Abstract
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome that develops in patients with severe liver dysfunction and/or portocaval shunting. Despite more than a century of research into the relationship between liver damage and development of encephalopathy, pathogenetic mechanisms of hepatic encephalopathy have not yet been fully elucidated. It is generally recognized, however, that the main trigger of neurologic complications in hepatic encephalopathy is the neurotoxin ammonia/ammonium, concentration of which in the blood increases to toxic levels (hyperammonemia), when detoxification function of the liver is impaired. Freely penetrating into brain cells and affecting NMDA-receptor-mediated signaling, ammonia triggers a pathological cascade leading to the sharp inhibition of aerobic glucose metabolism, oxidative stress, brain hypoperfusion, nerve cell damage, and formation of neurological deficits. Brain hypoperfusion, in turn, could be due to the impaired oxygen transport function of erythrocytes, because of the disturbed energy metabolism that occurs in the membranes and inside erythrocytes and controls affinity of hemoglobin for oxygen, which determines the degree of oxygenation of blood and tissues. In our recent study, this causal relationship was confirmed and novel ammonium-induced pro-oxidant effect mediated by excessive activation of NMDA receptors leading to impaired oxygen transport function of erythrocytes was revealed. For a more complete evaluation of “erythrocytic” factors that diminish brain oxygenation and lead to encephalopathy, in this study, activity of the enzymes and concentration of metabolites of glycolysis and Rapoport–Lubering shunt, as well as morphological characteristics of erythrocytes from the rats with acute hyperammoniemia were determined. To elucidate the role of NMDA receptors in the above processes, MK-801, a non-competitive receptor antagonist, was used. Based on the obtained results it can be concluded that it is necessary to consider ammonium-induced morphofunctional disorders of erythrocytes and hemoglobinemia which can occur as a result of alterations in highly integrated networks of metabolic pathways may act as an additional systemic “erythrocytic” pathogenetic factor to prevent the onset and progression of cerebral hypoperfusion in hepatic encephalopathy accompanied by hyperammonemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome that can develop as a result of acute or chronic liver diseases and/or portocaval collateral circulation, clinical manifestations of which vary from minimal disturbances of consciousness and behavior [1] to dementia and coma that usually leads to death [2]. Although there has been much research on the problem of central nervous system lesions, pathogenetic mechanisms of brain dysfunction in HE remains to be completely clarified.
Multiple etiological factors associated with the development of HE have been reported, which include bacterial and fungal infections [3], systemic inflammation [4], amino acid imbalance and products of their abnormal metabolism [5], some medications [6], different toxins [7], and many others [8].
Ammonia/ammonium is universally recognized to be a major neurotoxin that plays a pivotal role in the pathogenesis of HE [9]. In the case of impaired liver function associated with injury to hepatocytes and/or a portosystemic shunt, ammonia accumulates in the blood (hyperammonemia, HA) up to toxic levels and easily enters the brain, where acute ammonia toxicity mediated by hyperactivation of NMDA receptors (NMDA-R) [10] have an adverse effect on many energy-generating oxygen-dependent biochemical processes [2, 11], which underlie activity of nerve cells and organism as a whole [12].
It should be noted, however, that the available data that confirm presence of NMDA-R not only in the brain, but also in some peripheral organs, such as the liver, heart, pancreas, as well as erythrocytes [13], allow suggesting that toxic effects of ammonia/ammonium in HE, intensified by hyperactivation of NMDA-R in peripheral tissues, could be more generalized than previously thought.
Our recent findings that partially have confirmed this assumption, bring new insights into the mechanism of causal NMDA-R-dependent relationship between the liver dysfunction (“hepato”) and brain damage (“encephalopathy”). Knowing that brain cells are strictly dependent on glucose and ketones as essential energy substrates [14] formed exclusively in the liver due to gluconeogenesis and ketogenesis, our data show that the ammonia-induced and hyperactivated NMDA-R-mediated dysfunction of the mitochondrial respiratory chain of rat liver mitochondria leads to the rapid and abrupt inhibition of gluconeogenesis and ketogenesis. This, in turn, caused a significant drop in the level of both energy substrates in the liver and blood and preceded the initial stage of neurological disorders (lethargy, drowsiness, incoordination of movements, tremors and myoclonic convulsions) in animals with acute HA. Complete inhibition of gluconeogenesis and ketogenesis, resulting in pronounced hypoglycemia and hypoketonemia, and, thus, causing the levels of glucose and ketones to dramatically reduce in brains of these animals was the terminal event preceding the development of coma [15].
In addition, we were able to identify another, extrahepatic, ammonium-induced pathological change, namely, pro-oxidant – antioxidant imbalance in erythrocytes, which is directly related to the disruption of their oxygen transport function. In particular, this study showed that in animals with HA, most of the ammonia/ammonium accumulates not in plasma, but in erythrocytes, which makes these cells, devoid of ammonia-detoxifying enzymes, more sensitive (than other non-neuronal tissues) to the ammonia-induced NMDA-R-mediated oxidative stress [16].
As is known, oxidative stress has detrimental effect on the oxygen transport capacity of erythrocytes [17, 18], which is related to metabolic/energy processes that occur in the membranes and within erythrocytes [19] and regulate affinity of hemoglobin for oxygen, which should be high for maximizing hemoglobin-oxygen binding capacity in the lungs, but should be low for unhindered release of oxygen by hemoglobin and diffusion of oxygen into tissues [20, 21].
It is worthy of note that first reports on the relationship between the energy/metabolic processes in erythrocytes and ability of hemoglobin to bind, transport, and deliver oxygen to tissues were published more than 50 years ago [20, 22], but these scientific discoveries, as well as the fact that erythrocytes should be sufficiently “healthy” to perform their functions [23], have been mostly ignored. For instance, increase in the erythrocyte sedimentation rate (ESR, a hematological index that is necessarily determined in each patient, regardless of complaints) is usually associated with the appearance of inflammatory processes in the body [24], while decrease in the negative charge of the erythrocyte membrane (which underlies increase in ESR), leading to structural, biochemical disorders, aggregation of erythrocytes, increase in blood viscosity, “stagnation” in capillaries, which breaks microcirculatory blood flow and oxygen supply to tissues [25], is usually disregarded.
And erythrocytes, which are one of the important components in the integrated oxygen transport system (apart from cardiovascular and respiratory systems), due to the apparent “simplicity” of organization, are still considered to be little bags of hemoglobin, which, even under non-physiological conditions, easily bind to oxygen and easily transport oxygen from the lungs to tissues. This viewpoint regarding versatility and capacities of erythrocytes is supported in the situations, when the degree of hypoxemia/hypoxia is detected by estimates of SaO2 (oxygen saturation: percentage of oxygen-bound hemoglobin (Hb) in the arterial blood) and PaO2 (partial pressure of oxygen), which are usually measured in the clinical setting. In this case, despite the fact that deviation from the norm of at least one indicator of a highly integrated system of metabolic pathways in erythrocytes, which controls allosteric regulation, structure and, in general, oxygen-binding properties of Hb [26], can increase the affinity of Hb for oxygen and disrupt its transition to tissue. This can lead to multiorgan hypoxia [27, 28], irreversible brain damage, and persistent cognitive impairment [23, 29-31] even with normal values of SaO2 and PaO2 [32], which is not taken into account in medical practice. Hence, it should be admitted that there is no tool used nowadays to diagnose metabolic indicators in erythrocytes associated with impairment of their oxygen transport function and with the development of encephalopathy arising under the influence of ammonia/ammonium, concentration of which in blood and brain in many pathologies can multiply tenfold compared to the norm [33-35].
HE is not an exception. Despite the fact that the ammonia-induced encephalopathy, on the one hand, is accompanied by deviations from the norm of many hematological parameters [36] affecting oxygen delivery to tissues, and, on the other hand, is accompanied by multiorgan hypoxia [37], which includes reduced brain oxygenation [38] inevitably leading to brain pathology [39] and progression of persistent cognitive dysfunction, the causal relationship between energy metabolic alterations in erythrocytes and disorder of their functions has not been currently established.
To identify additional intracellular parameters of the energy metabolism in erythrocytes, which are necessary for more complete assessment of the effectiveness of their oxygen transport function and timely recognition of risk factors for the development of ammonia-induced encephalopathy, activity of the regulatory enzymes and concentration of the metabolites of glycolysis and Rapoport–Lubering shunt in erythrocytes of the rats with acute HA were measured. To examine involvement of NMDA-R, studies were conducted using MK-801, a non-competitive NMDA receptor antagonist.
MATERIALS AND METHODS
Materials. The following reagents were used: ammonium acetate, MK-801, NAD+, NADH, NADP+, NADPH, Tris, TEA, ATP, ADP, EGTA, EDTA, phosphoenolpyruvate, glucose, pyruvate, ouabain, saponin, myokinase, pyruvate kinase, lactate dehydrogenase, glucose-6-phosphate dehydrogenase, glyceraldehyde-3-phosphate dehydrogenase, alfa-cellulose, hemicrystalline cellulose type 50 came from Sigma Chemical Company (USA). A kit for determination of 2,3-DPG concentration was obtained from Roche (Austria).
All other reagents (Russia) were of extra pure and chemically pure grades.
Animals. Male Wistar rats (210 to 230 g) were used in the experiments. The rats were housed in the vivarium at room temperature. The vivarium was maintained under natural lighting conditions. The rats had access to food and water ad libitum. Rats were divided into groups of 10 rats each. Rats from the Group 1 (ammonium acetate) received a single intraperitoneal (i.p.) injection of ammonium acetate at a sublethal dose of 7 mmol/kg, were next decapitated 15 min after i.p. injection, usually after two seizure episodes [16].
The results of our previous study showed that the effects of acute ammonia intoxication on energy metabolism in the rat brain are clearly seen 15 min after i.p. injection [40].
So, for this experiment, this time interval (15 min) was used. Animals from the Group 2 (control) were injected with the sublethal dose of 9% (m/V) NaCl (7 mmol/kg; i.p.). Animals were decapitated 15 min after injection. Saline (physiological saline) was chosen as the normal control since it was found in our earlier experiments that injection of sodium acetate or physiological saline had no negative influence on the parameters measured. Rats from the Group 3 (group MK-801) were injected with MK-801 (i.p., 2.0 mg/kg), a dose which was needed to completely block the receptors. Rats from the Group 3 were decapitated 30 min after injection. Rats from the Group 4 (MK-801 + ammonium acetate) were injected with 2.0 mg/kg of MK-801 and then, 15 min after injection with MK-801, ammonium acetate injection at a dosage of 7 mmol/kg was administered. Decapitation was performed 15 min after ammonium acetate injection.
Plasma separation and purification of erythrocytes. To separate plasma and prepare purified erythrocytes, blood taken during decapitation (130 mM of 3Na-citrate was used as an anticoagulant, pH 7.4) was divided into two portions. Portion I was used to remove formed elements from plasma. Blood separation was performed by centrifugation for 10 min at 1000g (4°C). Level of free hemoglobin was immediately measured in the obtained supernatant. In order to prepare erythrocytes purified from leukocytes and platelets, the second portion of the blood was filtered through a column of alfa-cellulose/hemicrystalline cellulose type 50 at a 1 : 1 ratio and equilibrated with 0.9% (w/v) NaCl [41]. Elution was performed (1 : 5) at room temperature with a solution containing 10 mM KH2PO4, pH 7.4, 150 mM NaCl. Erythrocytes were precipitated during centrifugation (10 min, 1000g at 4°C), washed twice with a solution containing 10 mM KH2PO4, pH 7.4, 140 mM NaCl, 5 mM KCl, 2.8 mM glucose, 0.5 mM K-EDTA (10 min, 4°C, 1000g, 1500g, and 2000g), and resuspended in the same solution at a 1 : 5 ratio (v/v).
Preparation of lysates from erythrocytes for determination of enzyme activity. Erythrocyte samples (1 ml) purified from platelets and leukocytes were lysed in 2 ml of hypoosmotic lysis buffer (50 mM TEA, pH 7.4/0.15 mM K-EGTA, 3 mM beta-mercaptoethanol) containing 0.2% saponin. Enzyme activity was determined within the first two hours following preparation of lysates. Prior measurements, probes were stored at 4°C.
Determination of enzyme activity in lysates from erythrocytes. Activities of the Na+/K+-exchanging ATPase (EC 3.6.3.9), hexokinase (HK, EC 2.7.1.1), phosphofructokinase (PFK, EC 2.7.1.11), pyruvate kinase (PK, EC 2.7.1.40), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12), and lactate dehydrogenase (LDH, EC 1.1.1.27) were determined spectrophotometrically from the rates of NAD+ or NADPH formation monitored at 340 nm using the methods of enzymatic analysis for erythrocytes. These methods were developed by the international committee on standardization in hematology [41] and have been described in our previous study [42].
Preparation of erythrocyte extracts for determination of concentration of metabolites. Purified erythrocytes were mixed with a cooled mixture (–20°C) of 6% HClO4/40% C2H5OH at a ratio of 1 to 10. The resulting solution was centrifuged for 5 min at 4°C and 10,000g. The precipitate was removed, pH value in the supernatant was adjusted to 5-6 using 30% (m/m) KOH and dry KHCO3. The precipitate of potassium perchlorate was removed by centrifugation using the same conditions. The clear supernatant solution was immediately used for determination of metabolite concentration.
Determination of concentrations of ATP, ADP, AMP, lactate, pyruvate, and 2,3-diphosphoglycerate in erythrocyte extracts. Concentrations of adenine nucleotides, lactate, and pyruvate were measured by common spectrophotometry procedures [43] described in our previous study [42]. Energy charge (EC) was calculated using the Atkinson’s equation [44]: EC = (ATP + 0.5ADP)/(ATP + ADP + AMP).
NAD+/NADH ratio was estimated by the method of Williamson et al. [45], based on the equilibrium constant of 1.11×104 for lactate dehydrogenase reaction.
Concentration of 2,3-diphosphoglycerate was measured spectrophotometrically using a commercial kit (Roche, cat. # 10 148 334 001) containing a mixture of enzymes (phosphoglycerate mutase, phosphoglycerate kinase, glyceraldehyde-3-phosphate dehydrogenase, triosephosphate isomerase, and glycerol-3-phosphate dehydrogenase) according to the enclosed instructions.
Determination of hemoglobin concentration in blood and plasma. Osmotic fragility and morphology analysis of erythrocytes. Hb concentration was measured with a Dymind DH 36 Vet hematology analyzer (Dymind, China). Whole blood or plasma stabilized with 3-Na+-citrate was used in this study. The degree of hemolysis in erythrocytes was calculated using the formula: % hemolysis = [(1-Hct) × free Hb (g/dl) × 100]/total Hb (g/dl) [46].
A CELENA® S Digital Imaging System (LogosBio), lens × 40, was used for cell morphology analysis. Native whole blood smears were prepared using the conventional method on fat-free glass slides. The prepared blood smears were air-dried at room temperature, they were not additionally fixed or stained.
Osmotic fragility of erythrocytes was evaluated by the degree of erythrocyte cell lysis after incubation with different concentrations of NaCl (from 0 to 154 mM (0.9%) NaCl). The degree of erythrocyte lysis was estimated spectrophotometrically. For this purpose, concentration of the released Hb was determined from optical density at 540 nm after hemolysis was stopped by the addition of an equal amount of NaCl to the specimens at concentration needed for re-establishing of isotonicity. Concentration of NaCl, at which minimal (primary) and 50% cell lysis occurred, was used for quantitative assessment of osmotic fragility of erythrocytes.
Statistical analysis was performed using Prizm V8 (GraphPad, USA). The data were expressed as a mean and standard error of the mean. Normal distribution of variables was confirmed by the Kolmogorov–Smirnov test. Differences between the groups were analyzed with the Student’s t-test, whereas ANOVA test with a Bonferroni correction was used for multiple comparisons.
RESULTS AND DISCUSSION
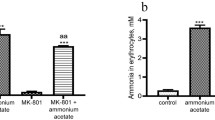
Influence of ammonia/ammonium and MK-801 on activity of the regulatory enzymes of rat erythrocyte glycolysis. In the first part of our study [16], it was shown that the ammonia content of rat erythrocytes from the control group was 0.300 ± 0.038 mmol/liter, which is within the acceptable range of values corresponding to physiological norm for the erythrocytes of these animals [47].
After a single injection with MK-801, no difference in the concentration of ammonia was observed between the experimental and control groups, while after injection of 7 mmol/kg of ammonium acetate, ammonia concentration in the animal erythrocytes increased to 3.602 ± 0.126 mM (p < 0.001). When MK-801 was injected in combination with ammonium acetate, concentration of ammonia in erythrocytes was lower (by 20%, p < 0.05), but remained at a rather high level (3.320 ± 0.06 mM) in comparison with that in control. In this situation, erythrocytes lacking ammonia-detoxifying enzymes become more susceptible (than other non-neuronal tissues) to ammonia-induced oxidative stress [48].
It is known that oxidative stress occurs, when the relationship between the oxygen transport function of erythrocytes and glycolytic system of erythrocytes [49] containing metabolites and enzymes that regulate affinity of hemoglobin for oxygen, is disturbed [20].
Therefore, in order to determine additional indicators, which are necessary for assessment of the capability of erythrocytes to deliver oxygen to tissues, the primary objective of our study was to evaluate whether accumulation of ammonia/ammonium in erythrocytes that retain NMDA-R has association with the disorders of glycolysis, a main metabolic pathway that is needed for maintenance of functional state and viability of erythrocytes. For this purpose, activities of the major regulatory enzymes such as HK, PFK, PK, as well as GAPDH and LDH were measured.
The results on the activities of enzymes measured in the rat erythrocytes from different groups under study are summarized in Fig. 1.
a-e) Activity of HK, PFK, PK, GAPDH, and LDH in the erythrocytes of rats from experiment groups (n = 10 rats in each group). Rats received intraperitoneal injection of 7 mmol/kg of ammonium acetate (ammonium acetate group), single intraperitoneal injection of 2 mg/kg of MK-801 (MK-801 group), and intraperitoneal injection of MK-801 15 min before the injection of ammonium acetate (MK-801 + ammonium acetate group). Rats from the control group (control) were injected with normal saline solution. Time of animal decapitation and methods for determining activity of the enzymes are presented in the Materials and Methods section. Enzyme activity is expressed as μmol/(min×g Hb). Results are expressed as mean ± SEM. *** p < 0.001 as compared to the control group of animals; a, p < 0.05; aa, p < 0.01; aaa, p < 0.001, as compared to the “ammonium acetate” group of animals. Differences between the groups were evaluated using ANOVA followed by Bonferroni corrections.
Figure 1 shows that injection of ammonium acetate induced decrease in the enzyme activities to different degrees in animal erythrocytes. For instance, activities of HK, PFK, PK, and GAPDH decreased by 38% (p < 0.001), 26% (p < 0.001), 22% (p < 0.001), and 30% (GAPDH, p < 0.001), respectively, whereas activity of LDH in the presence of ammonia intoxication remained the same as measured in the erythrocytes from the control group of animals (Fig. 1d). When MK-801 was injected, the values of measured activities of all the enzymes under study lay within the limits typical of the control values. When MK-801 was administered in combination with ammonium acetate, restoration of the activity of all enzymes under study to the levels observed in the control group of animals was observed. This indicates that the ammonium-induced inhibition of enzymes in glycolysis is mediated by activation of NMDA-R.
Additional evidence of the NMDA-R-dependent inhibition of glycolytic flux was also provided by restoration to control values of the sharply reduced lactate and pyruvate concentrations and increased NAD+/NADH ratio in the erythrocytes of animals with HA under the influence of MK-801 administered together with ammonium acetate (Fig. 2).
Concentration of lactate (a), pyruvate (b) and calculated NAD+/NADH ratio (c) in the erythrocytes of animals from experiment groups (n = 10 rats in each group). Regimes of drug administration, dosage, decapitation time are given in the legend to Fig. 1. Methods for determination of pyruvate and lactate concentrations are given in the Materials and methods section. Concentration of metabolites is presented as µmol/liter (µM). NAD+/NADH ratio was calculated according to the method [45]: NAD+/NADH = [pyruvate]/[lactate]×1/K, where K is the equilibrium constant of the reaction with lactate dehydrogenase and equal to 1.11×104. Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 as compared to the control group of animals; a, p < 0.05; aa, p < 0.01; aaa, p < 0.001, as compared to the “ammonium acetate” group of animals. Differences between the groups were evaluated using ANOVA followed by Bonferroni corrections.
These data partially explain the causes of inhibition of the glycolytic flux mainly related to the disorder of metabolic control in erythrocytes of the rats with HA leading to imbalance in the NAD+/NADH ratio (Fig. 2c) due to deficiency of pyruvate (Fig. 2b) and NADH, which are necessary for NAD+ regeneration in LDH reaction and glycolysis reestablishing at the stage of GAPDH-reaction [50].
According to the generally accepted opinion, the rate of glucose metabolism in erythrocytes is mainly regulated by the adsorption mechanism [51], based on reversible binding of glycolytic enzymes to the transmembrane band 3 protein (band 3). The essence of this mechanism is that disconnection of enzymes from the protein (activation of enzymes) or attachment to the protein (inactive form of enzymes) [53] mediated by posttranslational modifications of the band 3 protein [52] allows the mature erythrocytes devoid of mitochondria to instantly adaptively respond to endogenous or emerging factors in the bloodstream, and by switching the glucose flow from glycolysis to pentose phosphate pathway (PPP) (and in the opposite direction), to keep the state of energy metabolism and antioxidant system of red blood cells within the physiological norm [54].
As a consequence, decrease in the activity of glycolytic enzymes in the erythrocytes of rats with HA may be an adaptive response that maintains the intracellular antioxidant balance by inhibiting glycolytic flux and activating PPP [55], which ensures synthesis of NADPH necessary for preventing transition of ferroform (Fe2+) Hb to ferriform (Fe3+) in the presence of reactive oxygen species accumulated in erythrocytes of the animals with HA [56].
However, according to our recent studies, activity of glucose-6-phosphate dehydrogenase (G6PDH), a key PPP enzyme in the erythrocytes of rats with HA, significantly decreased compared with the control. Together with the sharp drop in the ratios of NADPH/NADP+ and GSH/GSSG, this indicated inhibition of PPP, which was one of the reasons for the triple accumulation of hydrogen peroxide and oxidative stress [16], which, in turn, excludes the possibility of considering the decrease in activity of glycolytic enzymes in the acute HA (Fig. 1) even in the form of a short-term as an adaptive response of red blood cells.
On the other hand, involvement of NMDA-R in the ammonium-induced inhibition of glycolysis, which has been discovered in our experiments, makes it possible to assume existence of a relationship between the Ca2+-dependent signaling cascade triggered by hyperactive NMDA-R, leading to increased formation of NO• in erythrocytes [13], and NO•-mediated posttranslational modification of the band 3 protein [52], which regulates the rate of glucose metabolism [54].
Given this, it can be assumed that one of the causes of the inhibition of regulatory glycolysis enzymes in erythrocytes of animals with HA under oxidative stress may be associated with the NO•-dependent inhibition of phosphotyrosine kinase, which, in combination with nitrosylation of tyrosine residues of the band 3 protein, leads to irreversible inhibition of glycolysis [57]. It is also possible that NO• and its derivatives can have a direct inhibitory effect on glycolytic enzymes in erythrocytes [58].
Although, if we take into account numerous causes leading to inhibition of the glycolytic pathway (inhibition of enzymes by reaction products [59] and posttranslational modifications [60], effect of pH, presence of deoxy-Hb competing with glycolysis enzymes for binding sites to the cytoplasmic domain of the band 3 protein [54], state of the glucose transporter [61], and presence of hormones in the blood [62]) it becomes obvious that in order to identify the mechanisms responsible for inhibition of glucose utilization in erythrocytes under conditions of oxidative stress, which we found in animals with HA, further detailed research is required that takes into consideration the discovered involvement of NMDA-R in the ammonium-induced inhibition of glycolytic enzymes.
Influence of ammonia/ammonium and MK-801 on the content of adenine nucleotides in rat erythrocytes. A distinctive feature of anaerobic glycolysis in erythrocytes is that it is the main pathway of ATP production, which is used by cells to support numerous vital functions. Therefore, inhibition of glycolysis observed in erythrocytes of the rats with HA (Figs. 1, 2) was predicted to cause decrease in ATP concentration and total adenine nucleotide pool.
Figure 3 represents the results obtained from determination of ATP, ADP, AMP concentrations, total content of adenine nucleotides (AN), ATP/ADP ratio, and calculation of the energy charge (EC), which is a metric indicating energetic status of the cell.
Concentrations of ATP (a), ADP (b), and AMP (c), ATP/ADP ratio (d), total amount of AN (e), and EC (f) in erythrocytes of the rats from experiment groups (n = 10 per group). Regimes of drug administration, dosage, decapitation time are given in the legend to Fig. 1. Methods for determination of ATP, ADP, and AMP concentrations are given in the Materials and methods section. Concentration of metabolites is presented as µmol/liter (µM). Energy charge was calculated according to Atkinson [44]: EC = (ATP + 0.5 ADP)/(ATP + ADP + AMP). Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 as compared to the control group of animals; a, p < 0.05; aa, p < 0.01, as compared to the “ammonium acetate” group of animals. Differences between the groups were evaluated using ANOVA followed by Bonferroni corrections.
As shown in Fig. 3, intracellular ATP concentration in the erythrocytes of rats with HA decreases (40%, p < 0.01) in comparison with the control as the level of activity of glycolytic flux goes down. The increased ATP hydrolysis contributed to elevations of ADP and AMP concentrations by 69% (p < 0.05) and 113% (p < 0.001), respectively (Fig. 3, a-c), and to the 3-fold decrease in the ATP/ADP ratio (Fig. 3e, p < 0.01). The sum of AH (Fig. 3d) and EC (Fig. 3f) became less than in the control by 27% (p < 0.05) and 20% (p < 0.001), respectively. Single injection of MK-801 did not affect these parameters (relative to the control), but after injection of MK-801 in combination with ammonium acetate injection, all measured parameters of adenine nucleotide contents were within the control range (Fig. 3, a-f). These findings suggest that the increased ATP utilization, which is not compensated by the slow glycolytic flux and leads to a metabolic alteration in erythrocytes of animals with HA, is mediated by the NMDA-R hyperactivation.
Influence of ammonia/ammonium and MK-801 on activity of Na+,K+-ATPase in rat erythrocytes. Most of the ATP energy generated in erythrocytes during glycolysis is used to activate Na+,K+-ATPase [63], main function of which is to maintain concentration gradient of Na+ and K+ necessary for regulation of aqueous homeostasis, cells volume and shape, and other numerous specialized vital functions of erythrocytes [64]. To identify relationship between the NMDA-R-dependent ammonium-induced ATP hydrolysis and functional ability of Na+,K+-ATPase, we measured activity of this enzyme in the erythrocytes of rats of all studied groups. The data are presented in Fig. 4.
a, b) Activity of Na+,K+-ATPase in erythrocytes of the rats from experimental groups (n = 10 rats in each group). Regimes of drug administration, dosage, decapitation time are given in the legend to Fig. 1. Methods for determination of the Na+,K+-ATPase activity are given in the Materials and Methods section. The enzyme activity is expressed as µmol/min × g of Hb. Results are expressed as mean ± SEM. * p < 0.05 as compared to the animals from the control group. Differences between the groups were evaluated using ANOVA followed by Bonferroni corrections. Pearson correlation coefficient (r) was used to determine association between the concentration of ammonia in erythrocytes and activity of Na+,K+-ATPase. Analysis was carried out using GraphPad Prism V8 software. Negative correlation between the ammonia concentration and the Na+,K+-ATPase activity in erythrocytes of rats with HA (r = – 0.899, p = 0.0004) was determined to be significant.
As shown in Fig. 4a, for erythrocytes of the animals injected with a sublethal dose of ammonium acetate, activity of Na+,K+-ATPase is almost two times lower (p < 0.05) than that in the control group of animals. Neither the single injection of MK-801 nor the MK-801 injection in combination with ammonium acetate injection affected the enzyme activity. This indicates that the ammonia-induced inhibition of Na+,K+-ATPase in erythrocytes is not dependent on NMDA-R but exclusively mediated by the effect of ammonia as evidenced by the presence of a significant negative correlation between the concentration of ammonia accumulated in erythrocytes of the animals with HA and Na+,K+-ATPase activity (r = –0.899, p = 0.0004, Fig. 4b).
In biological fluids, ammonia exists in two forms: in the form of NH3, an uncharged lipophilic form, which ensures its rapid permeability through the membrane of erythrocytes [65], and in a protonated form, NH4+ (ammonium ion), which is transported into the cells slower (compared with the gaseous NH3) [66] and with the help of specific transporters, localized on the membrane of erythrocytes [67].
Considering the fact that the transport of NH4+ into animal erythrocytes can lead to the decrease in intracellular concentration of K+ and increase in the concentration of Na+ and Cl– [68], we can assume that one of the reasons for the ammonium-induced drop of the activity of Na+,K+-ATPase is sharp decrease in the concentration of ATP, which becomes not sufficient for the increased energy requirement necessary for functional activity of the enzyme focused on maintaining ionic gradient, which is significantly distorted by the intense transport of both forms of ammonia into erythrocytes of the animals with HA. It is likely that the NH4+-dependent acidification of the intracellular environment of erythrocytes (inhibition of glycolysis), which replaces alkalization caused by the primary transport of NH3 into cells [67], plays an important role in inhibiting glycolysis, reducing ATP level, and inhibiting Na+,K+-ATPase in erythrocytes of the animals with HA [69].
It is also well known that the enzyme activity is largely inhibited under conditions of oxidative stress [70] that is partially explained by numerous free radical modifications leading to conformational changes and impaired function of the Na+,K+-ATPase [71], which is in agreement with the data showing that the non-specific inhibition of Na+,K+-ATPase under conditions of oxidative stress is typical not only for the red blood cells of the patients with liver diseases [72], but also for the patients with many other pathologies [73].
Interestingly, despite the fact that there are multiple mechanisms of inhibition of Na+,K+-ATPase and they may vary depending on specific conditions [64], blockade of the Na+/K+-pump in erythrocytes, observed in many pathologies, usually leads to the change in their shape, deterioration of the ability to deform, and intense lysis of erythrocytes in the bloodstream [74].
In this regard, it should be noted that, although it is generally recognized that the main cause of HE is HA, and the fact that ammonium salts increase the volume of red blood cells and cause their lysis has been known for more than a century [75], presence of intravascular hemolysis in the patients with HE is explained mainly by high pressure in the portal vein (portal hypertension), bleeding from the varicose veins of gastrointestinal tract [76], injury to the vein when taking blood for analysis [77], and many other reasons [78], whereas the role of ammonia/ammonium in the lysis of red blood cells in vivo is practically not considered in the routine clinical practice. To identify the missing link, in the next stage of our research we focused on the exploring the effect of HA on morphology of circulating erythrocytes, on their osmotic resistance, degree of lysis, and associated concentration of free hemoglobin in the blood plasma of animals.
Influence of HA and MK-801 on morphology, concentration of hemoglobin, and degree of erythrocyte lysis in rats. Microscopic examination of blood smears showed that the population of erythrocytes of animals with HA is characterized by some non-homogeneity arising from the transformation of discocytes-normocytes (Fig. 5a) into stomatocytes – cells that are practically unable not only to maintain strength of their membranes, but also to deform in the bloodstream [79]. MK-801 administered as a single injection did not noticeably change morphology of normal cells, and when combined with ammonium acetate only partially contributed to restoration of the disk shape of stomatocytes; as a result, as can be seen, most of the cells in the bloodstream of animals with HA in the presence of MK-801 remained in the shape of swollen stomatocytes [Fig. 5d (2)].
a-d) Changes in morphology of erythrocytes in rats with HA. Influence of MK-801. Regimes of drug administration, dosage, decapitation time (n = 6 rats in each group) are given in Fig. 1. Cell morphology was analyzed in the fluorescent microscopy image using the CELENA® S Digital Imaging System (LogosBio), lens ×40. The method of cell preparation for microscopy is described in the Materials and methods section. 1) Discocytes-normocytes, 2) stomatocytes (presence of transverse fissure or white spot in the center), 3) echinocytes, 4) ovalocytes.
The obtained results indicated that the ammonium-induced morphological changes and transition of the normal erythrocytes into stomatocytes in the bloodstream of animals with HA did not depend on NMDA-R, but was mainly associated with the action of ammonia/ammonium accumulated in animal erythrocytes.
According to the literature, there are multiple causes of the appearance of stomatocytes in the bloodstream of animals and humans [80]. It is generally assumed that transformation of normal discocytes into stomatocytes is associated with reduced transport of K+ to erythrocytes [81] and increased entry of Na+, leading to abnormally high-water content in the cells and their lysis in the bloodstream [82]. Considering that the concentration of ammonia/ammonium in animals with HA increased more than tenfold as compared to the control [16], and that its transport to erythrocytes, as mentioned above, is associated with the decrease in intracellular K+ concentration and increase in Na+ concentration [68], it could be suggested that one of the reasons for formation of stomatocytes, swollen pre-lysis cells, in the bloodstream of animals with HA may be associated with the ammonium-induced disorder of the Na+ and K+ concentration gradient, contributing to the increased entry of water, which, as has been found, does not depend on activation of NMDA-R. The ammonium-induced disorders such as excessive NH4+ and Cl– cotransport, decreased pH [83], conformational changes in integral membrane proteins, [84], inhibition of Na+,K+-ATPase activity [64], decreased ATP concentration [85], and oxidative stress [16, 86], directly or indirectly causing increased entry of water into erythrocytes, may also be associated with formation of stomatocytes in the bloodstream of animals with HA.
The obtained results are in agreement with the numerous literature data showing that the appearance of erythrocytes with atypical shape, in particular, stomatocytes, is observed in the general population of erythrocytes of the patients with various liver diseases [78, 87]. In this regard, it should be noted, however, that despite the fact that stomatocytes are cells that easily lyse in the bloodstream [88], the question of the role of ammonia/ammonium accumulated in erythrocytes in the cell hemolysis in vivo in pathologies with concomitant HA, as noted above, currently remains open. Since concentration of free hemoglobin in blood plasma is one of the main indicators of intravascular hemolysis [89], we measured concentration of this indicator and, taking into consideration other hematological parameters, calculated the degree of erythrocyte lysis (% hemolysis) [90], and determined osmotic resistance of the erythrocytes of animals of all studied groups based on the degree of resistance to the reduced NaCl concentration. The data are presented in Table 1.
The above data show that the Hct value, concentration of total and free Hb, osmotic resistance of erythrocytes in the rats from the control group lie within the permissible range of values that correspond to physiologically normal parameters for these animals [91].
In the animals with HA, there was a significant and almost equivalent decrease in the content of total Hb and Hct by 14% and 17% (*** p < 0.001) and (* p < 0.05), respectively, which, in combination with the observation of red-colored plasma, indicated the ongoing intravascular lysis of erythrocytes and hemoglobin release into plasma. Indeed, direct measurements showed that the content of free Hb in the plasma and the degree of lysis of erythrocytes of the animals with HA also increased almost equally (6-7 times, *** p < 0.001), when compared with the control (Table 1). High osmotic fragility of the erythrocytes of animals with HA due to ammonia/ammonium accumulated in erythrocytes was confirmed by the data showing that the initial lysis of cells (6%) was recorded in the solution with sufficiently high concentration of NaCl equal to 0.7%, whereas minimal lysis of erythrocytes in the control groups of animals (1.6%) was observed in the presence of 0.58% NaCl solution. 50%-Lysis of the erythrocytes of animals with HA also occurred at a higher concentration of NaCl (0.49 ± 0.011%, * p < 0.05), when compared with the control group (0.41 ± 0.0073%) (Table 1).
Single injection of MK-801 did not affect the measured values, and they corresponded to the control values (Table 1). MK-801, injected in combination with the ammonium acetate injection, partially restored the ammonium-induced disorder of hematological parameters, but their values remained significantly higher compared with the control. It can be seen that with the content of total Hb and Hct reduced by 7% (* p < 0.05) and 12% (* p < 0.05) relatively to the control, respectively, and increased concentration of free Hb (25%, *** p < 0.001), the degree of intravascular hemolysis of erythrocytes of the animals with HA in the presence of MK-801 remained twofold higher (0.64 ± 0.04, ** p < 0.01) as compared to the control (0.32 ± 0.02, *** p < 0.001). The obtained data indicate that morphological changes, as well as impaired regulation of the cell volume, osmotic resistance, and increased entry of water [92] contributing to intravascular hemolysis of erythrocytes and accumulation of free hemoglobin in the blood plasma of the animals with HA are the result of toxic effect of ammonia/ammonium (partially associated with hyperactive NMDA-R), which is intensively transported in vivo into erythrocytes of the rats with HA. It is possible that the oxidized forms of Hb, formed under conditions of oxidative stress [93] in the erythrocytes of animals with HA [16] may also participate in destabilization of the membranes and subsequent lysis of erythrocytes. Together, according to the literature data, the detected disorders could lead to the increase of blood viscosity, deterioration of blood flow in the microcirculatory bloodstream [94], impaired binding and release of oxygen by hemoglobin and, consequently, to hypoperfusion [95] underlying multiple organ damage [89] including neurological disorders and cognitive disorders [96, 97], which, moreover, could progress under the toxic effects of extracellular Hb [98].
In general, the obtained results made it possible to conclude that the morphofunctional disorders of erythrocytes and hemoglobinemia caused by the ammonium-induced disorder of a highly integrated system of metabolic pathways should be considered as an additional systemic pathogenetic factor associated with erythrocytes leading to progression of multiple organ hypoxia [99] in the case of HE.
Changes in concentration of 2,3-diphosphoglycerate in the erythrocytes of hyperammonemic rats. The effect of MK-801. Another erythrocyte marker of impaired tissue oxygenation is 2,3-diphosphoglycerate (2,3-DPG), which is formed in the Rapoport–Lubering shunt, a bypass stage of glycolysis. By reducing affinity of Hb to oxygen [100], 2,3-DPG provides an easier release of oxygen from the complex with Hb and its transfer to tissues. To assess further oxygen transport function of erythrocytes, the next step was to find out how concentration of 2,3-DPG in the erythrocytes of animals with HA, characterized by the reduced rate of glycolytic flux (Figs. 1 and 2), changes, and what is the role of MK-801 in maintaining normal intracellular concentrations of this metabolite.
The obtained data are summarized in Fig. 6. It can be seen that concentration of 2,3-DPG in the erythrocytes of the rats with HA decreased significantly (20%, *** p < 0.001), when compared to the control. Single injection of MK-801 did not affect the steady-state concentration of this metabolite, and MK-801 injection in combination with ammonium acetate injection did not restore the concentration of 2,3-DPG to the control value, and it remained low, as that in the erythrocytes of animals with HA (Fig. 6).
a, b) Concentration of 2,3-DPG in the erythrocytes of rats from the experimental group (n = 10 rats in each group). Regimes of drug administration, dosage, decapitation time are given in the legend to Fig. 1. Methods for determination of 2,3-DPG concentration are described in the Materials and Methods section. Concentration of the metabolite are presented as mmol/liter (mM). Results are expressed as mean ± SEM. *** p < 0.001 as compared to the control group of animals. Differences between the groups were examined using ANOVA followed by Bonferroni corrections. Pearson correlation coefficient (r) was used to determine association between concentration of ammonia in the erythrocytes and concentration of 2,3-DPG. Analysis was carried out using GraphPad Prism V8 software. Negative correlation between the ammonia concentration and concentration of 2,3-DPG in the erythrocytes of rats with HA (r = –0.854, p = 0.0016) was shown to be significant.
The obtained results indicated that the decrease in concentration of 2,3-DPG in the erythrocytes of the rats with HA occurs independent on the signaling cascade triggered by hyperactive NMDA-R, and is exclusively associated with the action of ammonia/ammonium accumulated in the cells, which was additionally confirmed by the significant negative correlation (r = –0.854, p = 0.0016) between these parameters (Fig. 6b).
Considering that the activity of the diphosphoglycerate mutase enzyme, which catalyzes formation of 2,3-DPG from 1,3-dysphosphoglycerate (1,3-DPG), is sharply inhibited with the decrease in intracellular pH [101], it could be assumed that the role of ammonia/ammonium in inhibition of DPGM, like other glycolytic enzymes (Fig. 1), is not direct, but is associated, as already mentioned above, with acidification of the intracellular environment of erythrocytes, caused by the transport of NH4+ into the cells [67]. The increased concentration of ADP observed in the erythrocytes of the rats with HA (Fig. 3b), redirecting 1,3-DPG to the reaction catalyzed by phosphoglycerate kinase, may also be an indirect rate-limiting factor in the synthesis of 2,3-DPG [102]. The NO-radical (NO•), excessively formed during oxidative stress [103] due to catalytic action of endothelial [104] and erythrocyte NO-synthase [105], could also suppress the synthesis of 2,3-DPG, since it inhibits GAPDH, which catalyzes formation of 1,3-DPG [106].
It should also be noted that similarity between the characteristics of erythrocytes of the animals with HA, which were revealed in our study, and characteristics of erythrocytes of the patients with enzymopathies [107], diabetic neuropathy [108], endotoxin shock [109], Down syndrome [110], patients with sporadic Alzheimer’s disease, as well as in elderly people [111] related [112] or unrelated to HA [113], made it possible to conclude that there are additional and currently unknown regulators of 2,3-DPG synthesis.
In fact, in addition to 2,3-DPG, other factors such as pH, pCO2, pO2, Cl–, ATP, Mg2+, and Pi [114], conformation and structure of Hb, temperature, etc., could affect affinity of Hb to oxygen [115]. However, it is generally recognized that 2,3-DPG is the primary biological indicator of tissue hypoxia [116], combining metabolism of erythrocytes with the systemic oxygen-dependent metabolic homeostasis, which underlies vital activity of all tissues and organs of the body.
High susceptibility of the rat erythrocytes to the damaging effects of ammonia/ammonium, which was revealed in this work, indicates that the changes of morphological characteristics, indicators of energy metabolism, and antioxidant status of erythrocytes [16] is the most important missing warning signs of an impairment of tissue oxygenation, which could result in neurological disorders in HE [117] with concomitant HA.
CONCLUSION
Based on our results, we concluded that by considering vital activity of the cells of the central nervous system in isolation from the integrated oxygen transport system, we overlook the fact that the brain has the highest level of oxidative metabolism [118] and, with limited oxygen supply [39], requires a constant supply oxygenated blood [119, 120].
Erythrocytes are the only cells in the integrated system of oxygen transport (in addition to cardiovascular system and respiratory system) that transport oxygen to the tissues and maintain the necessary level of oxidative metabolism in tissues. This study has revealed the order of events that show how the ammonia-induced impairments of energetic/metabolic processes, which control morphological, rheological features of erythrocytes [20, 22], could lead to disruption of their oxygen-transporting function and encephalopathy (Fig. 7).
A suggested order of changes at metabolic level that lead to interrelated dysfunction in erythrocytes and brain cells during the ammonia-induced hepatic encephalopathy. Designations: PCS, portacaval shunt; HK, hexokinase; PFK, phosphofructokinase; PK, pyruvate kinase; GAPDH, glyceraldehyde phosphate dehydrogenase; SOD, superoxide dismutase; GP, glutathione peroxidase; GT, glutathione transferase; G6PDG, glucose-6-phosphate dehydrogenase.
The obtained data demonstrate that the very first steps of deleterious toxic effects of ammonia/ammonium on erythrocytes are associated with their intracellular transport (both forms) resulting in disruption of ion homeostasis (Na+ and K+), inhibition of glycolysis, decreased ATP concentration, which lead to deficiency in energy supply necessary for the activity of Na+,K+-ATPase that facilitates restoration of the ion gradient considerably disturbed by the increased transport of ammonia/ammonium into erythrocytes of the rats with HA.
The results of our study show that the Na+,K+-pump blockade in the erythrocytes of the rats with HA does not depend on functional activity of NMDA-R, while morphology changes, transformation of normal erythrocytes in stomatocytes, disruption of the red cell volume regulation, osmotic resistance, increased lysis of erythrocytes, accumulation of free hemoglobin in blood plasma are the result of toxic effects of ammonia/ammonium, in part due to hyperactivation of NMDA-R. In contrast, the ammonia-induced decrease of the activity of glycolytic enzymes, intracellular ATP concentration, energy charge, and other indices of AH (not including 2,3-DPG) are, most likely, closely associated with the Ca2+-NO-dependent signaling cascade triggered by hyperactivation of NMDA-R.
The Na+,K+-pump blockade induced by ATP deficiency underlies changes in the erythrocyte shape (formation of stomatocytes), impaired erythrocyte’s deformability, increased blood viscosity, and deterioration of microcirculatory blood flow [74].
Decrease in the ATP- and 2,3-DPG-concentrations leads to the disorders that affect binding of oxygen by hemoglobin and release of oxygen from hemoglobin resulting in hypoperfusion [95], which is a hallmark of multiorgan damage [89] including neurological disorders and cognitive impairment [23, 96, 97], that may progress under the effects of oxidative stress [16] and toxic effects of extracellular Hb, accumulation of which results in the development of anomalies incompatible with life [89, 121].
Taking into account importance of causal relationship “HE–HA-acquired erythrocyte dysfunction–brain injury”, it has been suggested that the evidence demonstrating changes in biochemical parameters of erythrocytes should be considered as additional risk factors for the development of hypoxia and neurological deficits, as well as cognitive impairment caused by hypoxia.
This additional information is especially important for treatment of the patients with abnormal intracellular processes in erythrocytes considerably deviating from the norm, particularly in elderly [31], who may have these neurological dysfunctions present even at normal values of SaO2 and PaO2 [32]. Development of the science-based novel medical technologies for reestablishing intracellular metabolism in erythrocytes [122] as well as for decreasing ammonia/ammonium concentration in systemic circulation [123-125] may open new horizons in creating innovative medicines on the case-to-case basis. This has great significance for the use of new therapeutic strategies oriented to the improvement of oxygen transport to tissues thereby decreasing organ insufficiency and neurological dysfunction in the patients with diseases accompanied by HA.
Based on the obtained results, this paper presents a scheme of the order of events demonstrating how the initial cascade of ammonia-induced alterations in the energy metabolism processes that regulate morphological, rheological parameters of erythrocytes [20, 22], may result in the impairment of oxygen transport function, hypoperfusion, brain’s energy crisis, and encephalopathy (Fig. 7).
Abbreviations
- DPG:
-
diphosphoglycerate
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- HA:
-
hyperammonemia
- HE:
-
hepatic encephalopathy
- HK:
-
hexokinase
- LDH:
-
lactate dehydrogenase
- NMDA-R:
-
NMDA receptors
- PFK:
-
phosphofructokinase
- PK:
-
pyruvate kinase
- PPP:
-
pentose phosphate pathway
References
Reshetnyak, V. I. (2005) Hepatocytic insufficiency, Gen. Reanimatol., 1, 68-79, https://doi.org/10.15360/1813-9779-2005-3-68-79.
Häussinger, D., Dhiman, R. K., Felipo, V., Görg, B., Jalan, R., Kircheis, G., Merli, M., Montagnese, S., Romero-Gomez, M., Schnitzler, A., Taylor-Robinson, S. D., and Vilstrup, H. (2022) Hepatic encephalopathy, Nat. Rev. Dis. Primers, 8, 43, https://doi.org/10.1038/s41572-022-00366-6.
Merli, M., Lucidi, C., Pentassuglio, I., Giannelli, V., Giusto, M., Di Gregorio, V., Pasquale, C., Nardelli, S., Lattanzi, B., Venditti, M., and Riggio, O. (2013) Increased risk of cognitive impairment in cirrhotic patients with bacterial infections, J. Hepatol., 59, 243-250, https://doi.org/10.1016/j.jhep.2013.03.012.
Seyan, A. S., Hughes, R. D., and Shawcross, D. L. (2010) Changing face of hepatic encephalopathy: role of inflammation and oxidative stress, World J. Gastroenterol., 16, 3347-3357, https://doi.org/10.3748/wjg.v16.i27.3347.
Walker, C. O., and Schenker, S. (1970) Pathogenesis of hepatic encephalopathy with special reference to the role of ammonia, Am. J. Clin. Nutr., 23, 619-632, https://doi.org/10.1093/ajcn/23.5.619.
Walker, V. (2012) Severe hyperammonaemia in adults not explained by liver disease, Ann. Clin. Biochem., 49, 214-228, https://doi.org/10.1258/acb.2011.011206.
Lockwood, A. H. (1987) Hepatic encephalopathy: experimental approaches to human metabolic encephalopathy, Crit. Rev. Neurobiol., 3, 105-133.
Häussinger, D., and Schliess, F. (2008) Pathogenetic Mechanisms of hepatic encephalopathy, Gut, 57, 1156-1165, https://doi.org/10.1136/gut.2007.122176.
Butterworth, R. F., Giguère, J. F., Michaud, J., Lavoie, J., and Layrargues, G. P. (1987) Ammonia: key factor in the pathogenesis of hepatic encephalopathy, Neurochem. Pathol., 6, 1-12, https://doi.org/10.1007/bf02833598.
Kosenko, E., Kaminski, Y., Lopata, O., Muravyov, N., and Felipo, V. (1999) Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication, Free Radic. Biol. Med., 26, 1369-1374, https://doi.org/10.1016/s0891-5849(98)00339-6.
Kosenko, E., Felipo, V., Montoliu, C., Grisolía, S., and Kaminsky, Y. (1997) Effects of acute hyperammonemia in vivo on oxidative metabolism in nonsynaptic rat brain mitochondria, Metab. Brain Dis., 12, 69-82, https://doi.org/10.1007/bf02676355.
Monfort, P., Kosenko, E., Erceg, S., Canales, J.-J., and Felipo, V. (2002) Molecular mechanism of acute ammonia toxicity: role of NMDA receptors, Neurochem. Int., 41, 95-102, https://doi.org/10.1016/s0197-0186(02)00029-3.
Makhro, A., Wang, J., Vogel, J., Boldyrev, A. A., Gassmann, M., Kaestner, L., and Bogdanova, A. (2010) Functional NMDA receptors in rat erythrocytes, Am. J. Physiol. Cell Physiol., 298, 1315-1325, https://doi.org/10.1152/ajpcell.00407.2009.
Sherwin, R. S. (1980) Role of the liver in glucose homeostasis, Diabetes Care, 3, 261-265, https://doi.org/10.2337/diacare.3.2.261.
Kosenko, E., Tikhonova, L., Alilova, G., and Montoliu, C. (2020) A look into liver mitochondrial dysfunction as a hallmark in progression of brain energy crisis and development of neurologic symptoms in hepatic encephalopathy, J. Clin. Med., 9, 2259, https://doi.org/10.3390/jcm9072259.
Kosenko, E. A., Alilova, G. A., and Tikhonova, L. A. (2023) Impaired enzymatic antioxidant defense in erythrocytes of rats with ammonia-induced encephalopathy: role of NMDA receptors, Biochemistry (Moscow), 88, 1404-1415, https://doi.org/10.1134/s0006297923090195.
Sivilotti, M. L. A. (2004) Oxidant stress and haemolysis of the human erythrocyte, Toxicol. Rev., 23, 169-188, https://doi.org/10.2165/00139709-200423030-00004.
Gyawali, P., Richards, R. S., Bwititi, P. T., and Nwose, E. U. (2015) Association of abnormal erythrocyte morphology with oxidative stress and inflammation in metabolic syndrome, Blood Cells Mol. Dis., 54, 360-363, https://doi.org/10.1016/j.bcmd.2015.01.005.
Brewer, G. J., Oelshlegel, F. J., Moore, L. G., and Noble, N. A. (1974) In vivo red cell glycolytic control and DPG-ATP levels, Ann. N.Y. Acad. Sci., 241, 513-523, https://doi.org/10.1111/j.1749-6632.1974.tb21907.x.
Brewer, G. J., and Eaton, J. W. (1971) Erythrocyte metabolism: interaction with oxygen transport, Science, 171, 1205-1211, https://doi.org/10.1126/science.171.3977.1205.
Mairbäurl, H., Oelz, O., and Bärtsch, P. (1993) Interactions between Hb, Mg, DPG, ATP, and Cl determine the change in Hb-O2 affinity at high altitude, J. Appl. Physiol. (1985), 74, 40-48, https://doi.org/10.1152/jappl.1993.74.1.40.
Walder, J. A., Chatterjee, R., Steck, T. L., Low, P. S., Musso, G. F., Kaiser, E. T., Rogers, P. H., and Arnone, A. (2015) The Interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane, J. Biol. Chem., 259, 10238-10246.
Bosman, G. J. C. G. M. (2018) Disturbed red blood cell structure and function: an exploration of the role of red blood cells in neurodegeneration, Front. Med. (Lausanne), 5, 198, https://doi.org/10.3389/fmed.2018.00198.
Bray, C., Bell, L. N., Liang, H., Haykal, R., Kaiksow, F., Mazza, J. J., and Yale, S. H. (2016) Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine, WMJ, 115, 317-321.
Podzolkov, V., Koroleva, T., Bragina, A., Kudryavtseva, M., Druzhinina, N., and Pisarev, M. (2018) Change in the functional state of erythrocytes as a component of microcirculatory disorders in metabolic syndrome, Rational Pharmacother. Cardiol., 14, 184-189, https://doi.org/10.20996/1819-6446-2018-14-2-184-189.
Ahmed, M. H., Ghatge, M. S., and Safo, M. K. (2020) Hemoglobin: structure, function and allostery, Subcell. Biochem., 94, 345-382, https://doi.org/10.1007/978-3-030-41769-7_14.
Orvain, C., Joly, P., Pissard, S., Badiou, S., Badens, C., Bonello-Palot, N., Couque, N., Gulbis, B., and Aguilar-Martinez, P. (2017) Diagnostic approach to hemoglobins with high oxygen affinity: experience from France and Belgium and review of the literature, Ann. Biol. Clin. (Paris), 75, 39-51, https://doi.org/10.1684/abc.2016.1204.
Yusipovich, A. I., Braze, N. A., Luneva, O. G., Parshina, E. Yu., Churin, A. A., Rodnenkov, O. V., and Maksimov, G. V. (2013) Changes in the state of hemoglobin in patients with coronary heart disease and patients with circulatory failure, Bull. Exp. Biol. Med., 155, 233-235, https://doi.org/10.1007/s10517-013-2121-5.
Blass, J. P., and Gibson, G. E. (1999) Cerebrometabolic aspects of delirium in relationship to dementia, Dement. Geriatr. Cogn. Disord., 10, 335-338, https://doi.org/10.1159/000017165.
De la Torre, J. C. (2000) Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis, Neurobiol. Aging, 21, 331-342, https://doi.org/10.1016/s0197-4580(00)00111-1.
Kosenko, E., Tikhonova, L., Alilova, G., Urios, A., and Montoliu, C. (2020) The erythrocytic hypothesis of brain energy crisis in sporadic Alzheimer Disease: possible consequences and supporting evidence, J. Clin. Med., 9, 206, https://doi.org/10.3390/jcm9010206.
Zur, B., Bagci, S., Ludwig, M., and Stoffel-Wagner, B. (2012) Oxygen saturation in pulse oximetry in hemoglobin anomalies, Klin. Padiatr., 224, 259-265, https://doi.org/10.1055/s-0032-1312612.
Snyder, M. J., Bradford, W. D., Kishnani, P. S., and Hale, L. P. (2003) Idiopathic hyperammonemia following an unrelated cord blood transplant for mucopolysaccharidosis I, Pediatr. Dev. Pathol., 6, 78-83, https://doi.org/10.1007/s10024-001-0271-3.
Lichtenstein, G. R., Yang, Y. X., Nunes, F. A., Lewis, J. D., Tuchman, M., Tino, G., Kaiser, L. R., Palevsky, H. I., Kotloff, R. M., Furth, E. E., Bavaria, J. E., Stecker, M. M., Kaplan, P., and Berry, G. T. (2000) Fatal hyperammonemia after orthotopic lung transplantation, Ann. Intern. Med., 132, 283-287, https://doi.org/10.7326/0003-4819-132-4-200002150-00006.
Hoyer, S., Nitsch, R., and Oesterreich, K. (1990) Ammonia is endogenously generated in the brain in the presence of presumed and verified dementia of Alzheimer type, Neurosci. Lett., 117, 358-362, https://doi.org/10.1016/0304-3940(90)90691-2.
Gupte, P., and Nagral, A. (2009) Hematological problems and liver disease, Trop. Gastroenterol., 30, 65-70.
Fuhrmann, V., Kneidinger, N., Herkner, H., Heinz, G., Nikfardjam, M., Bojic, A., Schellongowski, P., Angermayr, B., Kitzberger, R., Warszawska, J., Holzinger, U., Schenk, P., and Madl, C. (2009) Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients, Intensive Care Med., 35, 1397-1405, https://doi.org/10.1007/s00134-009-1508-2.
Nakata, H., Miyamoto, T., Ogoh, S., Kakigi, R., and Shibasaki, M. (2017) Effects of acute hypoxia on human cognitive processing: a study using ERPs and SEPs, J. Appl. Physiol., 123, 1246-1255, https://doi.org/10.1152/japplphysiol.00348.2017.
Bailey, D. M., Willie, C. K., Hoiland, R. L., Bain, A. R., MacLeod, D. B., Santoro, M. A., DeMasi, D. K., Andrijanic, A., Mijacika, T., Barak, O. F., Dujic, Z., and Ainslie, P. N. (2017) Surviving without oxygen: how low can the human brain go? High Alt. Med. Biol., 18, 73-79, https://doi.org/10.1089/ham.2016.0081.
Kosenko, E., Kaminsky, Y., Grau, E., Miñana, M. D., Grisolía, S., and Felipo, V. (1995) Nitroarginine, an inhibitor of nitric oxide synthetase, attenuates ammonia toxicity and ammonia-induced alterations in brain metabolism, Neurochem. Res., 20, 451-456, https://doi.org/10.1007/bf00973101.
Beutler, E., Blume, K. G., Kaplan, J. C., Löhr, G. W., Ramot, B., and Valentine, W. N. (1977) International committee for standardization in haematology: recommended methods for red-cell enzyme analysis, Br. J. Haematol., 35, 331-340, https://doi.org/10.1111/j.1365-2141.1977.tb00589.x.
Kosenko, E. A., Aliev, G., and Kaminsky, Y. G. (2016) Relationship between chronic disturbance of 2,3-diphosphoglycerate metabolism in erythrocytes and Alzheimer disease, CNS Neurol. Disord. Drug Targets, 15, 113-123, https://doi.org/10.2174/1871527314666150821103444.
Beutler, E. (1971) Red Cell Metabolism: A Manual of Biochemical Methods, Grune & Stratton, ISBN 978-0-8089-0687-2.
Atkinson, D. E. (1968) The energy charge of the adenylate pool as a regulatory parameter. interaction with feedback modifiers, Biochemistry, 7, 4030-4034, https://doi.org/10.1021/bi00851a033.
Williamson, D. H., Lund, P., and Krebs, H. A. (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver, Biochem. J., 103, 514-527, https://doi.org/10.1042/bj1030514.
Borsakova, D. V., Koleva, L. D., Protasov, E. S., Ataullakhanov, F. I., and Sinauridze, E. I. (2022) Ammonium removal by erythrocyte-bioreactors based on glutamate dehydrogenase from Proteus Sp. Jointly with Porcine heart alanine aminotransferase, Sci. Rep., 12, 5437, https://doi.org/10.1038/s41598-022-09435-y.
Huizenga, J. R., Tangerman, A., and Gips, C. H. (1994) Determination of ammonia in biological fluids, Ann. Clin. Biochem., 31, 529-543, https://doi.org/10.1177/000456329403100602.
Kosenko, E., Tikhonova, L., Alilova, G., and Montoliu, C. (2022) Is NMDA-receptor-mediated oxidative stress in mitochondria of peripheral tissues the essential factor in the pathogenesis of hepatic encephalopathy? J. Clin. Med., 11, 827, https://doi.org/10.3390/jcm11030827.
Edwards, C. J., and Fuller, J. (1996) Oxidative stress in erythrocytes, Comp. Haematol. Int., 6, 24-31, https://doi.org/10.1007/bf00368098.
Tilton, W. M., Seaman, C., Carriero, D., and Piomelli, S. (1991) Regulation of glycolysis in the erythrocyte: role of the lactate/pyruvate and NAD/NADH ratios, J. Lab. Clin. Med., 118, 146-152.
Kurganov, B. I., Sugrobova, N. P., and Mil’man, L. S. (1985) Supramolecular organization of glycolytic enzymes, J. Theor. Biol., 116, 509-526, https://doi.org/10.1016/s0022-5193(85)80086-2.
Minetti, M., Mallozzi, C., and Di Stasi, A. M. M. (2002) Peroxynitrite activates kinases of the Src family and upregulates tyrosine phosphorylation signaling, Free Radic. Biol. Med., 33, 744-754, https://doi.org/10.1016/s0891-5849(02)00891-2.
Chu, H., Breite, A., Ciraolo, P., Franco, R. S., and Low, P. S. (2008) Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties, Blood, 111, 932-938, https://doi.org/10.1182/blood-2007-07-100180.
Chu, H., McKenna, M. M., Krump, N. A., Zheng, S., Mendelsohn, L., Thein, S. L., Garrett, L. J., Bodine, D. M., and Low, P. S. (2016) Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of Erythrocyte properties, Blood, 128, 2708-2716, https://doi.org/10.1182/blood-2016-01-692079.
Nemkov, T., Reisz, J. A., Xia, Y., Zimring, J. C., and D’Alessandro, A. (2018) Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport, Expert Rev. Proteomics, 15, 855-864, https://doi.org/10.1080/14789450.2018.1531710.
Mohorovic, L., Lavezzi, A., Jonjic, N., Stifter, S., Perry, G., Malatestinic, D., Micovic, V., Materljan, E., Haller, H., and Petrovic, O. (2017) Methemoglobinemia as biomarker and precursor of brain capillary oxidative damage link to ferric iron accumulation and originator of neurodegenerative diseases, J. Syst. Integr. Neurosci., 3, 1-5, https://doi.org/10.15761/jsin.1000180.
Harrison, M. L., Rathinavelu, P., Arese, P., Geahlen, R. L., and Low, P. S. (1991) Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis, J. Biol. Chem., 266, 4106-4111.
Ansari, F. A., Ali, S. N., and Mahmood, R. (2015) Sodium nitrite-induced oxidative stress causes membrane damage, protein oxidation, lipid peroxidation and alters major metabolic pathways in human erythrocytes, Toxicol. In Vitro, 29, 1878-1886, https://doi.org/10.1016/j.tiv.2015.07.022.
Chandel, N. S. (2021) Glycolysis, Cold Spring Harb. Perspect. Biol., 13, a040535, https://doi.org/10.1101/cshperspect.a040535.
Schmalhausen, E. V., Medvedeva, M. V., Serebryakova, M. V., Chagovets, V. V., and Muronetz, V. I. (2022) Products of S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase: relation between s-nitrosylation and oxidation, Biochim. Biophys. Acta Gen. Subj., 1866, 130032, https://doi.org/10.1016/j.bbagen.2021.130032.
Deng, D., Xu, C., Sun, P., Wu, J., Yan, C., Hu, M., and Yan, N. (2014) Crystal structure of the human glucose transporter GLUT1, Nature, 510, 121-125, https://doi.org/10.1038/nature13306.
Zancan, P., and Sola-Penna, M. (2005) Regulation of Human erythrocyte metabolism by insulin: cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function, Mol. Genet. Metab., 86, 401-411, https://doi.org/10.1016/j.ymgme.2005.06.011.
Clausen, T., Van Hardeveld, C., and Everts, M. E. (1991) Significance of cation transport in control of energy metabolism and thermogenesis, Physiol. Rev., 71, 733-774, https://doi.org/10.1152/physrev.1991.71.3.733.
Radosinska, J., and Vrbjar, N. (2016) The role of red blood cell deformability and Na+,K+-ATPase function in selected risk factors of cardiovascular diseases in humans: focus on hypertension, diabetes mellitus and hypercholesterolemia, Physiol. Res., 65, 43-54, https://doi.org/10.33549/physiolres.933402.
Cooper, A. J., and Plum, F. (1987) Biochemistry and physiology of brain ammonia, Physiol. Rev., 67, 440-519, https://doi.org/10.1152/physrev.1987.67.2.440.
Labotka, R. J., Lundberg, P., and Kuchel, P. W. (1995) Ammonia permeability of erythrocyte membrane studied by 14N and 15N saturation transfer NMR spectroscopy, Am. J. Physiol., 268, 686-699, https://doi.org/10.1152/ajpcell.1995.268.3.c686.
Westhoff, C. M., Ferreri-Jacobia, M., Mak, D.-O. D., and Foskett, J. K. (2002) Identification of the erythrocyte rh blood group glycoprotein as a mammalian ammonium transporter, J. Biol. Chem., 277, 12499-12502, https://doi.org/10.1074/jbc.c200060200.
Martinelle, K., and Häggström, L. (1993) Mechanisms of ammonia and ammonium ion toxicity in animal cells: transport across cell membranes, J. Biotechnol., 30, 339-350, https://doi.org/10.1016/0168-1656(93)90148-g.
Whittam, R., and Ager, M. E. (1965) The connexion between active cation transport and metabolism in erythrocytes, Biochem. J., 97, 214-227, https://doi.org/10.1042/bj0970214.
Tozzi-Ciancarelli, M. G., Di Giulio, A., Troiani-Sevi, E., D’Alfonso, A., Amicosante, G., and Oratore, A. (1989) Human erythrocyte damage at the initial stages of oxidative stress, Cell Biophys., 15, 225-234, https://doi.org/10.1007/bf02989685.
Liu, J., Nie, Y., Chaudhry, M., Bai, F., Chuang, J., Sodhi, K., and Shapiro, J. I. (2020) The redox-sensitive Na+/K+-ATPase signaling in uremic cardiomyopathy, Int. J. Mol. Sci., 21, 1256, https://doi.org/10.3390/ijms21041256.
Owen, J. S., and McIntyre, N. (1978) Erythrocyte lipid composition and sodium transport in human liver disease, Biochim. Biophys. Acta, 510, 168-176, https://doi.org/10.1016/0005-2736(78)90138-4.
Korff, J. M., Siebens, A. W., and Gill, J. R. (1984) Correction of hypokalemia corrects the abnormalities in erythrocyte sodium transport in Bartter’s syndrome, J. Clin. Invest., 74, 1724-1729, https://doi.org/10.1172/jci111590.
Maturu, P., Vaddi, D. R., Pannuru, P., and Nallanchakravarthula, V. (2010) Alterations in erythrocyte membrane fluidity and Na+/K+-ATPase activity in chronic alcoholics, Mol. Cell Biochem., 339, 35-42, https://doi.org/10.1007/s11010-009-0367-z.
Hedin, S. G. (1897) Ueber die Permeabilität der Blutkörperchen [in German], Pflüger. Arch., 68, 229-338, https://doi.org/10.1007/bf01661862.
Gonzalez, R., Zamora, J., Gomez-Camarero, J., Molinero, L.-M., Bañares, R., and Albillos, A. (2008) Meta-analysis: combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis, Ann. Intern. Med., 149, 109-122, https://doi.org/10.7326/0003-4819-149-2-200807150-00007.
Lazebnik, L. B., Golovanova, E. V., Alekseenko, S. A., Bueverov, A. O., Plotnikova, E. Y., Dolgushina, A. I., Ilchenko, L. Y., Ermolova, T. V., Tarasova, L. V., Lee, E. D., Tsyganova, Y. V., Akhmedov, V. A., Ageeva, E. A., Losev, V. M., Kupriyanova, I. N., Serikova, S. N., Korochanskaya, N. V., Vologzhanina, L. G., Zimmerman, Y. S., Sas, E. I., Zhuravel, S. V., Okovitiy, S. V., Osipenko, M. F., Radchenko, V. G., Soldatova, G. S., Sitkin, S. I., Seliverstov, P. V., Shavkuta, G. V., Butova, E. N., and Kozhevnikova, S. A. (2021) Russian Consensus “Hyperammonemia in Adults” (Version 2021) [in Russian], Experimental and Clinical Gastroenterology, 97-118, https://doi.org/10.31146/1682-8658-ecg-187-3-97-118.
Morse, E. E. (1990) Mechanisms of hemolysis in liver disease, Ann. Clin. Lab. Sci., 20, 169-174.
Diez-Silva, M., Dao, M., Han, J., Lim, C.-T., and Suresh, S. (2010) Shape and biomechanical characteristics of human red blood cells in health and disease, MRS Bull., 35, 382-388, https://doi.org/10.1557/mrs2010.571.
Lim, H. W. G., Wortis, M., and Mukhopadhyay, R. (2002) Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer- couple hypothesis from membrane mechanics, Proc. Natl. Acad. Sci. USA, 99, 16766-16769, https://doi.org/10.1073/pnas.202617299.
Oski, F. A., Naiman, J. L., Blum, S. F., Zarkowsky, H. S., Whaun, J., Shohet, S. B., Green, A., and Nathan, D. G. (1969) Congenital hemolytic anemia with high-sodium, low-potassium red cells. Studies of three generations of a family with a new variant, N. Engl. J. Med., 280, 909-916, https://doi.org/10.1056/nejm196904242801701.
Parker, J. C., and Welt, L. G. (1972) Pathological alterations of cation movements in red blood cells, Arch. Intern. Med., 129, 320-332, https://doi.org/10.1001/archinte.129.2.320.
Gedde, M. M., Davis, D. K., and Huestis, W. H. (1997) Cytoplasmic PH and human erythrocyte shape, Biophys. J., 72, 1234-1246, https://doi.org/10.1016/s0006-3495(97)78770-8.
Wong, P. (1999) A basis of echinocytosis and stomatocytosis in the disc-sphere transformations of the erythrocyte, J. Theor. Biol., 196, 343-361, https://doi.org/10.1006/jtbi.1998.0845.
Wolf, P. L., and Koett, J. (1980) Hemolytic anemia in hepatic disease with decreased erythrocyte adenosine triphosphate, Am. J. Clin. Pathol., 73, 785-788, https://doi.org/10.1093/ajcp/73.6.785.
Antonelou, M. H., Kriebardis, A. G., Velentzas, A. D., Kokkalis, A. C., Georgakopoulou, S.-C., and Papassideri, I. S. (2011) Oxidative stress-associated shape transformation and membrane proteome remodeling in erythrocytes of end stage renal disease patients on hemodialysis, J. Proteomics, 74, 2441-2452, https://doi.org/10.1016/j.jprot.2011.04.009.
Wislöff, F., and Boman, D. (1979) Acquired stomatocytosis in alcoholic liver disease, Scand. J. Haematol., 23, 43-50, https://doi.org/10.1111/j.1600-0609.1979.tb02852.x.
Wiley, J. S. (1976) Hereditary stomatocytosis: a disease of cell water regulation, in Membranes and Disease (Bolis, L., Hoffman, J. F., and Leaf, A., eds) Raven Press, N.Y., pp. 89-94.
Rother, R. P., Bell, L., Hillmen, P., and Gladwin, M. T. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease, JAMA, 293, 1653-1662, https://doi.org/10.1001/jama.293.13.1653.
Kumukova, I. B., Trakhtman, P., Starostin, N. N., Borsakova, D. V., Ignatova, A., Fedotov, A. Y., Plakhotnik, M. E., and Ataullakhanov, F. I. (2018) Comparison of laboratory parameters of X-ray irradiated erythrocyte suspensions and suspensions, prepared from whole blood pre-treated with ultraviolet in the presence of riboflavin, Vopr. Gematol. Onkol. Immunopatol. Pediatr., 17, 64-74, https://doi.org/10.24287/1726-1708-2018-17-1-64-74.
He, Q., Su, G., Liu, K., Zhang, F., Jiang, Y., Gao, J., Liu, L., Jiang, Z., Jin, M., and Xie, H. (2017) Sex-specific reference intervals of hematologic and biochemical analytes in Sprague-Dawley rats using the nonparametric rank percentile method, PLoS One, 12, e0189837, https://doi.org/10.1371/journal.pone.0189837.
Bruce, L. J. (2009) hereditary stomatocytosis and cation-leaky red cells – recent developments, Blood Cells Mol. Dis., 42, 216-222, https://doi.org/10.1016/j.bcmd.2009.01.014.
Kosmachevskaya, O. V., and Topunov, A. F. (2018) Alternate and additional functions of erythrocyte hemoglobin, Biochemistry (Moscow), 83, 1575-1593, https://doi.org/10.1134/s0006297918120155.
Helms, C. C., Gladwin, M. T., and Kim-Shapiro, D. B. (2018) Erythrocytes and vascular function: oxygen and nitric oxide, Front. Physiol., 9, 125, https://doi.org/10.3389/fphys.2018.00125.
Berisavac, I. I., Jovanović, D. R., Padjen, V. V., Ercegovac, M. D., Stanarčević, P. D. J., Budimkić-Stefanović, M. S., Radović, M. M., and Beslać-Bumbaširević, L. G. (2017) How to recognize and treat metabolic encephalopathy in neurology intensive care unit, Neurol. India, 65, 123-128, https://doi.org/10.4103/0028-3886.198192.
Stevenson, A., Lopez, D., Khoo, P., Kalaria, R. N., and Mukaetova-Ladinska, E. B. (2017) Exploring erythrocytes as blood biomarkers for Alzheimer’s disease, J. Alzheimers Dis., 60, 845-857, https://doi.org/10.3233/jad-170363.
Kosenko, E. A., Tikhonova, L. A., Montoliu, C., Barreto, G. E., Aliev, G., and Kaminsky, Y. G. (2017) Metabolic Abnormalities of erythrocytes as a risk factor for Alzheimer’s disease, Front. Neurosci., 11, 728, https://doi.org/10.3389/fnins.2017.00728.
Oh, D. J., Kim, J. S., Lee, S., Yang, H. W., Bae, J. B., Han, J. W., and Kim, K. W. (2022) Association between serum free hemoglobin level and cerebral white matter hyperintensity volume in older adults, Sci. Rep., 12, 3296, https://doi.org/10.1038/s41598-022-07325-x.
Moreau, R., Lee, S. S., Soupison, T., Roche-Sicot, J., and Sicot, C. (1988) Abnormal tissue oxygenation in patients with cirrhosis and liver failure, J. Hepatol., 7, 98-105, https://doi.org/10.1016/s0168-8278(88)80512-9.
Benesch, R., and Benesch, R. E. (1967) The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin, Biochem. Biophys. Res. Commun., 26, 162-167, https://doi.org/10.1016/0006-291x(67)90228-8.
Rapoport, I., Berger, H., Elsner, R., and Rapoport, S. (1977) pH-dependent changes of 2,3-bisphosphoglycerate in human red cells during transitional and steady states in vitro, Eur. J. Biochem., 73, 421-427, https://doi.org/10.1111/j.1432-1033.1977.tb11333.x.
Duhm, J., Deuticke, B., and Gerlach, E. (1968) Metabolism of 2,3-diphosphoglycerate and glycolysis in human red blood cells under the influence of dipyridamole and inorganic sulfur compounds, Biochim. Biophys. Acta, 170, 452-454, https://doi.org/10.1016/0304-4165(68)90033-0.
Hsu, L. L., Champion, H. C., Campbell-Lee, S. A., Bivalacqua, T. J., Manci, E. A., Diwan, B. A., Schimel, D. M., Cochard, A. E., Wang, X., Schechter, A. N., Noguchi, C. T., and Gladwin, M. T. (2007) Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability, Blood, 109, 3088-3098, https://doi.org/10.1182/blood-2006-08-039438.
Tejero, J., Shiva, S., and Gladwin, M. T. (2019) Sources of vascular nitric oxide and reactive oxygen species and their regulation, Physiol. Rev., 99, 311-379, https://doi.org/10.1152/physrev.00036.2017.
Cortese-Krott, M. M., and Kelm, M. (2014) Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol., 2, 251-258, https://doi.org/10.1016/j.redox.2013.12.027.
Bertrand, R. (2012) Nitric oxide-mediated suppression of 2,3-bisphosphoglycerate synthesis: therapeutic relevance for environmental hypoxia and sickle cell disease, Med. Hypotheses, 79, 315-318, https://doi.org/10.1016/j.mehy.2012.05.020.
Valentine, W. N., Tanaka, K. R., and Paglia, D. E. (1985) Hemolytic anemias and erythrocyte enzymopathies, Ann. Intern. Med., 103, 245-257, https://doi.org/10.7326/0003-4819-103-2-245.
Nakamura, J., Koh, N., Sakakibara, F., Hamada, Y., Wakao, T., Hara, T., Mori, K., Nakashima, E., Naruse, K., and Hotta, N. (1995) Polyol pathway, 2,3-diphosphoglycerate in erythrocytes and diabetic neuropathy in rats, Eur. J. Pharmacol., 294, 207-214, https://doi.org/10.1016/0014-2999(95)00531-5.
Matsumoto, Y. (1995) The function of red blood cells during experimental hemorrhagic and endotoxic shock, Masui, 44, 342-348.
Kedziora, J., Hübner, H., Kański, M., Jeske, J., and Leyko, W. (1972) Efficiency of the glycolytic pathway in erythrocytes of children with Down’s syndrome, Pediatr. Res., 6, 10-17, https://doi.org/10.1203/00006450-197201000-00002.
Kaminsky, Y. G., Reddy, V. P., Ashraf, G. M., Ahmad, A., Benberin, V. V., Kosenko, E. A., and Aliev, G. (2013) Age-related defects in erythrocyte 2,3-diphosphoglycerate metabolism in dementia, Aging Dis., 4, 244-255, https://doi.org/10.14336/ad.2013.0400244.
Kosenko, E. A., Solomadin, I. N., Tikhonova, L. A., Reddy, V. P., Aliev, G., and Kaminsky, Y. G. (2014) Pathogenesis of Alzheimer disease: role of oxidative Stress, amyloid-β peptides, systemic ammonia and erythrocyte energy metabolism, CNS Neurol. Disord. Drug Targets, 13, 112-119, https://doi.org/10.2174/18715273113126660130.
MacDonald, R. (1977) Red cell 2,3-diphosphoglycerate and oxygen affinity, Anaesthesia, 32, 544-553, https://doi.org/10.1111/j.1365-2044.1977.tb10002.x.
Larsen, V. H., Waldau, T., Gravesen, H., and Siggaard-Andersen, O. (1996) Erythrocyte 2,3-diphosphoglycerate depletion associated with hypophosphatemia detected by routine arterial blood gas analysis, Scand. J. Clin. Lab. Invest. Suppl., 224, 83-87, https://doi.org/10.3109/00365519609088626.
Lichtman, M. A., Miller, D. R., Cohen, J., and Waterhouse, C. (1971) Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia, Ann. Intern. Med., 74, 562-568, https://doi.org/10.7326/0003-4819-74-4-562.
Juel, R. (1979) 2,3-diphosphoglycerate: its role in health and disease, CRC Crit. Rev. Clin. Lab. Sci., 10, 113-146, https://doi.org/10.3109/10408367909147131.
Cordoba, J., Ventura-Cots, M., Simón-Talero, M., Amorós, À., Pavesi, M., Vilstrup, H., Angeli, P., Domenicali, M., Ginés, P., Bernardi, M., Arroyo, V., and Canonic Study Investigators of EASL-CLIF Consortium (2014) Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF), J. Hepatol., 60, 275-281, https://doi.org/10.1016/j.jhep.2013.10.004.
Hall, C. N., Klein-Flügge, M. C., Howarth, C., and Attwell, D. (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing, J. Neurosci., 32, 8940-8951, https://doi.org/10.1523/jneurosci.0026-12.2012.
Leithner, C., and Royl, G. (2014) The oxygen paradox of neurovascular coupling, J. Cereb. Blood Flow Metab., 34, 19-29, https://doi.org/10.1038/jcbfm.2013.181.
Peers, C., Dallas, M. L., Boycott, H. E., Scragg, J. L., Pearson, H. A., and Boyle, J. P. (2009) Hypoxia and neurodegeneration, Ann. N.Y. Acad. Sci., 1177, 169-177, https://doi.org/10.1111/j.1749-6632.2009.05026.x.
Meegan, J. E., Bastarache, J. A., and Ware, L. B. (2021) Toxic effects of cell-free hemoglobin on the microvascular endothelium: implications for pulmonary and nonpulmonary organ dysfunction, Am. J. Physiol. Lung Cell. Mol. Physiol., 321, 429-439, https://doi.org/10.1152/ajplung.00018.2021.
Papassotiriou, I., Kister, J., Griffon, N., Abraham, D. J., Kanavakis, E., Traeger-Synodinos, J., Stamoulakatou, A., Marden, M. C., and Poyart, C. (1998) Synthesized allosteric effectors of the hemoglobin molecule: a possible mechanism for improved erythrocyte oxygen release capability in hemoglobinopathy H disease, Exp. Hematol., 26, 922-926.
Kosenko, E. A., Venediktova, N. I., Kudryavtsev, A. A., Ataullakhanov, F. I., Kaminsky, Y. G., Felipo, V., and Montoliu, C. (2008) Encapsulation of glutamine synthetase in mouse erythrocytes: a new procedure for ammonia detoxification, Biochem. Cell Biol., 86, 469-476, https://doi.org/10.1139/o08-134.
Godfrin, Y., Horand, F., Franco, R., Dufour, E., Kosenko, E., Bax, B. E., Banz, A., Skorokhod, O. A., Lanao, J. M., Vitvitsky, V., Sinauridze, E., Bourgeaux, V., and Gunter, K. C. (2012) International seminar on the red blood cells as vehicles for drugs, Expert Opin. Biol. Ther., 12, 127-133, https://doi.org/10.1517/14712598.2012.631909.
Protasov, E. S., Borsakova, D. V., Alexandrovich, Y. G., Korotkov, A. V., Kosenko, E. A., Butylin, A. A., Ataullakhanov, F. I., and Sinauridze, E. I. (2019) Erythrocytes as bioreactors to decrease excess ammonium concentration in blood, Sci. Rep., 9, 1455, https://doi.org/10.1038/s41598-018-37828-5.
Acknowledgments
The authors of this study are grateful to the Center of Collective Use of research equipment at the Institute of Theoretical and Experimental Biophysics for providing access to the equipment (Varian Cary Eclipse fluorescence spectrophotometer, Australia) throughout this study.
Funding
The work was financially supported by the Russian Science Foundation (project no. 23-25-00133; https://rscf.ru/project/23-25-00133/ [in Russian]).
Author information
Authors and Affiliations
Contributions
E.A.K. conceived and planned this research, wrote the text of the article; L.A.T. and G.A.A. carried out the experiments; E.A.K., L.A.T., and G.A.A. discussed the results of this research and commented on the text of the article.
Corresponding author
Ethics declarations
The experimental procedures were adhered to the policy on humane care and use of the animals in compliance with European guidelines on the use of laboratory animals adopted in 1986 (it was replaced by Directive 2010/63/EU of European Council). All experimental protocols were approved by the Commission on Biological Safety and Bioethics at the Institute of Theoretical and Experimental Biophysics (ITEB) (experimental protocol № 31/2023, February 15, 2023; № 10/2024, March 18, 2024). The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alilova, G.A., Tikhonova, L.A. & Kosenko, E.A. NMDA Receptors and Indices of Energy Metabolism in Erythrocytes: Missing Link to the Assessment of Efficiency of Oxygen Transport in Hepatic Encephalopathy. Biochemistry Moscow 89, 1490–1508 (2024). https://doi.org/10.1134/S000629792408008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792408008X