N4 + NaCl. Increasing the NaCl content of the starting mixture to 3 wt % causes no decrease in the percentage of α-Si3N4.
REFERENCES
Atkinson, A., Moulson, A.J., and Roberts, E.W., Nitridation of high purity silicon, J. Am. Ceram. Soc., 1976, vol. 59, no. 3, pp. 285–289.
Zhang, S.C. and Cannon, W.R., Preparation of silicon nitride from silica, J. Am. Ceram. Soc., 1984, vol. 67, no. 10, pp. 691–695.
Ekelund, M., Forslund, B., and Zheng, J., Control of particle size in Si3N4 powders prepared by high-pressure carbothermal nitridation, J. Mater. Sci., 1996, vol. 21, no. 21, pp. 5749–5757.
Yamada, T., Preparation and evaluation of sinterable silicon nitride powder by imide decomposition method, Am. Ceram. Soc. Bull., 1993, vol. 72, no. 5, pp. 99–106.
Zakorzhevsky, V.V. and Borovinskaya, I.P., Some regularities of α-Si3N4 synthesis in a commercial SHS reactor, Int. J. Self-Propag. High-Temp. Synth., 2000, vol. 9, no. 2, pp. 171–191.
Zakorzhevsky, V.V. and Borovinskaya, I.P., Combustion synthesis of silicon nitride using ultrafine silicon powders, Powder Metall. Met. Ceram., 2009, vol. 48, nos. 7–8, pp. 375–380.
Amosov, A.P. and Bichurov, G.V., Azidnaya tekhnologiya samorasprostranyayushchegosya vysokotemperaturnogo sinteza mikro- i nanoporoshkov nitridov (Azide Route in Self-Propagating High-Temperature Synthesis of Nitride Micro- and Nanopowders), Moscow: Mashinostroenie-1, 2007.
Per-Olov Käll, Quantitative phase analysis of Si3N4-based materials, Chem. Scr., 1988, vol. 28, pp. 439–446.
Givargizov E.I. Rost nitevidnykh i plastinchatykh kristallov iz para (Vapor Growth of Filamentary and Platelike Crystals), Moscow: Nauka, 1977.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zakorzhevskii, V.V., Mukhina, N.I. Combustion Synthesis of α-Si3N4 with the Participation of Low-Melting-Point Salt Additives. Inorg Mater 55, 1195–1200 (2019). https://doi.org/10.1134/S002016851912015X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016851912015X