Abstract
Deep phenotyping is an emerging conceptual paradigm and experimental approach aimed at measuring and linking many aspects of a phenotype to understand its underlying biology. To date, deep phenotyping has been applied mostly in cultured cells and used less in multicellular organisms. However, in the past decade, it has increasingly been recognized that deep phenotyping could lead to a better understanding of how genetics, environment and stochasticity affect the development, physiology and behavior of an organism. The nematode Caenorhabditis elegans is an invaluable model system for studying how genes affect a phenotypic trait, and new technologies have taken advantage of the worm’s physical attributes to increase the throughput and informational content of experiments. Coupling of these technical advancements with computational and analytical tools has enabled a boom in deep-phenotyping studies of C. elegans. In this Review, we highlight how these new technologies and tools are digging into the biological origins of complex, multidimensional phenotypes.
Similar content being viewed by others
Main
One of the great drivers of biological research in the twentieth century was the desire to understand how information encoded in an organism’s genome gives rise to its physical and behavioral phenotypes, which are collectively referred to as its phenome1. However, the phenotype of an organism is not merely a reflection of its genes, but the integrated product of its genotype interacting with the environmental conditions and stochastic effects to which the individual is exposed before observation2,3. Consequently, an organism’s phenome contains a large, multidimensional set of observable characteristics, and the phenospace in a population of individuals is vast. Understanding the specific contribution of the genome, environment and stochastic processes to the position of an individual in the phenospace is a formidable technical challenge. Fortunately, technological innovation is beginning to provide the tools necessary for quantifying these multidimensional characteristics and their underlying causes, opening up a new experimental paradigm known as deep phenotyping4. Considering that it is fairly new, the term deep phenotyping is broadly used within the scientific literature. In this Review, we define deep phenotyping as the coupling of high-throughput experimental techniques with computational analyses to enable the generation, examination and interpretation of high-dimensional biological data.
C. elegans has many attributes that make this organism an ideal model system for deep-phenotyping studies. The worm is a small poikilotherm with an adult body length of approximately 1 mm that feeds on bacteria; it has a rapid life cycle, developing from egg to reproductive adult in approximately 3 days at 20 °C, and it has a deterministic developmental lineage5. In addition, the worm primarily reproduces as a self-fertilizing hermaphrodite, which means that large, near-isogenic populations can be grown in highly controlled environmental conditions. Additionally, because at least 38% of the protein-coding genes in the worm genome have a human ortholog6, insights gained from worm studies can inform us about human biology. Genes involved in apoptosis7 and axonal migration8,9, microRNAs10,11, and RNA interference (RNAi)12, for instance, were all initially identified in C. elegans and have since been shown to have roles in human disease13,14,15.
In this Review, we outline the technological and analytical developments that have enabled deep-phenotyping studies in C. elegans. We then examine with several examples how these tools have yielded greater insight into the biology of complex phenotypes. Finally, we offer a prospective outlook on the future of deep-phenotyping experiments in C. elegans and in other systems.
Tools and techniques for deep phenotyping
Historically, the phenotypic analysis of C. elegans involved the manual measurement of morphometric or behavioral features such as changes in body shape or defects in movement16. However, modern biological techniques have now dramatically expanded the definition of phenotype1. RNA sequencing and mass spectrometry, for example, have become more widely available and less cost prohibitive, enabling the transcriptomic and proteomic characterization of individuals. Similarly, advancements in both hardware and computational tools have increased both the throughput and informational content of experiments. In this section, we examine in three specific areas the developments that have enabled deep-phenotyping studies of the worm.
Manipulating the genome
A sophisticated set of techniques, including both forward and reverse genetic approaches, can be used to manipulate the worm genome and elicit phenotypes5. RNAi of gene expression can be achieved by feeding worms bacteria engineered to express double-stranded RNA corresponding to a gene of interest17,18. This technique has enabled multiple reverse-genetic screens to identify phenotypes associated with various gene inactivations19,20. More recently, several research groups have introduced CRISPR-Cas9 gene editing methods optimized for manipulation of the C. elegans genome21,22. These methods allow rapid and efficient knockout of genes or introduction of fluorescent markers of gene or protein activity.
Hardware for deep phenotyping
Two different types of hardware development have enabled deep-phenotyping studies in worms. The first is improvement in devices for handling worms and the second is improvement in the imaging technology that records the output of an experiment. Both developments have substantially reduced component costs and produced many designs and proof-of-principle experiments, which increases the accessibility of hardware needed for deep-phenotyping experiments.
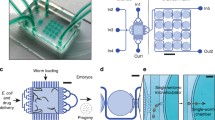
Manual handling of worms is labor intensive and limits the scale and scope of an experiment. Fortunately, the small size of the worm makes it very easy to manipulate with microfluidics23,24. In the past decade, microfluidic devices used by the C. elegans research community have been fabricated mostly from polydimethylsiloxane (PDMS). PDMS is nontoxic to worms, and fabrication of devices with this material is relatively cheap and straightforward. PDMS is also optically transparent and is therefore compatible with many types of microscopy used to study the worm. Many microfluidic devices include on-chip valves that take advantage of the elastic properties of PDMS to control the flow of fluid containing the worms. This design enables automation of animal handling and higher sample throughput23,25,26,27. In addition, microfluidics can be used to tightly control the microenvironment surrounding the worm within the device, which is hard to do on an agar plate. Device and system designs widely used for worm experiments include arena or multi-chamber arrays28,29,30,31, imaging and sorting devices24,27,32,33,34,35,36,37, and devices enabling more complex manipulations38,39,40,41 (Fig. 1).
Examples of microfluidic devices routinely used in worm deep-phenotyping studies. a, Multi-chamber arrays for simultaneously studying large numbers of individual worms. b, Sorting devices used to rapidly isolate worms with specific characteristics. c, Arena devices for behavioral assays. Panel a adapted from ref. 29; panel b adapted from ref. 24; panel c adapted from ref. 28.
C. elegans has proven to be amenable to multiple imaging modalities. Because the worm is optically transparent, early in vivo studies of worm development used Nomarski optics to provide sufficient contrast to observe individual cell nuclei42,43. Various types of electron microscopy have been used for ultrastructural studies of C. elegans44. High-throughput experiments typically require the use of automated imaging systems that can quantify the intensity of fluorescent reporters. The demonstration that fluorescent protein reporters can be expressed and imaged in C. elegans paved the way for new types of fluorescence microscopy that are compatible with high-throughput experimentation45. Examples include the use of light-sheet and lattice light-sheet microscopy (LLSM) platforms to study embryogenesis46,47,48,49 as well as protein dynamics in adult worms50. In the past year, a new form of LLSM with adaptive optics has been used for highly detailed imaging of cell movements during vulva development51. Several new microscopy platforms, including two-photon-based52 and light-field53,54 systems, have enabled fast volumetric whole-brain imaging of Ca2+ dynamics in C. elegans. A new form of volumetric flow cytometry using line excitation array detection has been used to monitor protein aggregation in worm models of Huntington’s disease55. Several super-resolution microscopy systems have also been used to study embryogenesis56,57, muscle structure58 and the distribution of glutamate receptors59 in C. elegans.
Unlike imaging techniques used to examine cellular structures or activities, many high-content behavioral studies utilize dark-field or transmission imaging to enhance the contrast of transparent worms and allow longitudinal tracking and quantification of whole-animal phenotypes60,61. The hardware needed for such systems is becoming cheaper and more readily available62,63,64,65. Behavior-tracking systems have also been integrated into microscopy systems that use targeted illumination of specific cells for optogenetic experiments in worms66,67. Such systems have been used to determine how the mechanosensory system of the worm encodes spatial and temporal information about stimuli to ensure appropriate behavioral responses68,69. The greater availability of these systems has been central to the rapid increase in behavioral deep-phenotyping studies.
Computational software for high-content phenotypic information
Due to the hardware-related gains in throughput, many experimentalists now face a dramatic increase in the amount of data produced from experiments. Making sense of these data can be beyond the analytical capability of human vision or comprehension. Therefore, extracting useful information from large volumes of data requires workflows that can parse the content efficiently and in an interpretable manner. For image-based data, the typical structure of a deep-phenotyping workflow includes a segmentation step to identify objects of interest within each image70,71. This step is then followed by feature extraction and classification, which provides a quantitative breakdown of the differences between images and allows the assignment of phenotypic profiles70,71. Open-source image analysis platforms such as ImageJ72,73 and CellProfiler74, both of which have C. elegans-specific plugins75,76,77, conveniently offer many of the computational tools required to analyze high-content data.
Several different segmentation algorithms have been applied in C. elegans imaging informatics. These include algorithms that segment fluorescently labeled nuclei, which have been used for cell identification and tracking during embryonic development78,79,80,81 or within the context of a digital atlas of the L1-stage larva82,83, as well as for tracking Ca2+ transients across neurons in whole-brain imaging data84,85. Algorithms also exist for segmenting fluorescently labeled synapses to study synaptogenesis32,86 and for automatically tracing fluorescently labeled neurites87 in the worm. Bright-field images can also be automatically segmented to calculate various size-related metrics of worms using the ImageJ plug-in WormSizer88.
Machine learning is increasingly used to automate segmentation, feature extraction and classification of images in high-content workflows70,89. DevStaR is a machine learning-based platform that has been developed to score phenotypes automatically in worms90. This system can segment bright-field images and count animals belonging to different developmental stages in a mixed population. Zhan and colleagues have used a modular image-processing pipeline for the rapid development of custom supervised learning-based support-vector machines (SVM) classifiers 32,91. This pipeline has been used to train a classifier to identify the head of the worm in bright-field images91, as well as to train classifiers to identify neurons and differentiate between ASI and ASJ neurons91 and between ASI, ADF and NSM neurons92 in fluorescence microscopy images. WorMachine is another modular pipeline that can automatically score a wide range of phenotypes using trained classifiers93. This system can identify the sex of the animal as well as quantify complex, partially penetrant phenotypes or intracellular protein aggregation in a group of worms93. The development of these image analysis pipelines allows researchers without machine-vision expertise to design and perform deep-phenotyping studies in C. elegans. Additional examples of machine learning in imaging informatics and behavioral classification are provided below.
Applications of deep phenotyping
With the development of hardware and computational tools, deep phenotyping is now commonly used in multiple areas of worm biology. Examples of such studies are reviewed below.
Embryogenesis and lineage tracing
C. elegans is one of the few metazoans for which the entire somatic cell lineage can be traced from single-cell embryo to adult42,43. However, tracing cell lineage in the developing worm embryo is exceptionally challenging given the rapidity of cell division and morphological similarity across different cell types94. Because it is more complicated to identify phenotypic aberrations in the embryonic lineage than in post-embryonic cells, the mechanisms governing embryonic cell division and differentiation have been harder to elucidate. The advent of 4D imaging systems95,96 removed the need for manual observation of embryogenesis but not for curation of the resultant images into lineages.
Deep phenotyping can be done with manually curated data. As an example, a whole-genome RNAi screen identified 661 genes involved in early embryogenesis97, and a subsequent study integrated transcriptional, protein interaction and visual phenotypic data for these 661 genes to create predictive models of cellular events during embryogenesis98 (Fig. 2a). The use of transgenic worms that ubiquitously express fluorescently tagged histone proteins has enabled the development of automated tracing algorithms that track embryonic lineages up to the 350-cell stage47,79,80,99,100,101 (Fig. 2b).
a, Transcriptomic, proteomic and phenotypic data can be integrated to create models of cellular events in early embryogenesis. b, Use of two-color imaging can inform automated embryonic lineaging (top), which can then be used to create spatiotemporal maps of gene expression (bottom). c, Deep phenotyping can reveal more of the phenotypic spectrum than traditional screens. Subtle mutants (labeled in pink) lie closest to the wild type (WT), whereas previously identified mutants are farther from the WT. Panel a adapted from ref. 98; panel b adapted from ref. 108; panel c adapted from ref. 86.
Automated image collection and curation allows researchers to examine different aspects of embryonic cell division. Several studies have used automated lineage tracing to identify the precise cellular expression patterns of known embryonic genes102,103,104. This approach has allowed the construction of a single-cell-resolution atlas of gene expression, revealing when and where transcription factors are expressed in the developing embryo103,104.
It is now also possible to investigate how different genetic perturbations affect embryogenesis. Early studies, for example, have identified the subtle roles of a single transcription factor105 and the distinct roles of highly related and recently duplicated genes106 in defining an embryonic lineage. More recent studies have demonstrated the roles of several hundred genes in cell fate choice107,108 and in the regulation of asynchronous cell division109. A specific screen of chromatin regulators has also revealed distinct roles for several chromatin-modifying complexes during embryogenesis110. Together these studies are revealing the genetic programs and molecular mechanisms that specify how a single fertilized oocyte becomes an embryo containing a complex collection of differentiated cell types.
Whereas tools are available for deep phenotyping of embryonic development, high-throughput lineaging of post-embryonic worms has been lagging, mainly due to technical challenges imposed by the need to immobilize animals prior to imaging. However, in 2017, Keil and colleagues presented a microfluidic device that allows long-term culturing of larvae and routine immobilization of the animals, enabling high-resolution imaging with a variety of microscopy techniques111. Using this platform, the investigators imaged animals from the L1 larval stage through to reproductive adulthood and examined vulva precursor cell development, apoptosis during larval molting, neuron differentiation and neurite outgrowth. The emergence of new technologies such as this type of platform, coupled with future developments in post-embryonic phenotyping, is likely to lead to a complete description of the biological events involved in the development of a multicellular animal.
Automated high-throughput genetic screens
One of the most useful aspects of C. elegans as a model system has been that it allows forward-genetic screens to identify mutations affecting all elements of a gene, such as its promoter, exons, introns and other regulatory elements. However, most genetic screens rely on visually identifiable phenotypic differences from the control, which inherently limits the ability to identify mutations that have weak or non-obvious phenotypes but that still provide valuable information about the function of a gene. The power of deep phenotyping for identifying subtle mutants that might be missed by visual inspection has been demonstrated in automated screens to find mutations affecting synaptogenesis32,86,112. Using a microfluidic sorting system32 (Fig. 1b), hundreds to thousands of worms were continuously imaged, processed and sorted in real time. An online SVM-based image-processing algorithm that had been developed to classify multidimensional features of fluorescently labeled synapses in the fly was used to process the images32,86. Clustering of multidimensional features for all the worms revealed the phenospace of the entire mutational spectrum32,86 (Fig. 2c). The clusters indicate where each new mutant resides in the phenospace, allowing inference of their potential genetic relationships. This study is an example of how deep phenotyping integrates high-throughput hardware with computational tools to provide mechanistic insights that would not have been possible for a human observer.
Measuring aging and age-related decline
Over the past 30 years, scientists have made a concerted effort to understand the biology of aging. C. elegans is the premier model organism for the study of longevity because of its short lifespan and powerful genetics113. The majority of lifespan studies in worms are performed manually; animals maintained on agar plates are periodically examined under a stereomicroscope for either spontaneous or stimulated movement114. These manual experiments constrain the types of phenotypic data and the scale of demographic data that a human observer can obtain. Deep-phenotyping technologies offer more efficient and cost-effective collection of high-throughput and high-temporal-resolution lifespan data.
The first automated aging studies in worms used scanners to capture time-lapse data showing worm movement on agar plates115,116. In these systems, death is defined as a persistent absence of movement, as the worms cannot be stimulated to test for induced movement. Stroustrup and colleagues have used their automated system, termed the lifespan machine116, to examine the demographic features of large populations117. At the population level, in groups that had very different lifespans after exposure to factors such as changes in ambient temperature or chemically induced stress, aging appeared to scale temporally; plots of survival against time for worms in different conditions could be superimposed by rescaling the time axis. This scaling implies that a single effective constant rate governs aging in C. elegans and that interventions that altered longevity did so by changing this rate117.
These scanner-based lifespan studies cannot gather data at the level of the individual because their temporal resolution does not allow tracking of each worm until it dies116. As a result, these systems cannot assess inter-individual variation in lifespan within a population, which can be large even for isogenic worms raised in identical conditions118. In the past 3 years, two groups have achieved robust high-throughput automated acquisition of lifespan data from individual animals30,31,119. Pittman and colleagues used a polyethylene glycol (PEG) hydrogel substrate seeded with spots of Escherichia coli as a food source for developing C. elegans embryos, each of which is individually placed in a single spot; once the embryos are deposited, the substrate is sealed using a layer of PDMS31. The PDMS acts as a barrier confining each animal upon hatching to its local spot of E. coli. The PEG substrate can then be imaged using a wide array of microscopy platforms, allowing researchers to track multiple features of the development and lifespan of each worm individually. Zhang and colleagues have used this system to show that when tracked at the level of the individual, aging does not display temporal scaling, with long-lived animals showing an extended decrepitude phase—termed twilight—compared with short-lived individuals119. The WorMotel platform30,62 can record both spontaneous and stimulated movement; stimulated movement is evoked by a brief pulse of blue light to assess whether a worm is alive or not. Using this system, Churgin and colleagues have also demonstrated that short-lived and long-lived individual lifespans from an isogenic population do not temporally scale30. The differences seen between population-level measures of longevity and measures that account for inter-individual variability demonstrate the increasing power of deep-phenotyping technologies to study biological processes.
Dietary restriction (DR) has been shown to extend robustly lifespan in an evolutionarily conserved manner120. There is considerable interest, from researchers in both academia and the pharmaceutical industry, in finding drugs that can induce the phenotypic effects of DR in humans without the concomitant need for drastic diet change. Using the lifespan machine116, Lucanic and colleagues designed a high-throughput screen to identify potential DR mimetics in C. elegans121. The investigators screened a library of 30,000 small molecules, identifying 57 compounds that repeatedly extended the lifespan of treated animals compared with control worms. Several of these compounds were structurally related and contained a nitrophenyl piperazine moiety; further analysis of the most effective of the nitrophenyl piperazine compounds, NP1, suggested that it extended longevity by inducing DR-like effects121.
One of the reasons for the rising interest in the study of aging is that the incidence of both cognitive impairment and neurodegenerative disease increases with old age, which imposes a substantial societal cost as the proportion of elderly individuals relative to those of working age continues to grow in the general population122. The research community seeks to develop treatments that can counteract the debilitating neurological effects of aging. C. elegans also displays age-related declines in cognitive ability and in the morphological structure of its nervous system123. Bazopoulou and colleagues have developed a microfluidic platform to monitor the Ca2+ responses of a specific polymodal neuron, ASH, as it ages124. They used this system to conduct a pilot screen to identify small molecules that could delay the age-related decline of ASH activity. Several molecules from a panel of 107 FDA-approved compounds delayed the decline of Ca2+ responses in ASH during aging; the most effective of these compounds were tiagabine and honokiol124. This study demonstrates that deep-phenotyping technologies can acquire dynamic readouts, such as neural activity, in a high-content manner.
Drug discovery and small-molecule biology
Deep phenotyping is increasingly being embraced by researchers in the field of drug discovery because it allows rapid screening of the pleiotropic effects of different molecules. Due to its ease of culture and amenability to high-throughput experimentation, the worm has been used to model many different human diseases125,126. Although many researchers are developing methodologies to speed up drug discovery using worm models of disease, these platforms do not fit our definition of deep phenotyping as they tend to analyze single and relatively simple phenotypes. However, we review these methodologies here because they may suggest how newer deep-phenotyping platforms will transform drug discovery screens in model organisms.
The phenotype of diseased hepatocytes has been replicated in a worm model of cirrhosis expressing a mutant human α1-antitrypsin (ATZ) fused to green fluorescent protein (GFP) that aggregates within the endoplasmic reticulum of intestinal cells126. A plate reader-based screen of a commercially available compound library yielded 33 compounds that decreased the rate of GFP aggregation within this worm’s intestinal cells126. More recently, a high-throughput genome-scale RNAi screen of ATZ model worms was performed to find gene inactivations that alter intestinal GFP aggregation; the study identified 100 genes whose inactivation decreased GFP aggregation in the ATZ worms. Using an in silico approach, the investigators identified drugs known to target the mammalian orthologs of the worm genes and tested them for rescue of the GFP-aggregation phenotype127.
Polyglutamine expansion (polyQ) diseases, such as Huntington’s disease, are another example of protein-aggregation disorders that have been modeled successfully in worms128. A 2016 study unveiled a high-resolution microfluidic system for high-throughput drug discovery using a worm polyQ model as a demonstration of the platform’s potential129. With this platform, Mondal and colleagues rapidly screened around 100,000 worms to test 983 FDA-approved compounds for their ability to reduce yellow fluorescent protein (YFP) aggregation in a high-polyQ strain129. The screen identified four compounds that induced a significant reduction in protein aggregation. Another high-resolution microfluidic system has been developed to identify compounds that affect axon regeneration in C. elegans130. This platform was used to perform high-throughput laser microsurgery specifically on the axons of the PLM neuron and then move the axotomized worms into media containing different compounds to test the effects on neurite regrowth. This screen revealed that the compounds staurosporine and prostratin could, respectively, inhibit and promote axonal regeneration130.
The use of deep-phenotyping technologies might also enable cost-effective studies of widespread but often neglected diseases. Parasitic nematodes, for example, are thought to infect a billion people worldwide and are also a substantial source of infection in many animals and plants that humans depend on for their food and livelihoods. However, the development of anthelmintic drugs has not kept pace with the acquisition of drug resistance by these nematodes, and therefore new therapeutics are urgently needed131,132. Since parasitic nematodes are difficult to work with directly because they need to grow in a host system, C. elegans has become a model system for anthelmintic toxicology. The WormScan platform can simultaneously measure the mobility, brood size, body size and lifespan of worms either on agar plates or in liquid culture115,131. This system was used to screen 26,000 compounds, resulting in the identification of 14 potential anthelmintic compounds. The Invertebrate Automated Phenotyping Platform (INVAPP) also utilizes a 96-well-plate format for culturing worms in liquid. However, the automated image capture relies on a high-frame-rate camera instead of a scanner, giving this system increased sensitivity in the detection of drug-induced motility defects132. A proof-of-concept drug screen using INVAPP with a 400-compound library identified 14 molecules that impaired worm growth132. A separate compound-library screen against the parasitic nematode Trichuris muris using the INVAPP platform and the Paragon algorithm uncovered an entirely new class of promising anthelmintics133. Such deep-phenotyping studies of C. elegans allow researchers to rapidly identify potential anthelminthic drugs and their modes of action without performing the complex in-host assays required to study parasitic worms.
Small-molecule screens are not only a tool to identify therapeutic chemicals; they can also be used to study the biology of genetic pathways. The gene skn-1 encodes a transcription factor that regulates the worm’s response to oxidative and xenobiotic stress134. Leung and colleagues performed a plate reader-based screen for small-molecule activators of the protein skinhead-1 (SKN-1) by measuring the induction of a GFP-based transcriptional reporter of the skn-1 target gene gst-4135. The investigators then performed an ultra-high-throughput screen for inhibitors of SKN-1 in a compound library containing >364,000 small molecules136. This screen identified 125 molecules that specifically lowered the fluorescence signal of the gst-4::gfp reporter compared with a control, suggesting that these molecules are SKN-1 inhibitors136. A similar strategy was used to study the male-specific linker cell, which undergoes cell death just after the molt between the fourth larval stage and adulthood137. The death of this cell is independent of caspases, therefore non-apoptotic, and shares many morphological features with other non-apoptotic cell death observed in vertebrate development137. Schwendeman and Shaham performed a proof-of-concept small-molecule screen to identify potential inhibitors of linker-cell death that might shed further light on the biology of this phenomenon138. A screen of 23,797 compounds using a laser-scanning cytometer identified six compounds that caused persistence of the linker cell by inducing some form of global developmental delay in the worms that was rescuable upon removal of the worms from the drug138. Together these studies demonstrate how the use of deep-phenotyping technologies might enable quantitative measurement of multiple morphometric traits to gain new insight into biology.
Behavioral analyses of freely moving worms
One of the original motivations that drove the development of C. elegans as a model system was the desire to understand how the nervous system of an animal gives rise to its behaviors16. The history and biological significance of behavioral studies of C. elegans were extensively reviewed by McDiarmid and colleagues in 201761. In this Review, we briefly discuss improvements in worm-tracking hardware and software in the past 20 years and show how deep-phenotyping studies might reveal mechanisms underlying emergent behaviors.
The rapidity of behavior changes in freely moving worms makes it nearly impossible for a human observer to record all events in real time. Therefore, the majority of behavioral studies use some form of automated imaging. The earliest tracking systems could record the spatial position, speed and turning rate of individual worms139. Newer tracking platforms offer more comprehensive descriptions of worm behavior. Worm-tracking systems fall into two categories: single-worm trackers for high-resolution analysis of individual behavior140,141,142,143 and multi-worm systems for population-level studies65,144,145,146,147,148,149 (Fig. 3). These systems have elucidated the behavioral genetics of several sensory modalities. Explicit analyses of different behavioral features of thousands of animals from 239 genotypes uncovered 87 genes involved in locomotion, including components of the Gαq signaling pathway, and predicted 370 specific genetic interactions among them150. Similar studies have identified genes involved in thermotaxis151, chemotaxis144,152 and mechanosensation144. Most of these studies were performed on agar plates. However, the development of microfluidic arenas now allows for precise spatiotemporal control of the chemical environment, revealing behaviors that are not observable in plate-based experiments28.
In addition to the improvements in worm-tracker hardware, the development of new algorithms has led to a more comprehensive description of worm behaviors. For example, principal component analysis has been used to decompose the postural space of worms into eigenvectors referred to as eigenworms153. Surprisingly, the study showed that the postural space of locomoting worms on agar plates is low dimensional and that the superposition of just four eigenworms is sufficient to describe the majority of the worm’s locomotory postures. This analysis, which dramatically reduces the complexity of quantifying behavioral patterns, has been built into many worm-tracking systems141,144,153,154. The approach has enabled deep-phenotyping studies of behavioral dynamics, such as the creation of a dictionary of behavioral motifs curated from both wild-type and 307 mutant strains154. Similarly, an extensive phenotypic database of locomotory behaviors for a large number of strains was compiled through comprehensive behavioral recording for multiple alleles of the same gene as well as some double and triple mutants141. These dictionaries154 and databases141 uncover subtle behavioral phenotypes that cannot be discerned by manual observation, underscoring the importance of deep-phenotyping pipelines.
There is still limited understanding of how behaviors emerge from the integration of information from the external environment and the worm’s internal state. This problem has been studied extensively in food-related behaviors155. The behavioral state transitions that a worm undergoes while foraging, as well as the informational value of the food it encounters, can be modeled mathematically156,157,158, and these models can be used to predict the worm’s response to food156,158. We anticipate that the integration of such predictive models into deep-phenotyping studies will lead to greater mechanistic insight into the genetics of foraging behavior. Increasing experimental throughput is also essential to achieve deep phenotyping of foraging behavior. WorMotel allows for highly parallelized monitoring of individual worms under uniformly controlled environmental conditions30,62. This platform has been used to examine the relationship between food abundance and roaming (active) or dwelling (sedentary) behaviors159,160. These studies reveal the biological complexity of foraging behaviors. For example, serotonin produced by ADF neurons promotes roaming, whereas serotonin produced by NSM neurons promotes dwelling160. Other biological amines such as dopamine promote dwelling159, whereas octopamine is involved in roaming behaviors160.
Similar experimental parallelization has been performed in plate-based foraging assays with the use of multiple cheap cameras161. Stern and colleagues examined roaming and dwelling behaviors in individual worms continuously across their development161. The pattern of behaviors varied reproducibly across different developmental stages as well as between the onset and exit phases of each larval stage. The study showed that a suite of neuromodulators is responsible for the regulation of these behavioral patterns161. Interestingly, by tracking individual worms throughout their development, the investigators also identified striking inter-individual variation in roaming behavior. Even though the worms were derived from isogenic populations, some animals consistently roamed less across all developmental stages, whereas others consistently roamed more161. Quantifying this type of stochastic variation in any phenotype and understanding its biological origins is challenging given the large numbers of individuals that must be surveyed. Deep phenotyping offers a way to integrate high-throughput experimentation with comprehensive behavioral analysis.
Whole-brain imaging: the next frontier in deep phenotyping
How the nervous system encodes external environmental information to modulate animal behavior is the subject of intense examination in biology. This question becomes even more challenging to answer in natural habitats, where the environment contains a myriad of conflicting cues that an animal must successfully integrate to perform behaviors that maximize its chance of survival. In C. elegans, the nervous system is essentially divided into three layers: sensory neurons that detect external stimuli, interneurons that integrate information from sensory neurons, and motor neurons that control the behavior of the worm, with a few polymodal neurons that perform several of these functions162. It is a complex problem to understand how the different layers receive and process information and communicate with one another to ensure the appropriate outcome. For example, the aversive chemotactic response to isoamyl alcohol exposure is stochastic, even though the chemosensory neuron that detects the stimulant depolarizes in a deterministic way163. The behavioral stochasticity of this chemotaxis circuit is generated at the level of interneurons via their collective activity163. The development of genetically encoded Ca2+ indicators has allowed imaging of neuronal activity in the worm, ranging from a single neuron all the way up to the entire brain164. This development, in turn, has paved the way for whole-brain imaging platforms for long-term observation and analysis of large numbers of neurons. The goal of these systems is to dissect neuronal activity and identify not only neural circuits associated with specific behaviors, but also global states that reflect the functional architecture of the brain.
Several studies have successfully characterized whole-brain dynamics under various experimental conditions. Kato and colleagues used a microfluidic imaging platform to carry out whole-brain recordings from immobilized worms, which revealed that the time evolution of network dynamics among neurons is directional and cyclical165. Different phases in this cyclical activity regulate motor commands that drive certain locomotory behaviors. Using a spinning-disk confocal system, Venkatachalam and colleagues developed a whole-brain imaging platform and studied representations of sensory input and motor output of individual neurons upon thermosensory stimulus in freely moving worms166. Similarly, using a simultaneous worm-tracking and whole-brain imaging system, Nguyen and colleagues recorded whole-brain activity of freely moving worms without stimulation85,167; in a related effort, Nguyen and colleagues used machine learning to track neurons in the freely moving heads of worms, which is an important step toward robustly and automatically analyzing such large sets of dynamical data85. More recently, Nichols and colleagues investigated global brain dynamics during lethargus, a sleep-like state in the worm168. The study showed that global brain activity becomes quiescent during lethargus; however, specific neurons remain active as these cells promote the establishment of the quiescence. By examining whole-brain dynamics, this work demonstrated that the transition to the wakeful state is carried out via reestablishment of activity in specific neurons that drives global brain dynamics back to the aroused state168.
The examples reviewed above show the benefit of studying the entire nervous system rather than analyzing specific subsets of neurons, given that researchers have not identified all the neurons or functional connections in circuits governing various sensorimotor behaviors. These examples also point to opportunities for future technological and theoretical developments in analyzing and understanding such complex and dynamical systems. For instance, the field still needs better imaging systems that allow coupling of other experimental techniques such as optogenetics. Automatic identification of neurons is also needed, along with new tracking algorithms that are more accurate, faster or both. Furthermore, better theories might be needed in the future for interpreting large volumes of curated data and making sense of how the brain processes information and makes decisions.
Future outlook
In this Review, we have highlighted many recent conceptual and methodological developments for deep phenotyping using C. elegans as a model system. The success of these studies clearly indicates that by measuring many aspects of the morphology, functional output and behavior of cells, as well as circuits, tissues and individual animals, we can expand the scope of biological studies. Furthermore, by using appropriate mathematical and statistical tools, we can better understand the mechanisms underlying many biological processes. We believe that these approaches will become more ubiquitous as improved microscopy and other experimental tools as well as analytical pipelines using advanced computational and theoretical techniques become more accessible.
Although many of the tools discussed above were used in a C. elegans-specific context, they are applicable to a broad range of biological systems. For example, microfluidics is widely used in the analysis of single cells, with platforms such as DropMap providing high-content, single-cell-resolution analysis of IgG-secreting cells169. Substantial progress has also been made in developing automated animal handling and tracking systems that can work with diverse organisms. The MAPLE robotic system and its derivatives can perform deep-phenotyping experiments on Saccharomyces cerevisiae, Physarum polycephalum, Bombus impatiens and Drosophila melanogaster in addition to C. elegans65,170. Several studies have also demonstrated the feasibility of deep phenotyping in the vertebrate model system Danio rerio. High-throughput optical-projection tomography has been used to measure gene expression changes across the brains of different mutants of zebrafish171. Similarly, micron-scale tomography172 has been used to study the determinants of skeletal development in zebrafish173. We predict that the future integration of efforts in different disciplines such as biology, engineering and computational sciences will considerably accelerate progress in linking phenotypes to genotypes, environmental conditions and stochasticity.
References
Houle, D., Govindaraju, D. R. & Omholt, S. Phenomics: the next challenge. Nat. Rev. Genet. 11, 855–866 (2010).
Raj, A. & van Oudenaarden, A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226 (2008).
Honegger, K. & de Bivort, B. Stochasticity, individuality and behavior. Curr. Biol. 28, R8–R12 (2018).
Tracy, R. P. ‘Deep phenotyping’: characterizing populations in the era of genomics and systems biology. Curr. Opin. Lipidol. 19, 151–157 (2008).
Corsi, A. K., Wightman, B. & Chalfie, M. A transparent window into biology: a primer on Caenorhabditis elegans. Genetics 200, 387–407 (2015).
Shaye, D. D. & Greenwald, I. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One 6, e20085 (2011).
Ellis, H. M. & Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829 (1986).
Hedgecock, E. M., Culotti, J. G. & Hall, D. H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61–85 (1990).
Ishii, N., Wadsworth, W. G., Stern, B. D., Culotti, J. G. & Hedgecock, E. M. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9, 873–881 (1992).
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Fadeel, B. & Orrenius, S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 258, 479–517 (2005).
Lekka, E. & Hall, J. Noncoding RNAs in disease. FEBS Lett. 592, 2884–2900 (2018).
Van Battum, E. Y., Brignani, S. & Pasterkamp, R. J. Axon guidance proteins in neurological disorders. Lancet Neurol. 14, 532–546 (2015).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395, 854 (1998).
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Rual, J. F. et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14, 2162–2168 (2004).
Dickinson, D. J. & Goldstein, B. CRISPR-based methods for Caenorhabditis elegans genome engineering. Genetics 202, 885–901 (2016).
Farboud, B. Targeted genome editing in Caenorhabditis elegans using CRISPR/Cas9. Wiley Interdiscip. Rev. Dev. Biol. 6, e287 (2017).
Crane, M. M., Chung, K., Stirman, J. & Lu, H. Microfluidics-enabled phenotyping, imaging, and screening of multicellular organisms. Lab. Chip 10, 1509–1517 (2010).
Chung, K., Crane, M. M. & Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637–643 (2008).
Ben-Yakar, A., Chronis, N. & Lu, H. Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C. elegans. Curr. Opin. Neurobiol 19, 561–567 (2009).
Cornaglia, M., Lehnert, T. & Gijs, M. A. M. Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans. Lab. Chip 17, 3736–3759 (2017).
Lee, H. et al. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab. Chip 14, 4513–4522 (2014).
Albrecht, D. R. & Bargmann, C. I. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat. Methods 8, 599–605 (2011).
Chung, K. et al. Microfluidic chamber arrays for whole-organism behavior-based chemical screening. Lab. Chip 11, 3689–3697 (2011).
Churgin, M. A. et al. Longitudinal imaging of Caenorhabditis elegans in a microfabricated device reveals variation in behavioral decline during aging. Elife 6, e26652 (2017).
Pittman, W. E., Sinha, D. B., Zhang, W. B., Kinser, H. E. & Pincus, Z. A simple culture system for long-term imaging of individual C. elegans. Lab. Chip 17, 3909–3920 (2017).
Crane, M. M. et al. Autonomous screening of C. elegans identifies genes implicated in synaptogenesis. Nat. Methods 9, 977–980 (2012).
Krajniak, J., Hao, Y., Mak, H. Y. & Lu, H. C.L.I.P.—continuous live imaging platform for direct observation of C. elegans physiological processes. Lab. Chip 13, 2963–2971 (2013).
Kopito, R. B. & Levine, E. Durable spatiotemporal surveillance of Caenorhabditis elegans response to environmental cues. Lab. Chip 14, 764–770 (2014).
Gokce, S. K. et al. A multi-trap microfluidic chip enabling longitudinal studies of nerve regeneration in Caenorhabditis elegans. Sci. Rep. 7, 9837 (2017).
Rouse, T., Aubry, G., Cho, Y., Zimmer, M. & Lu, H. A programmable platform for sub-second multichemical dynamic stimulation and neuronal functional imaging in C. elegans. Lab. Chip 18, 505–513 (2018).
Casadevall i Solvas, X. et al. High-throughput age synchronisation of Caenorhabditis elegans. Chem. Commun. (Camb) 47, 9801–9803 (2011).
Cho, Y., Oakland, D. N., Lee, S. A., Schafer, W. R. & Lu, H. On-chip functional neuroimaging with mechanical stimulation in Caenorhabditis elegans larvae for studying development and neural circuits. Lab. Chip 18, 601–609 (2018).
Cho, Y. et al. Automated and controlled mechanical stimulation and functional imaging in vivo in C. elegans. Lab. Chip 17, 2609–2618 (2017).
Rahman, M. et al. NemaFlex: a microfluidics-based technology for standardized measurement of muscular strength of C. elegans. Lab. Chip 18, 2187–2201 (2018).
Lockery, S. R. et al. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab. Chip 12, 2211–2220 (2012).
Sulston, J. E. & Horvitz, H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56, 110–156 (1977).
Sulston, J. E., Schierenberg, E., White, J. G. & Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 (1983).
Hall, D. H., Hartwieg, E. & Nguyen, K. C. Modern electron microscopy methods for C. elegans. Methods Cell. Biol. 107, 93–149 (2012).
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C. Green fluorescent protein as a marker for gene expression. Science 263, 802–805 (1994).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Mace, D. L., Weisdepp, P., Gevirtzman, L., Boyle, T. & Waterston, R. H. A. High-fidelity cell lineage tracing method for obtaining systematic spatiotemporal gene expression patterns in Caenorhabditis elegans. G3 (Bethesda) 3, 851–863 (2013).
Wu, Y. et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc. Natl Acad. Sci. U S A 108, 17708–17713 (2011).
Wu, Y. et al. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 31, 1032–1038 (2013).
Rieckher, M. et al. A customized light sheet microscope to measure spatio-temporal protein dynamics in small model organisms. PLoS One 10, e0127869 (2015).
Liu, T. L. et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392 (2018).
Schrodel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 10, 1013–1020 (2013).
Prevedel, R. et al. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat. Methods 11, 727–730 (2014).
Shaw, M., Elmi, M., Pawar, V. & Srinivasan, M. A. Investigation of mechanosensation in C. elegans using light field calcium imaging. Biomed. Opt. Express 7, 2877–2887 (2016).
Martin, C. et al. Line excitation array detection fluorescence microscopy at 0.8 million frames per second. Nat. Commun. 9, 4499 (2018).
Gao, L. et al. Noninvasive imaging beyond the diffraction limit of 3D dynamics in thickly fluorescent specimens. Cell 151, 1370–1385 (2012).
Ingaramo, M. et al. Two-photon excitation improves multifocal structured illumination microscopy in thick scattering tissue. Proc. Natl Acad. Sci. U S A 111, 5254–5259 (2014).
Qadota, H. et al. High-resolution imaging of muscle attachment structures in Caenorhabditis elegans. Cytoskeleton (Hoboken) 74, 426–442 (2017).
Vangindertael, J. et al. Super-resolution mapping of glutamate receptors in C. elegans by confocal correlated PALM. Sci. Rep. 5, 13532 (2015).
Husson, S.J., Costa, W.S., Schmitt, C. & Gottschalk, A. Keeping track of worm trackers. WormBook https://doi.org/10.1895/wormbook.1.156.1 (2013).
McDiarmid, T. A., Yu, A. J. & Rankin, C. H. Beyond the response—high throughput behavioral analyses to link genome to phenome in Caenorhabditis elegans. Genes Brain Behav. 17, e12437 (2018).
Churgin, M. A. & Fang-Yen, C. An imaging system for C. elegans behavior. Methods Mol. Biol. 1327, 199–207 (2015).
Liu, Z., Tian, L., Liu, S. & Waller, L. Real-time brightfield, darkfield, and phase contrast imaging in a light-emitting diode array microscope. J. Biomed. Opt. 19, 106002 (2014).
Yu, C. C., Raizen, D. M. & Fang-Yen, C. Multi-well imaging of development and behavior in Caenorhabditis elegans. J. Neurosci. Methods 223, 35–39 (2014).
Alisch, T., Crall, J. D., Kao, A. B., Zucker, D. & de Bivort, B. L. MAPLE (modular automated platform for large-scale experiments), a robot for integrated organism-handling and phenotyping. Elife 7, e37166 (2018).
Leifer, A. M., Fang-Yen, C., Gershow, M., Alkema, M. J. & Samuel, A. D. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat. Methods 8, 147–152 (2011).
Stirman, J. N. et al. Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nat. Methods 8, 153–158 (2011).
Liu, M., Sharma, A. K., Shaevitz, J. W. & Leifer, A. M. Temporal processing and context dependency in Caenorhabditis elegans response to mechanosensation. Elife 7, e36419 (2018).
Porto, D. A., Giblin, J., Zhao, Y. & Lu, H. Reverse-correlation analysis of the mechanosensation circuit and behavior in C. elegans reveals temporal and spatial encoding. Sci. Rep. 9, 5182 (2019).
Grys, B. T. et al. Machine learning and computer vision approaches for phenotypic profiling. J. Cell Biol. 216, 65–71 (2017).
Wollmann, T., Erfle, H., Eils, R., Rohr, K. & Gunkel, M. Workflows for microscopy image analysis and cellular phenotyping. J. Biotechnol. 261, 70–75 (2017).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
McQuin, C. et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Wahlby, C. et al. An image analysis toolbox for high-throughput C. elegans assays. Nat. Methods 9, 714–716 (2012).
Jung, S. K., Aleman-Meza, B., Riepe, C. & Zhong, W. QuantWorm: a comprehensive software package for Caenorhabditis elegans phenotypic assays. PLoS One 9, e84830 (2014).
Labocha, M. K., Jung, S. K., Aleman-Meza, B., Liu, Z. & Zhong, W. WormGender—Open-source software for automatic Caenorhabditis elegans sex ratio measurement. PLoS One 10, e0139724 (2015).
Chen, L., Chan, L. L., Zhao, Z. & Yan, H. A novel cell nuclei segmentation method for 3D C. elegans embryonic time-lapse images. BMC Bioinformatics 14, 328 (2013).
Santella, A., Du, Z., Nowotschin, S., Hadjantonakis, A. K. & Bao, Z. A hybrid blob-slice model for accurate and efficient detection of fluorescence labeled nuclei in 3D. BMC Bioinformatics 11, 580 (2010).
Santella, A., Du, Z. & Bao, Z. A semi-local neighborhood-based framework for probabilistic cell lineage tracing. BMC Bioinformatics 15, 217 (2014).
Zichuan, L. et al. NucleiNet: A convolutional encoder-decoder network for bio-image denoising. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 1986–1989 (2017).
Long, F., Peng, H., Liu, X., Kim, S. K. & Myers, E. A 3D digital atlas of C. elegans and its application to single-cell analyses. Nat. Methods 6, 667–672 (2009).
Qu, L. et al. Simultaneous recognition and segmentation of cells: application in C. elegans. Bioinformatics 27, 2895–2902 (2011).
Toyoshima, Y. et al. Accurate automatic detection of densely distributed cell nuclei in 3D space. PLoS Comput. Biol. 12, e1004970 (2016).
Nguyen, J. P., Linder, A. N., Plummer, G. S., Shaevitz, J. W. & Leifer, A. M. Automatically tracking neurons in a moving and deforming brain. PLoS Comput. Biol. 13, e1005517 (2017).
San-Miguel, A. et al. Deep phenotyping unveils hidden traits and genetic relations in subtle mutants. Nat. Commun. 7, 12990 (2016).
Peng, H., Ruan, Z., Atasoy, D. & Sternson, S. Automatic reconstruction of 3D neuron structures using a graph-augmented deformable model. Bioinformatics 26, i38–46 (2010).
Moore, B. T., Jordan, J. M. & Baugh, L. R. WormSizer: high-throughput analysis of nematode size and shape. PLoS One 8, e57142 (2013).
Wang, M. F. Z. & Fernandez-Gonzalez, R. (Machine-)learning to analyze in vivo microscopy: support vector machines. Biochim. Biophys. Acta Proteins Proteom. 1865, 1719–1727 (2017).
White, A. G. et al. DevStaR: high-throughput quantification of C. elegans developmental stages. IEEE Trans. Med. Imaging 32, 1791–1803 (2013).
Zhan, M. et al. Automated processing of imaging data through multi-tiered classification of biological structures illustrated using Caenorhabditis elegans. PLoS Comput. Biol. 11, e1004194 (2015).
Entchev, E. V. et al. A gene-expression-based neural code for food abundance that modulates lifespan. Elife 4, e06259 (2015).
Hakim, A. et al. WorMachine: machine learning-based phenotypic analysis tool for worms. BMC Biol. 16, 8 (2018).
Zacharias, A. L. & Murray, J. I. Combinatorial decoding of the invariant C. elegans embryonic lineage in space and time. Genesis 54, 182–197 (2016).
Fire, A. A four-dimensional digital image archiving system for cell lineage tracing and retrospective embryology. Comput. Appl. Biosci. 10, 443–447 (1994).
Thomas, C., DeVries, P., Hardin, J. & White, J. Four-dimensional imaging: computer visualization of 3D movements in living specimens. Science 273, 603–607 (1996).
Sonnichsen, B. et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434, 462–469 (2005).
Gunsalus, K. C. et al. Predictive models of molecular machines involved in Caenorhabditis elegans early embryogenesis. Nature 436, 861–865 (2005).
Bao, Z. et al. Automated cell lineage tracing in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 103, 2707–2712 (2006).
Dzyubachyk, O., Jelier, R., Lehner, B., Niessen, W. & Meijering, E. Model-based approach for tracking embryogenesis in Caenorhabditis elegans fluorescence microscopy data. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 5356–5359 (2009).
Giurumescu, C. A. et al. Quantitative semi-automated analysis of morphogenesis with single-cell resolution in complex embryos. Development 139, 4271–4279 (2012).
Hunt-Newbury, R. et al. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 5, e237 (2007).
Murray, J. I. et al. Automated analysis of embryonic gene expression with cellular resolution in C. elegans. Nat. Methods 5, 703–709 (2008).
Murray, J. I. et al. Multidimensional regulation of gene expression in the C. elegans embryo. Genome Res. 22, 1282–1294 (2012).
Moore, J. L., Du, Z. & Bao, Z. Systematic quantification of developmental phenotypes at single-cell resolution during embryogenesis. Development 140, 3266–3274 (2013).
Boeck, M. E. et al. Specific roles for the GATA transcription factors end-1 and end-3 during C. elegans E-lineage development. Dev. Biol. 358, 345–355 (2011).
Du, Z. et al. The regulatory landscape of lineage differentiation in a metazoan embryo. Dev. Cell. 34, 592–607 (2015).
Du, Z., Santella, A., He, F., Tiongson, M. & Bao, Z. De novo inference of systems-level mechanistic models of development from live-imaging-based phenotype analysis. Cell 156, 359–372 (2014).
Ho, V. W. et al. Systems-level quantification of division timing reveals a common genetic architecture controlling asynchrony and fate asymmetry. Mol. Syst. Biol. 11, 814 (2015).
Krüger, A. V. et al. Comprehensive single cell-resolution analysis of the role of chromatin regulators in early C. elegans embryogenesis. Dev. Biol. 398, 153–162 (2015).
Keil, W., Kutscher, L. M., Shaham, S. & Siggia, E. D. Long-term high-resolution imaging of developing C. elegans larvae with microfluidics. Dev. Cell. 40, 202–214 (2017).
Crane, M. M., Chung, K. & Lu, H. Computer-enhanced high-throughput genetic screens of C. elegans in a microfluidic system. Lab. Chip 9, 38–40 (2009).
Uno, M. & Nishida, E. Lifespan-regulating genes in C. elegans. NPJ. Aging Mech. Dis. 2, 16010 (2016).
Sutphin, G. L. & Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. J/of Vis Exp. 12, e1152 (2009).
Mathew, M. D., Mathew, N. D. & Ebert, P. R. WormScan: a technique for high-throughput phenotypic analysis of Caenorhabditis elegans. PLoS One 7, e33483 (2012).
Stroustrup, N. et al. The Caenorhabditis elegans lifespan machine. Nat. Methods 10, 665–670 (2013).
Stroustrup, N. et al. The temporal scaling of Caenorhabditis elegans ageing. Nature 530, 103–107 (2016).
Rea, S. L., Wu, D., Cypser, J. R., Vaupel, J. W. & Johnson, T. E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 37, 894–898 (2005).
Zhang, W. B. et al. Extended twilight among isogenic C. elegans causes a disproportionate scaling between lifespan and health. Cell Syst. 3, 333–345.e334 (2016).
Kapahi, P., Kaeberlein, M. & Hansen, M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 39, 3–14 (2017).
Lucanic, M. et al. Chemical activation of a food deprivation signal extends lifespan. Aging Cell 15, 832–841 (2016).
Hullinger, R. & Puglielli, L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav. Brain Res. 322, 191–205 (2017).
Arey, R. N. & Murphy, C. T. Conserved regulators of cognitive aging: from worms to humans. Behav. Brain Res. 322, 299–310 (2017).
Bazopoulou, D., Chaudhury, A. R., Pantazis, A. & Chronis, N. An automated compound screening for anti-aging effects on the function of C. elegans sensory neurons. Sci. Rep. 7, 9403 (2017).
Markaki, M. & Tavernarakis, N. Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 5, 1261–1276 (2010).
Gosai, S. J. et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin α1-antitrypsin Z. PLoS One 5, e15460 (2010).
O’Reilly, L. P. et al. A genome-wide RNAi screen identifies potential drug targets in a C. elegans model of α1-antitrypsin deficiency. Hum. Mol. Genet. 23, 5123–5132 (2014).
Morley, J. F., Brignull, H. R., Weyers, J. J. & Morimoto, R. I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 99, 10417–10422 (2002).
Mondal, S. et al. Large-scale microfluidics providing high-resolution and high-throughput screening of Caenorhabditis elegans poly-glutamine aggregation model. Nat. Commun. 7, 13023 (2016).
Samara, C. et al. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc. Natl Acad. Sci. U S A 107, 18342–18347 (2010).
Mathew, M. D. et al. Using C. elegans forward and reverse genetics to identify new compounds with anthelmintic activity. PLoS Negl. Trop. Dis. 10, e0005058 (2016).
Partridge, F. A. et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug. Resist. 8, 8–21 (2018).
Partridge, F. A. et al. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity. in vivo. PLoS Negl. Trop. Dis. 11, e0005359 (2017).
Sykiotis, G. P. & Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 3, re3 (2010).
Leung, C. K., Deonarine, A., Strange, K. & Choe, K. P. High-throughput screening and biosensing with fluorescent C. elegans strains. J. Vis. Exp. 51, 2745 (2011).
Leung, C. K. et al. An ultra high-throughput, whole-animal screen for small molecule modulators of a specific genetic pathway in Caenorhabditis elegans. PLoS One 8, e62166 (2013).
Abraham, M. C., Lu, Y. & Shaham, S. A morphologically conserved nonapoptotic program promotes linker cell death in Caenorhabditis elegans. Dev. Cell 12, 73–86 (2007).
Schwendeman, A. R. & Shaham, S. A high-throughput small molecule screen for C. elegans .inker cell death inhibitors. PLoS One 11, e0164595 (2016).
Pierce-Shimomura, J. T., Morse, T. M. & Lockery, S. R. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 19, 9557–9569 (1999).
Cronin, C. J. et al. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 6, 5 (2005).
Yemini, E., Jucikas, T., Grundy, L. J., Brown, A. E. & Schafer, W. R. A database of Caenorhabditis elegans behavioral phenotypes. Nat. Methods 10, 877–879 (2013).
Chronis, N., Zimmer, M. & Bargmann, C. I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods 4, 727–731 (2007).
Feng, Z., Cronin, C. J., Wittig, J. H., Sternberg, P. W. & Schafer, W. R. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics 5, 115 (2004).
Swierczek, N. A., Giles, A. C., Rankin, C. H. & Kerr, R. A. High-throughput behavioral analysis in C. elegans. Nat. Methods 8, 592–598 (2011).
Itskovits, E., Levine, A., Cohen, E. & Zaslaver, A. A multi-animal tracker for studying complex behaviors. BMC Biol. 15, 29 (2017).
Restif, C. et al. CeleST: computer vision software for quantitative analysis of C. elegans swim behavior reveals novel features of locomotion. PLoS Comput. Biol. 10, e1003702 (2014).
Winter, P.B. et al. A network approach to discerning the identities of C. elegans in a free moving population. Sci. Rep. 6 (2016).
Perez-Escudero, A., Vicente-Page, J., Hinz, R. C., Arganda, S. & de Polavieja, G. G. idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743–748 (2014).
Perni, M. et al. Massively parallel C. elegans tracking provides multi-dimensional fingerprints for phenotypic discovery. J. Neurosci. Methods 306, 57–67 (2018).
Yu, H. et al. Systematic profiling of Caenorhabditis elegans locomotive behaviors reveals additional components in G-protein Gαq signaling. Proc. Natl Acad. Sci. USA 110, 11940–11945 (2013).
Ghosh, R., Mohammadi, A., Kruglyak, L. & Ryu, W. S. Multiparameter behavioral profiling reveals distinct thermal response regimes in Caenorhabditis elegans. BMC Biol 10, 85 (2012).
McGrath, P. T. et al. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron 61, 692–699 (2009).
Stephens, G. J., Johnson-Kerner, B., Bialek, W. & Ryu, W. S. Dimensionality and dynamics in the behavior of C. elegans. PLoS Comput. Biol. 4, e1000028 (2008).
Brown, A. E., Yemini, E. I., Grundy, L. J., Jucikas, T. & Schafer, W. R. A dictionary of behavioral motifs reveals clusters of genes affecting Caenorhabditis elegans locomotion. Proc. Natl Acad. Sci. USA 110, 791–796 (2013).
Sengupta, P. The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Curr. Opin. Neurobiol. 23, 68–75 (2013).
Calhoun, A. J. et al. Neural mechanisms for evaluating environmental variability in Caenorhabditis elegans. Neuron 86, 428–441 (2015).
Calhoun, A. J., Chalasani, S. H. & Sharpee, T. O. Maximally informative foraging by Caenorhabditis elegans. Elife 3, e04220 (2014).
Roberts, W. M. et al. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. Elife 5, e12572 (2016).
McCloskey, R. J., Fouad, A. D., Churgin, M. A. & Fang-Yen, C. Food responsiveness regulates episodic behavioral states in Caenorhabditis elegans. J. Neurophysiol. 117, 1911–1934 (2017).
Churgin, M. A., McCloskey, R. J., Peters, E. & Fang-Yen, C. Antagonistic serotonergic and octopaminergic neural circuits mediate food-dependent locomotory behavior in Caenorhabditis elegans. J. Neurosci. 37, 7811–7823 (2017).
Stern, S., Kirst, C. & Bargmann, C. I. Neuromodulatory control of long-term behavioral patterns and individuality across development. Cell 171, 1649–1662.e10 (2017).
Bargmann, C. I. Genetic and cellular analysis of behavior in C. elegans. Annu. Rev. Neurosci. 16, 47–71 (1993).
Gordus, A., Pokala, N., Levy, S., Flavell, S. W. & Bargmann, C. I. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161, 215–227 (2015).
Cho, Y., Zhao, C. L. & Lu, H. Trends in high-throughput and functional neuroimaging in Caenorhabditis elegans. Wiley Interdiscip. Rev. Syst. Biol. Med. 9, e1376 (2017).
Kato, S. et al. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669 (2015).
Venkatachalam, V. et al. Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E1082–1088 (2016).
Nguyen, J. P. et al. Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proc Natl Acad. Sci. U S A 113, E1074–1081 (2016).
Nichols, A. L. A., Eichler, T., Latham, R. & Zimmer, M. A global brain state underlies C. elegans sleep behavior. Science 356, eaam6851 (2017).
Eyer, K. et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat. Biotechnol. 35, 977–982 (2017).
Crall, J. D. et al. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683–686 (2018).
Allalou, A., Wu, Y., Ghannad-Rezaie, M., Eimon, P. M. & Yanik, M. F. Automated deep-phenotyping of the vertebrate brain. Elife 6, e23379 (2017).
Cheng, K. C., Xin, X., Clark, D. P. & La Riviere, P. Whole-animal imaging, gene function, and the Zebrafish phenome project. Curr. Opin. Genet. Dev. 21, 620–629 (2011).
Hur, M. et al. MicroCT-based phenomics in the zebrafish skeleton reveals virtues of deep phenotyping in a distributed organ system. Elife 6, e26014 (2017).
Acknowledgements
We thank K.E. Bates, D.A. Porto and T. Rouse for their suggestions on relevant literature, and the US National Institutes of Health (grants AG056436, DC015652, NS096581, GM088333, EB021676, EB020424 and GM10896) and National Science Foundation (grants 1707401 and 1764406) for funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, D.S., Xu, N. & Lu, H. Digging deeper: methodologies for high-content phenotyping in Caenorhabditis elegans. Lab Anim 48, 207–216 (2019). https://doi.org/10.1038/s41684-019-0326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-019-0326-6
- Springer Nature America, Inc.