Abstract
A method has been developed for producing analogs of fermented milk drinks from pumpkin seed meal, which is a massive waste product from oilseed production, using new strains of lactic acid bacteria (LAB) isolated from different samples of kumiss. Based on the results of screening 50 LAB isolates capable of fermenting milk and aqueous meal extracts in a wide pH range, three strains with the best growth characteristics were selected. These strains were identified as representatives of the genus Lacticaseibacillus, most closely related to L. rhamnosus and L. casei (with 99.93 and 99.65% similarity in 16S rRNA gene sequences). An optimal scheme for producing drinks has been selected, including grinding meal, optimized extraction with alkaline solutions, heat treatment of the extract to remove foreign microflora, introduction of the inoculum (3–5% vol/vol) of new LAB strains, and fermentation at 37oС for ten hours. Compared with the fermented milk product obtained by fermenting milk with the same microorganisms, the drink made from meal extracts was distinguished by the absence of lactose and cholesterol, and increased content of unsaturated fatty acids (2.3 times) and protein (1.7 times), and the presence of essential amino acids in proteins. Thus, pumpkin seed meal, which is still used ineffectively, is a good basis for obtaining analogs of fermented milk products with beneficial properties. The developed method for producing lacto-fermented drinks can be adapted for processing other types of meals and cakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, there has been a significant increase in interest in obtaining plant-based analogs of milk and fermented milk products due to the high content of protein and unsaturated fatty acids and the absence of lactose and cholesterol, which is attractive to certain groups of the population [1, 2]. Sources of milk analogs and fermented products obtained from it are oilseeds, nuts, and beans which are valuable for food production [3–5]. The main stages of obtaining milk analogs include grinding of plant raw materials, extraction with aqueous solutions, and removal of large particles [6–8].
Plant extracts with a good content of protein, vitamins, mineral components, and trace elements are suitable substrates for fermentation by monocultures or consortia of lactic acid bacteria (LAB) of the genera Lactobacillus ((L. acidophilus, L. brevis, L. helveticus, L. delbrueckii), Lacticaseibacillus (L. сasei) and Levilactobacillus (L. brevis), and Streptococcus (Str. salivarius) and the production of fermented beverages [9, 10]. Their properties are determined by many parameters, including the composition of the initial plant raw materials, its preliminary preparation, and the microorganisms themselves—fermentation agents.
A common and significant limitation of the mass production of new fermented beverages is the value of whole seeds for the food and processing industry; therefore, the search for affordable and suitable raw materials is relevant. Such raw materials could include pumpkin seed meal with a relatively rich composition, a high content of protein, and unsaturated fatty acids and other components [11], which is mainly used as a feed additive for farm animals [12]. Based on the estimated annual pumpkin harvest of tens of millions of tons [12] and the growing global production of pumpkin seed oil, the meal produced by pumpkin seed production is a massive waste. However, to date, no studies on obtaining analogs of fermented milk products directly from pumpkin seed meal extract have been performed.
This study was aimed at isolating and selecting strains of lactic acid bacteria, the most effective agents of extract fermentation, since the LAB cultures used are not necessarily adapted to new substrates, as was shown earlier [13, 14]. Among the sources of isolation of potentially valuable strains, kumiss is of interest. It is the national drink in the regions of Asia, with a high titer of bacteria and yeast (5 × 107 CFU/mL and 1–2 × 107 CFU/mL) [15] as well as a wide variety of LAB, depending on the place of origin and method of preparation [16, 17].
The purpose of this study was to isolate new strains of lactic acid bacteria from kumiss and use them for the fermentation of pumpkin seed meal extract, to select the optimal scheme for obtaining a lacto-fermented drink, and to determine its characteristics.
MATERIALS AND METHODS
Seven samples of kumiss produced in the summer period in Bashkiria (Russia) were selected as a starting point for the isolation of LAB. Samples of pumpkin seed meal, from the harvest of 2020, were provided by “Maslodel,” Russia.
Aliquots of kumiss were diluted in a series of tenfold dilutions in sterile water and plated on a dense nutrient medium MRS (Merck KGaA, Germany) with a pH of 5.7 or wort-agar. The dishes with cultures were incubated at 37°C for two days.
The affiliation of the grown and re-plated colonies to lactic acid bacteria was determined by the fermentation test of sterile skimmed milk. Working LAB cultures grown in an MRS liquid medium were stored at 4°C. The number of viable LAB cells was estimated by colony unit (CFU) titers after inoculation on MRS agar dishes and incubation at 37°C for 48 h. The most valuable strains for use in the production of fermented milk products were deposited in the Collection of Unique and Extremophilic Microorganisms (UNIQEM) of the Center for Collective Usage of Federal Research Center of Biotechnology of the Russian Academy of Sciences under registration numbers UQM 41618, UQM 41619, and UQM 41620 and stored in cryopreserved form.
The cell morphology of the isolated LAB isolates was studied by microscopy using an Axioplan microscope (Carl Zeiss, Germany). Resistance to different pH was assessed based on growth results on standard MRS and other liquid media (milk, slightly alkaline protein extracts). Acidity was measured using a 150 MI pH meter (OOO Measuring Technics, Russia).
The isolated strains were identified based on the results of the analysis of the nucleotide sequence of fragments of the 16S rRNA gene, performed at the Center for Collective Use, Federal Research Center of Biotechnology, Russian Academy of Sciences, by the described methods previously [18].
The scheme of preparation of raw materials for fermentation included grinding pumpkin seed meal to flour at the Stegler LM-1000 laboratory mill, extraction of components with alkaline solutions from pH 6.2 to 9.0, upon heating up to 90°С (30 min) with stirring, and removal of insoluble particles by filtration. For optimal extraction, the ratio of extractant to flour has been selected. To remove foreign microflora, the obtained extracts were subjected to heat treatment at 0.5 atm for 30 min and then cooled to 37°С.
Seed meal extracts were inoculated with new strains from kumiss and strains of L. acidophilus, L. casei, L. bulgaricus, and Streptococcus sp. from the collection of the Laboratory of Microorganism Survival, Vinogradskii Institute of Microbiology, Russian Academy of Sciences. Cultures for inoculation were grown on sterile skimmed milk for 7 to 24 h at 37°С and introduced into the medium at a ratio of 0.3%–0.5% (vol/vol) until an initial cell titer of 104 CFU/mL was reached. In control experiments, regular sterile milk was used instead of meal extract. Fermentation of extracts and milk was carried out under static conditions (without mixing) at 37°С for 7 to 24 h.
The weight of dry matter in the samples obtained by the fermentation of extract from the meal, whole milk, and pumpkin seed flour was determined gravimetrically with drying at 105°C until a constant weight was reached. The total protein content was determined using the Lowry method or based on the sum of amino acids. The lactose content was determined using a method based on enzymatic hydrolysis and colorimetric determination of its cleavage products [19]. The content of dietary fiber (g/100 g) was determined by the gravimetric method after precipitation in ethanol and drying [20].
Samples of fermentation products for further analysis were dried using a freeze dryer FreeZone (Labconco, United States) in the Center for Collective Usage of the UNIQEM Collection in a vacuum at –80°C. The amino acid composition was determined using a liquid chromatograph manufactured by Hitachi (Japan) in the standard mode of analysis of protein hydrolysates with a sulfonated copolymer of styrene and divinylbenzene and a step gradient of a sodium-citrate buffer solution with an increasing pH and molarity value. Lipids from lyophilized samples were extracted with a mixture of chloroforms: hydrochloric acid methanol (2 : 1) (Methanolic-HCl 0.5, n, Supelco, Germany), according to the Folch method. The fatty acid composition of lipids was studied using a chromatograph with a Simadzu GCMS-QP2010 Ultra mass detector (Shimadzu, Japan). Studies of the amino acid composition and profile of fatty acids were carried out in the Center for Collective Use for Industrial Biotechnology, Federal Research Center of Biotechnology, Russian Academy of Sciences, and the Belozerskii Institute of Physico-Chemical Biology of Moscow State University. The basic experiments were carried out in three repetitions.
RESULTS AND DISCUSSION
Based on the results of cultures of seven samples of Bashkir kumiss on MRS and wort-agar, its total bacterial content (5.0 ± 0.7) × 108 CFU/mL and yeast (1.5 ± 0.7) × 107 CFU/mL was established, which corresponded to the abundance of microorganisms in kumiss from other regions [15]. The share of LAB in the studied kumiss ranged from 46 to 68%, and the average abundance ranged from 2.3 × 108 to 3.4 × 108 CFU/mL according to the fermentation test of sterile milk. Using multiple (3–5 times) passages on MRS agar, 50 pure LAB isolates were selected, which were inoculated into sterile milk and adapted to a new substrate—an extract from pumpkin seed meal. All isolates from kumiss were capable of fermenting extracts at different rates at 37°C for 7–7–24 h. Based on the best growth rate and organoleptic properties of the resulting products, three new strains were selected and deposited in the UNIQEM collection under the numbers UQM 41618, UQM 41619, and UQM 41620.
The selected LAB were represented by rod-shaped, non-spore-forming, non-motile, solitary, or chained cells and grew at 27°C–37°C with the formation of a white precipitate in liquid media. Based on the results of molecular genetic identification by analyzing a fragment of the 16S rRNA gene, it was established that the strains belong to the genus Lacticaseibacillus, recently bred based on the genus Lactobacillus and validly described [21]. The strains turned out to be the closest to L. rhamnosus and L. casei, based on the similarity of their sequences by 99.93 and 99.65% with the known deposited sequences NR_113332.1 and NR_041893.1, respectively. According to the results of phylogenetic analysis, a high relatedness of the UQM 41618, UQM 41619, and UQM 41620 strains with each other and with the typical Lacticaseibacillus rhamnosus strain was established (Fig. 1). An important feature of the isolated Lacticaseibacillus strains was tolerance to changes in the pH of the environment with an upper limit of up to 9.5. The optimal pH range for lactobacilli growth was between 5.5 and 6.2 [22]. Tolerance to increased alkalinity of the medium is known, and the upper pH limit varied in different Lactobacillus species and even strains [23]. Thus, new isolates of lactic acid bacteria were obtained from kumiss, and the potential for the use is discussed below.
According to the efficiency of fermentation of pumpkin seed meal with the new LAB strain, as well as other starter cultures, the need to optimize the primary stages of obtaining milk analogs has been established. The use of water with a pH value close to neutral as an extractant, sufficient to obtain milk analogs from whole seeds or nuts [1, 24], turned out to be ineffective for pumpkin meal: aqueous extracts were not fermented by LAB, which was probably due to the low protein yield. The use of alkaline extraction, as in the study on obtaining milk analogs from lupine seeds [25], turned out to be more effective for pumpkin meal only after the selection of optimal conditions. For example, alkalinizing the meal flour suspension from the initial pH value of 6.2 to 7.5 improved fermentability; however, the resulting product had unsatisfactory organoleptic properties. An increase in the pH of the extractable mixture to 9.0 supported the production of a substrate efficiently fermented at 37°С for 10 h with new LAB strains from kumiss with the formation of a homogeneous product with dense consistency with a fermented milk taste and odor. Use of other LAB strains like L. acidophilus, L. casei, L. bulgaricus, and Streptococcus sp. turned out to be less effective: fermentation took place within 24 h, the LAB titer did not exceed 105 CFU/mL, and the final product did not have good qualities. To improve extraction, optimal meal-extractant ratios were also selected. These and other technological parameters of extraction were previously described in [26].
Due to the contamination of pumpkin seeds with bacteria and fungi, based for example on the average cumulative data of ~1.4 × 105 CFU/g [27], the heat treatment stage was included in the scheme of preparation of the substrate for fermentation, without which spoilage of the final product occurred. It was found that the heat treatment modes at 80–85°С and 90–95°С for 10 min were insufficient to destroy foreign microflora; a longer (30 min) warm-up period was required at 115°С. Thus, the optimal scheme for obtaining an extract from pumpkin meal for effective fermentation was selected, the main elements of which, considering the modifications, are presented in Fig. 2.
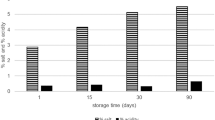
An important property that determined the choice of this raw material for the preparation of a suitable substrate was the high content (about 30%) of total protein (Fig. 3). According to the data obtained by the Lowry method, the degree of protein extraction from meal flour under selected optimal conditions corresponded to 50%. Its content was sufficient for the development of LAB. Strains of Lacticaseibacillus sp. nov. UQM 41618, UQM 41619, and UQM 41620 effectively fermented pumpkin meal seed extract: LAB abundance increased from 104 CFU/mL from inoculation to 108 CFU/mL after 10 h of incubation at 37°C under steady-state conditions. During growth, the environment was acidified from 8.5–9.5 to neutral pH values. It should be noted that the abundance of viable lactic acid bacteria in the cultured product remained stable (108 CFU/mL) during storage for one month at 4°С. In the resulting fermentation products, there was a 1.7-fold increase in the proportion of water-soluble proteins and a 1.3-fold decrease in the proportion of alkaline-soluble proteins. In addition, the proportion of essential amino acids and valuable unsaturated fatty acids (UFA) increased compared to the initial seed meal (Table 1, Fig. 3). Thus, pumpkin seed meal extract may be a suitable substrate for fermentation with newly selected LAB strains. Based on the analysis of studies on the production of fermented milk products from plant raw materials, pumpkin meal has so far been used only as a component of a mixture with camel milk [28].
The following advantages of the new fermented product based on pumpkin meal compared to the fermented milk product obtained using the same LAB strains were revealed. The new product was characterized by an increased content of protein (by 1.7 times) and valuable food components, especially several essential amino acids and polyunsaturated linoleic acid.
Other important properties of the new product were the high ratio of UFA/SFA, the absence of lactose and cholesterol (which is not surprising taking into consideration the composition of the initial raw materials), the presence of dietary fiber (Fig. 3; Table 1), and good flavor. The results of the analyses of the total composition of pumpkin seed meal and the total content of polyunsaturated fatty acids (Fig. 3; Table 1) corresponded to the literature data [29]. According to the above properties, the obtained lacto-fermented beverage may be attractive to certain groups of the population. Thus, based on the example of pumpkin seed meal, an original approach to the valorization of mass food waste from the oil extraction industry has been developed and a simple and effective scheme for obtaining an extract suitable for fermentation and obtaining analogs of fermented milk products with useful properties has been developed. The results indicate a high adaptive potential of new lactobacilli strains to new growth conditions, fluctuations in pH, and the ability to maintain survival during storage.
REFERENCES
Silva, A.R., Silva, M.M., and Ribeiro, B.D., Food Res. Int., 2020, vol. 131, no. 1, p. 108972. https://doi.org/10.1016/j.foodres.2019.108972
Vanga, S.K. and Raghavan, V., J. Food Sci. Technol., 2018, vol. 55, no. 1, pp. 10–20.
Kundu, P., Dhankhar, J., and Sharma, A., Curr. Res. Nutr. Food Sci. J., 2018, vol. 6, no. 1, pp. 203–210.
Mortas, M., Besir, A., Tok, Z., Keles, M., and Yazici, F., Plant Foods Hum. Nutr., 2023, vol. 78, pp. 358–365.
Bastıoğlu, A.Z., Tomruk, D., Koç, M., and Ertekin, F.K., J. Food Sci. Technol., 2016, vol. 53, no. 5, pp. 2396–2404.
Makinen, O.E., Wanhalinna, V., Zannini, E., and Arendt, E.K., Crit. Rev. Food Sci. Nutr., 2016, vol. 56, no. 3, pp. P. 339–349.
Ma, W., Zhang, C., Kong, X., Li, X., Chen, Y., and Hua, Y., Food Biosci., A, 2021, vol. 44, p. 101416. https://doi.org/10.1016/j.fbio.2021.101416
Ahmadian-Kouchaksaraei, Z., Mohammad, M.V., Varidi, J., and Pourazarang, H., LWT—Food Sci. Technol., 2014, vol. 57, no. 1, pp. 299–305.
Hickisch, A., Beer, R., Vogel, R.F., and Toelstede, S., Food Res. Int., vol. 84, pp. 180–188.
Garro, M.S., de Valdez, G.F., and de Giori, G.S., Food Microbiol., vol. 21, no. 5, pp. 511–518.
Vlaicu, P.A. and Panaite, T.D., Anim. Biosci., 2022, vol. 35, no. 2, pp. 236–246.
Valdez-Arjona, L.P. and Ramirez-Mella, M., Animals (Basel), 2019, vol. 9, no. 10, p. 769. https://doi.org/10.3390/ani9100769
Ulanova, R. and Kravchenko, I., Int. J. Eng. Sci. Innov. Technol., 2013, vol. 2, no. 6, pp. 618–624.
Ulanova, R.V., Kravchenko, I.K., Gorelova, O.P., and Nikolaeva, O.S., RF Patent No. 2557404, 2015.
Afzaal, M., Saeed, F., Anjum, F., Waris, N., Husaain, M., Ikram, A., et al., Food Sci. Nutr., 2021, vol. 9, no. 11, pp. 6421–6428.
Tang, H., Ma, H., Hou, Q., Li, W., Xu, H., Liu, W., and Menghe, B., BMC Microbiol., 2020, vol. 20, no. 1, pp. 1–11.
Meng, Y., Chen, X., Sun, Z., Li, Y., Chen, D., Fang, S., and Chen, J., LWT, 2021, vol. 135, p. 110049. https://doi.org/10.1016/j.lwt.2020.110049
Filippova, S.N., Surgucheva, N.A., Kolganova, T.V., Cherbunina, M.Yu., Brushkov, A.V., Mulyukin, A.L., and Gal’chenko, V.F., Biol. Bull. (Moscow), 2019, vol. 46, no. 3, pp. 234–241.
Ostroumov, L.A. and Gavrilov, V.G., Biotransformation of lactose by β-galactosidase enzyme preparations, Tekhn. Tekhnol. Pishch. Proizv., 2013, no. 1, pp. 26–30.
McCleary, B.V., J. AOAC Int., 2019, vol. 102, no. 1, pp. 196–207.
Zheng, J., Wittouck, S., Salvetti, E., Franz, C.M., Harris, H.M., Mattarelli, P., et al., Int. J. Syst. Evol. Microbiol., 2020, vol. 70, no. 4, pp. 2782–2858.
Śliżewska, K. and Chlebicz-Wójcik, A., Biology (Basel), 2020, vol. 9, no. 12, p. 423. https://doi.org/10.3390/biology9120423
Sawatari, Y. and Yokota, A., Appl. Environ. Microbiol., 2007, vol. 73, no. 12, pp. 3909–3915.
Kluczkovski, A., Lima, N., and Oliveira, M.K., J. Food Proc. Preserv., 2017, vol. 41, no. 5, p. 13147. https://doi.org/10.1111/jfpp.13147
Jiménez-Martínez, C., Hernández-Sánchez, H., and Dávila-Ortiz, G., J. Sci. Food Agricult., vol. 83, no. 6, pp. 515–522.
Ulanova, R.V., Nikolaev, Yu.A., Kolpakova, V.V., Galuza, O.A., and Sinel’nikov, A.V., RF Patent No. RU2784723.25, 2022.
Silva, D., Nunes, P., Melo, J., and Quintas, C., AIMS Microbiol., 2022, vol. 8, no. 1, pp. 42–52.
Shahein, M.R., Atwaa, E.S.H., Alrashdi, B.M., Ramadan, M.F., Abd El-Sattar, E.S., Siam, A.A.H., et al., Fermentation, 2022, vol. 8, no. 5, p. 223. https://doi.org/10.1016/j.fbio.2021.101416
Vlaicu, P.A. and Panaite, T.D., Anim. Biosci., 2022, vol. 35, no. 2, pp. 236–246.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of State Assignment no. 122040800164-6 of the Federal Research Center of Biotechnology, Russian Academy of Sciences, and Agreement no. 075-15-2021-1051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human or animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by V. Mittova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sinelnikov, A.V., Kolganova, T.V. & Ulanova, R.V. Obtaining Analogues of Fermented Milk Products from Seed Meal Using New Strains of Lactic Acid Bacteria. Appl Biochem Microbiol 60, 476–482 (2024). https://doi.org/10.1134/S0003683824603664
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683824603664