Abstract
DNA methylation plays an important role in modulating plant growth plasticity in response to stress, but mechanisms involved in such control need further investigation. We used drm1 drm2 cmt3 mutant of Arabidopsis thaliana, defective in DNA methylation, to explore metabolic pathways downstream epigenetic modulation under cadmium (Cd) stress. To this aim, a transcriptomic analysis was performed on ddc and WT plants exposed to a long-lasting (21 d) Cd treatment (25/50 µM), focusing on hormone genetic pathways. Growth parameters and hormones amount were also estimated. Transcriptomic data and hormone quantification showed that, under prolonged Cd treatment, level and signalling of growth-sustaining hormones (auxins, CKs, GAs) were enhanced and/or maintained, while a decrease was detected for stress-related hormones (JA, ABA, SA), likely as a strategy to avoid the side effects of their long-lasting activation. Such picture was more effective in ddc than WT, already at 25 µM Cd, in line with its better growth performance. A tight relationship between methylation status and the modulation of hormone genetic pathways under Cd stress was assessed. We propose that the higher genome plasticity conferred to ddc by DNA hypomethylated status underlies its prompt response to modulate hormones genetic pathways and activity and assure a flexible growth.
Similar content being viewed by others
Introduction
Plants, as sessile organisms, are under constant influence of environment, which modulates their growth and development through a multiplicity of signals. Therefore, plants evolved molecular mechanisms to sense and rapidly adapt to the wide range of environmental changes occurring at diurnal, seasonal and stochastic level, thus exhibiting high growth plasticity1.
A role in such plasticity is paid by epigenetic mechanisms, including DNA methylation, which act on the chromatin status allowing a simultaneous and wide regulation of gene expression. Indeed, studies on both the model Arabidopsis thaliana and fruit crops demonstrated that methylome dynamic, beside playing a role at evolution level, is involved in the control of plant ontogenesis and modulate plant response to external cues, including multiple stresses2. In particular, either hypermethylation or hypomethylation were detected in plants under different stressors3. However, despite all this information, many aspects of the mechanisms that translate the information superimposed by DNA methylation into downstream regulation of gene expression remain still unclear.
A suitable tool to investigate these aspects is provided by Arabidopsis thaliana methylation-defective mutants, many of which exhibit phenotype pleiotropic alterations4,5. In plants, DNA cytosine methylation occurs in all sequence context and is driven by three enzyme families: the METHYLTRANSFERASES (MET), acting in maintaining methylation in the symmetric CG context, the DOMAINS REARRANGED METHYLTRANSFERASES (DRMs), acting as de novo methyltransferases in the asymmetric CHH context (H = A, C or T) and the plant specific CHROMOMETHYLASES (CMTs), acting primarily in the maintenance of CHG symmetric and CHH asymmetric methylation, but also playing a role in de novo DNA methylation5,6. DNA glycosylases contribute to the overall methylome pattern by removing methylated cytosines5. In this work, we used the triple Arabidopsis thaliana drm1 drm2 cmt3 (ddc) mutant, combining mutations on DRM1, DRM2, and CMT3 genes, defective in both maintenance and de novo DNA methylation7. The aim was to investigate molecular and cellular mechanisms that are modulated by DNA methylation in response to stressful factors, such as heavy metal presence.
Heavy metals are naturally present in soils and some of them are required at trace quantities as essential elements. However, at high levels they affect cell homeostasis and are harmful for all organisms8. In our study we selected Cd, one of the most harmful and not essential heavy metals, as a stressor due to its ubiquitous presence, long incubation period, ability to migrate and strong ecotoxicity8. Moreover, Cd in the soil is easily adsorbed by plants and at high concentration inhibits their growth and development by impacting on several metabolic processes through a wide range of structural and molecular changes, including epigenetic modifications9.
On this basis, Arabidopsis ddc mutant and wild type (WT) lines were exposed, from germination to 21 d after germination (DAG), to 25 and 50 µM Cd concentrations, whose growth inhibitory effect was previously documented in WT10. Plant growth parameters were monitored, and a transcriptome approach was applied to compare how genetic networks and related pathways were affected by Cd in ddc mutant compared to WT. The obtained results clearly showed that methylation status is involved in modulating plant response to Cd stress.
Results
Plant growth
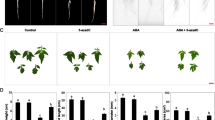
Primary root length and rosette size were estimated. Control root length, measured until 21 DAG, was lightly minor in ddc vs WT (Fig. 1A). Cd differentially inhibited root growth in the two samples: at 21 DAG, 25 and 50 µM Cd-treated roots were 1.2 and 2.2 fold shorter than control roots in ddc, while in the WT Cd-treated roots were 1.8 and 2.8 fold shorter than control ones (Fig. 1A). Consequently, at 21 DAG root of Cd-treated samples was longer in ddc vs WT, particularly at the lowest Cd concentration.
(A) Primary root length (B) Picture of rosette leaf series and (C) rosette leaf area (cm2) of WT and ddc plants of A. thaliana, germinated and grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl). Root length was monitored up to 21 days after germination (DAG) every two days from germination. The results represent the mean value (± SD) of three independent biological replicates (n = 45). Asterisks indicate significant pairwise differences using Student’s t-test (*P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001), performed between ddc vs WT subjected to the same treatment. Bars, 0.5 cm.
Rosette size was estimated at 21 DAG, corresponding to the period necessary for its full development11, by evaluating leaf number and area. Control plants of both ddc and WT exhibited a complete leaf series, although most leaves resulted smaller in ddc (Fig. 1B,C). Cd affected rosette development reducing leaf number and area, less in ddc than WT, resulting into a higher leaf area and/or number in ddc under both Cd concentrations (Fig. 1B,C).
Gene expression profile
RNA-Seq analysis provided an overview of gene expression profile of Cd-treated and control plants of both ddc and WT. The following comparisons were performed: ddc vs WT under control (Ctrl) conditions (ddc vs WT-Ctrl) and 25 and 50 µM Cd treatment (ddc vs WT-25 µM Cd; ddc vs WT-50 µM Cd); 25/50 µM Cd-treated vs Ctrl in ddc (25 µM Cd vs Ctrl-ddc; 50 µM Cd vs Ctrl-ddc); 25/50 µM Cd-treated vs Ctrl in the WT (25 µM Cd vs Ctrl-WT; 50 µM Cd vs Ctrl-WT).
After DEGs identification (see Supplementary Fig. S1 online) 14 of them were analysed through qRT-PCR to validate transcriptomic analysis (see Supplementary Fig. S2 online). Results were fully consistent with RNA-seq data. Gene Enrichment analysis was also performed, evidencing that Cd strongly impacted on transcriptome in both ddc and WT, but in a largely different way (see Supplementary Figs. S3–S9 online). Notwithstanding, a common aspect was that in both ddc and WT the genetic pathways (GPs) more impacted by Cd dealt with photosynthesis, stress responses and hormone biosynthesis and signalling.
Expression pattern of genetic pathways related to hormones
In view of hormones pivotal role in plant development and stress response and considering the assessed epigenetic control on their action and signalling12, in this work we analysed in depth how the expression pattern (EP) of hormone-related GPs was modulated in ddc vs WT under Cd stress. The most relevant differences are discussed.
Auxins
Under control conditions, GPs related to auxin biosynthesis showed comparable EP in ddc and WT and no DEGs were detected (Fig. 2A). 25 µM Cd induced significant changes only in ddc resulting into: i) TAA1 and YUC5 downregulation along indole-3-pyruvic acid (IPA) pathway; ii) CYP71A13 and NIT2 overexpression along indole-3-acetaldoxime (IAOX) auxiliary pathway, while CYP79B3 was downexpressed (Fig. 2A). Differently, 50 µM Cd induced similar changes in ddc and WT consisting in: (i) a downexpression of YUC2 along IPA pathway in both samples and YUC5 and YUC9 in ddc and WT, respectively; (ii) overexpression of CYP71A12, CYP71A13, NIT2, NIT4 and downexpression of CYP71A16 along IAOX pathway in ddc and WT (Fig. 2A).
Genes differentially expressed (DEGs) along the pathway of (A) auxin biosynthesis, auxin conjugation, (B) indole-3-acetyl-amino acid biosynthesis, (C) methyl-indole-3-acetate interconversion and (D) auxin signalling in ddc and WT plants identified through a transcriptomic approach. For each comparison, the log2(fold change) of the analysed DEGs was shown in orange and in blue for the upregulated and downregulated genes, respectively. Plants were grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl).
Auxin level and homeostasis also depend on its oxidative degradation, conjugation and methylation13. Under control conditions, GPs related to auxin conjugation and methylation showed comparable EPs in ddc and WT and no DEGs were detected (Fig. 2B,C), but were differentially impacted by Cd, mainly at 25 µM concentration. Namely, at 25 µM Cd several genes related to auxin conjugation (GH3.3, GH3.17, YDK1) and methylation (MES7, MES17) were downregulated in ddc, whereas in WT only MES18, involved in methyl-indole-3-acetate production, was downregulated (Fig. 2B,C). At 25 µM Cd most of the above genes were downregulated in ddc vs WT (Fig. 2B,C), while at 50 µM Cd only two genes, working in auxin methylation were differentially modulated in ddc and WT (Fig. 2B,C).
Under control conditions, GP related to auxin signalling exhibited similar EP in ddc and WT (Fig. 2D). Differences were induced by Cd. Namely, at 25 µM Cd, AUX/IAA family genes, which acts in signalling repression, were globally downregulated in ddc, while in WT the repressor IAA34 was overexpressed (Fig. 2D). Differently, 50 µM Cd effects on ddc and WT were quite similar, dealing with AUX/IAA family genes downregulation (Fig. 2D). In ddc vs WT comparisons, at 25 µM Cd above genes were downregulated, while no differences were found at 50 µM Cd (Fig. 2D). Moreover, and somehow unexpectedly, following 50 µM Cd it was observed in both ddc and WT a downregulation of several SAURs members, belonging to a large family of auxin responsive genes, which in turn can also have an impact on auxin pathway14 (Fig. 2D). Interestingly, such effect was more pronounced in ddc than WT. However, it must be mentioned that, although most of them are induced by auxin, several other hormones and co-factors acts upstream SAUR genes, regulating their activity in response to both endogenous stimuli and environmental cues14.
In summary, in both ddc and WT, Cd induced: i) a downregulation of IPA pathway, which is the main auxin biosynthetic pathway15 and a simultaneous upregulation of IAOX auxiliary biosynthetic pathway; ii) an enhancement of hormone signalling. However, in the WT such effects occurred only at 50 μM Cd. Moreover, in ddc Cd also induced a downregulation of GPs related to auxin conjugation.
Cytokinins
In all comparisons, GPs related to CKs biosynthesis showed similar EPs, unless for the downregulation in 50 µM Cd-treated WT vs Ctrl of IPT5, encoding rate-limiting enzyme along the pathway16 (Fig. 3A).
Genes differentially expressed (DEGs) along the pathway of (A) trans-zeatin biosynthesis, (B) CKs degradation, (C) CKs N7- and N9-glucoside biosynthesis, (D) CKs O-glycosylation and (E) CKs signalling in ddc and WT plants identified through a transcriptomic approach. For each comparison, the log2(fold change) of the analysed DEGs was shown in orange and in blue for the upregulated and downregulated genes, respectively. Plants were grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl).
Major differences were observed for GPs related to CKs catabolism and conjugation, occurring through cleavage by oxidation and glycosylation, respectively16. Under control conditions, these GPs also exhibited similar EPs in ddc and WT (Fig. 3B-D). At both 25 and 50 µM Cd a downregulation of CKX5 and CKX6, encoding cytokinin-oxidases, occurred only in ddc (Fig. 3B). Differently, Cd impact on GPs related to CKs N-glycosylation was almost comparable in ddc and WT, resulting into the overexpression of two different genes working in N7- and N9-glycosylation pathways at 25 µM Cd, and one gene at the higher concentration (Fig. 3C).
Note that at 25 µM Cd the above genes were both overexpressed in ddc vs WT, while no differences were found at 50 µM Cd (Fig. 3C). Cd impact on GP related to cytokinin O-glycosylation was major, especially in ddc, involving at 25 µM Cd the overexpression of seven genes along this pathway compared to Ctrl (Fig. 3D), and one gene in WT 25 µM Cd vs Ctrl (Fig. 3D). By contrast, at 50 μM Cd the expression pattern along this pathway was similar in ddc and WT, being characterised by the upregulation of the same seven genes above mentioned and the downregulation of AT5G38010 (Fig. 3D). Finally, at 25 μM Cd, five genes along these pathways resulted upregulated in ddc vs WT while a similar EP occurred in ddc and WT under 50 μM Cd treatment (Fig. 3D).
Concerning GP involved in CKs signalling, under control conditions A-ARRs, encoding negative regulators of CKs signalling17, were downregulated and signalling was likely enhanced in ddc vs WT (Fig. 3E). 25 µM Cd induced a downregulation of ARR11 A-type ARRs and B-ARR family ARR10 transcription factors, which control primary plant response to CKs, only in ddc (Fig. 3E). Whereas, at 50 µM Cd both ddc and WT showed A-ARR downregulation, supposedly leading to pathway upregulation (Fig. 3E). At 25 µM Cd AHP1, encoding positive regulators of CKs signalling18, was downregulated in ddc vs WT, while ARR17 was overexpressed, suggesting that signalling was downregulated also in ddc vs WT (Fig. 3E). No differences occurred between ddc and WT at 50 µM Cd (Fig. 3E).
In summary, transcriptomic analysis evidenced that GP related to the biosynthesis of trans-zeatin, the most relevant CK, was negatively affected by Cd only in the WT at 50 μM Cd. In response to Cd, GPs related to CKs inactivation were enhanced in both ddc and WT, but in ddc a downregulation of GP related to CKs cleavage also occurred. Finally, hormone signalling was differentially modulated by Cd in relation to both the sample (ddc vs WT) and heavy metal concentration, resulting into a downregulation at 25 µM Cd only in ddc and an enhancement in ddc and WT at 50 µM Cd.
Gibberellins
Under control conditions, GPs related GAs biosynthesis showed similar EPs in ddc vs WT (Fig. 4A). 25 μM Cd induced in ddc: i) a downregulation of GA2 encoding the ent-kaurene synthase, a pivotal enzyme along the early GAs biosynthetic pathways to synthetize GA12; ii) a downregulation of GA4, a key gene of GAs biosynthesis, along which bioactive GAs are synthetized19 (Fig. 4A). No Cd-induced modulation was observed in the WT (Fig. 4A). On the contrary, at 50 μM Cd both ddc and WT showed a downregulation of GA5 (Fig. 4A). Finally, in ddc vs WT the only difference dealt with GA2 downregulation at 25 µM Cd (Fig. 4A).
Genes differentially expressed (DEGs) along the pathway of (A) GAs biosynthesis, (B) GAs inactivation and (C) GAs signalling in ddc and WT plants identified through a transcriptomic approach. For each comparison, the log2(fold change) of the analysed DEGs was shown in orange and in blue for the upregulated and downregulated genes, respectively. Plants were grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl).
GPs controlling GAs inactivation also showed a comparable transcriptional pattern in ddc and WT under control conditions, and no DEGs were detected (Fig. 4B). 25 μM Cd induced a downregulation of DAO2 and AOP1, encoding GA2ox enzymes, only in ddc (Fig. 4B). Accordingly, in ddc vs WT these genes were downregulated only at the lowest Cd concentration (Fig. 4B).
Under control conditions, also GP related to GAs signalling was not differentially modulated in ddc vs WT (Fig. 4C). A downregulation of genes encoding DELLA proteins, which act as repressors20, was induced only in ddc by 25 µM Cd (Fig. 4C) and in both ddc and WT at 50 µM Cd (Fig. 4C), suggesting an enhancement of hormone signalling. Consequently, DELLA-codifying genes resulted downregulated also in ddc vs WT only at the lowest Cd concentration (Fig. 4C).
In summary, in ddc the lowest Cd treatment negatively affected GPs related to GAs biosynthesis but, at the same time, hormone signalling resulted enhanced. In the WT similar effects were observed only at 50 µM Cd.
Jasmonic acid
Under control conditions, six genes along the GP related to JA biosynthesis were downregulated in ddc vs WT (Fig. 5A). 25 µM Cd induced in ddc a downregulation of this GP, except for LOX4 upregulation (Fig. 5A) and a downregulation involving eight genes in WT (Fig. 5A). At 50 μM, Cd effects were limited to LOX5 downregulation and LOX4 and OPR1 upregulation in ddc and LOX5 and AOS downregulation in WT (Fig. 5A). No Cd–induced differences were found in ddc vs WT (Fig. 5A).
Genes differentially expressed (DEGs) along the pathway of (A) JA biosynthesis, (B) JA signalling, (C) ABA biosynthesis, (D) ABA degradation, (E) ABA glucose ester biosynthesis and (F) ABA signalling in ddc and WT plants identified through a transcriptomic approach. For each comparison, the log2(fold change) of the analysed DEGs was shown in orange and in blue for the upregulated and downregulated genes, respectively. Plants were grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl).
Concerning the JA signalling-related GP, in control conditions JAZ5 gene, encoding a protein acting as repressor21, was downregulated in ddc vs WT, highlighting a signalling enhancement (Fig. 5B). Interestingly, JAZ10 and JAZ9 were differentially impacted by 25 µM Cd in ddc and WT resulting upregulated and downregulated, respectively. (Fig. 5B). At 50 µM Cd, JAZs were overexpressed in both ddc and WT (Fig. 5B). No differences were detected in ddc vs WT exposed to Cd (Fig. 5B).
Globally, Cd negatively impacted on the GP related to JA biosynthesis especially in WT. Under Cd treatment hormone signalling was downregulated more in ddc than in WT, whatever concentration was applied.
Abscisic acid
Under control conditions, ABA biosynthesis-related GP showed comparable EP in ddc and WT, and no DEGs were detected (Fig. 5C). The only significant Cd effect dealt with NCED3 downregulation both in ddc and WT, regardless of applied concentration (Fig. 5C). Under control conditions, also the GPs related to ABA catabolism showed a comparable EP in ddc and WT and no DEGs were detected (Fig. 5D), but Cd differentially impacted on CYP genes, involved in phaseic acid degradative production22. Namely, at 25 μM Cd, CYP707A3 was downregulated only in ddc (Fig. 5D). Moreover, also CYP707A2 appeared downregulated in ddc vs WT (Fig. 5D). At 50 µM Cd it was observed an upregulation of both CYP707A2 and CYP707A4 in ddc, and of only CYP707A4 in WT (Fig. 5D).
Under control conditions, GP related to ABA inactivation through glucose conjugation showed similar EP in ddc vs WT (Fig. 5E). 25 μM Cd determined AT4G15260 upregulation and UGT71C3 downregulation only in ddc (Fig. 5E). At 50 μM, Cd equally impacted on ddc and WT, resulting into UGT71C1 and UGT2 downregulation, AT5G49690, UGT71B5, UGT71B6 upregulation and, limited to WT, AT4G15260 upregulation (Fig. 5E). No differences were highlighted in ddc vs WT exposed to Cd (Fig. 5E).
Under control conditions, the GP related to hormone signalling also presented a comparable EP in ddc and WT (Fig. 5F). In ddc, 25 µM Cd impact on this GP appeared rather complex, resulting in an upregulation of PYL3, encoding ABA receptor, and a downregulation of PP2Cs (PP2CA and HAI1) encoding negative regulators of ABA signalling23. Moreover, ABI5, codifying a key transcription factor in ABA signalling24 belonging to AREBs/ABFs family, was upregulated. However, SnRK2.7 gene, codifying a protein which activate the AREBs/ABFs transcription factors24, was downregulated. Based on the prominent role of SnRK2s in plant response to ABA, it is likely that at 25 µM Cd ABA signalling was downregulated in ddc (Fig. 5F). Instead, 25 µM Cd determined in WT the upregulation of PYL6 and the downregulation of PP2Cs, suggesting an enhancement of ABA signalling (Fig. 5F). At 50 µM Cd, PYL6 was upregulated and SnRK2.7 downregulated in both ddc and WT, while HAI1 was downregulated only in ddc (Fig. 5F). When comparing ddc vs WT, at 25 µM Cd only SnRK2.7 was downregulated, while no differences occurred at 50 μM Cd (Fig. 5F).
In summary, Cd determined a slight downregulation of GP related to ABA biosynthesis in both ddc and WT regardless of its concentration. ABA catabolic pathway was lightly downregulated in ddc at 25 µM Cd but upregulated in both samples at 50 μM Cd. At the transcriptomic level, ABA signalling featured as enhanced in WT and downregulated in ddc regardless of Cd concentration.
Ethylene
Along GP related to ethylene biosynthesis, under control conditions ACS8 and ACS11 were upregulated in ddc vs WT (Fig. 6A). 25 µM Cd determined ACS8 and ACO5 downregulation in ddc and ACS4 upregulation in the WT (Fig. 6A). 50 μM Cd induced ACS7 upregulation and ACO5 downregulation in both ddc and WT and ACS2 and ACS11 overexpression only in WT (Fig. 6A). Finally, the only Cd-induced difference in ddc vs WT dealt with ACO1 downregulation at 25 µM Cd (Fig. 6A).
Genes differentially expressed (DEGs) along the pathway of (A) ethylene biosynthesis, (B) ethylene signalling and (C) SA signalling in ddc and WT plants identified through a transcriptomic approach. For each comparison, the log2(fold change) of the analysed DEGs was shown in orange and in blue for the upregulated and downregulated genes, respectively. Plants were grown for 21 DAG in long day condition: (i) on growth medium added with 25 or 50 µM Cd; (ii) on growth medium without Cd as control (Ctrl).
GP related to ethylene signalling showed similar EP in ddc and WT both under control conditions (Fig. 6B) and at 25 µM Cd, except for the upregulation of ETR2, encoding ethylene receptor25 in WT (Fig. 6B). At 50 µM Cd, both ddc and WT exhibited ETR2 and ERF1 overexpression suggesting an upregulation of ethylene signalling (Fig. 6B); no differences occurred in ddc vs WT (Fig. 6B).
In summary, in control conditions GP related to ethylene biosynthesis was upregulated in ddc vs WT. Cd determined a downregulation and upregulation of this GP in ddc and WT, respectively. Concerning hormone signalling, at the highest Cd concentration in both ddc and WT an upregulation of this GP occurred.
Salicylic acid
Regarding SA, only the GP related to signalling resulted differentially expressed. Under control conditions, the GP related to SA signalling showed similar EP in ddc and WT. However, PR1, a useful molecular marker for the systemic acquired resistance (SAR) in response to pathogens26, was downregulated in ddc vs WT (Fig. 6C). 25 μM Cd induced a downregulation of genes codifying TGA10 transcription factor only in the ddc (Fig. 6C). Whereas, 50 μM Cd induced a downregulation of PRB1 in both ddc and WT and of TGA8 only in ddc (Fig. 6C). In ddc vs WT, differences were found only at 25 µM Cd, with the downregulation of TGA10 and CAPE3 (Fig. 6C).
Altogether, these results evidenced a Cd-induced downregulation of this GP, likely resulting in an impairment of hormone signalling in both WT and ddc, but in the latter this effect already occurred at the lowest Cd concentration.
Phytohormone level
Based on the major effects induced by 25 µM Cd treatment, hormone quantification was carried out on plants exposed to this concentration, compared to untreated control plants.
Under control conditions, IAA amount was higher in ddc than WT, although not significantly. After Cd treatment, a decreasing trend was observed only in WT, resulting into a significant lower level as compared to ddc (Fig. 7A).
(A) IAA, (B–G) CKs, (H–R) GAs, (S) JA, (T) ABA, (U) SA and (V) SAG amount in A. thaliana ddc mutant and WT plants grown in Ctrl conditions and treated with 25 µM Cd estimated by GC–MS. The results represent the mean value (± SD) of three independent biological replicates. Statistical analysis was performed by using two-way ANOVA with Tukey post hoc test (P ≤ 0.05) after Shapiro–Wilk normality test. Means with the same letter are not significantly different at P ≤ 0.05.
Concerning CKs, both biological active (tZ) and inactive conjugate (tZR, cZR, tZOG, cZOG, iPR) forms were analysed (Fig. 7B–G). Under control conditions, all analysed CKs were present in ddc, but CKs conjugate forms and above all O-glycosylated exhibited the highest levels (Fig. 7B–G). By contrast, in WT tZ was not detectable and all the other CKs forms exhibited a lower level compared to the mutant, which appeared significant for tZR and cZOG (Fig. 7B–G). Following Cd treatment, CKs levels increased in ddc, except for tZ decrease. In WT, the unique Cd effect dealt with tZR increase and tZOG decrease. Consequently, under Cd treatment the level of all CKs forms remained higher (from 0.25 to 3 times) in ddc than in WT (Fig. 7B–G).
Concerning GAs, precursors (GA9, GA19, GA20), biologically active forms (GA1, GA3, GA4, GA7) and catabolites (GA8, GA34, GA29, GA51) were analysed (Fig. 7H–R). Under control conditions, GA19 amount was significantly higher in ddc vs WT, while the amount of GA20, the other in serie precursor of hydroxylated forms, was comparable between the samples (Fig. 7H,I). Following Cd treatment, GA19 amount significantly increased in WT while a slight downtrend occurred in ddc, leading to comparable values in the two samples. The same trend was observed, but at less extent, for GA20 (Fig. 7H,I). Consistently, under control conditions also the amount of the active hydroxylated forms GA1 and GA3 was higher in WT than in ddc (Fig. 7J,K). Following Cd treatment, a decrease of their amount was detected only in WT, globally leading to a higher level of these GAs in ddc mutant compared to the WT (Fig. 7J,K). In addition, in ddc mutant also the related catabolites GA8 and GA29 were globally lower than in WT, under both control conditions and Cd treatment (Fig. 7L,M).
Differences were observed also for GA9, precursor of non-hydroxylated GAs: under Cd treatment its amount decreased in the WT and was instead induced in ddc mutant, resulting in a quite comparable value between the two samples (Fig. 7N). Consistently, the amount of active non-hydroxylated forms, GA4 and GA7, increased under Cd treatment only in ddc mutant; also in this case, at the end of heavy metal treatment, comparable values were detected in ddc and WT (Fig. 7O,P) In agreement with these results, following Cd treatment, the amount of catabolites GA51 and GA34 did not change in the WT, whereas in ddc it increased and decreased, respectively (Fig. 7Q,R).
As evident in Fig. 7S–V, differences were reported also for JA, ABA, SA and its predominant inactive conjugate, SA 2-O-β-D-glucoside (SAG). Under control conditions both JA and ABA amount was significantly lower in ddc vs WT and significantly decreased following Cd treatment only in the WT (Fig. 7S,T). Notwithstanding, under such condition the ABA amount remained lower in ddc than in WT while JA values were comparable in the two samples due its light, but not significant, increase in ddc (Fig. 7S,T). By contrast, under control conditions both SA and SAG amounts were significantly higher in ddc than in WT (Fig. 7U,V). Following Cd treatment, their amounts significantly decreased more in ddc than in WT, leading to an opposite condition (Fig. 7U,V).
Testing of the involvement of SUPPRESSOR OF DRM1 DRM2 CMT3 (SDC) gene in ddc response to Cd
Finally, we planned to inquire on the possible involvement of SDC gene in the response of ddc triple mutant to Cd exposure. Indeed, it has been reported that in ddc mutant the misexpression of such gene, which encodes a F-Box protein, is ultimately responsible of the developmental phenotypes of ddc, such as curled leaves and reduced growth, as evidenced by its reversion in the drm1 drm2 cmt3 sdc quadruple mutant27. Note that in the WT SDC is silenced, being methylated in all its sequence contexts because of the redundant action of DRM2 and CMT3 enzymes. By contrast, in ddc, where DRM2 and CMT3 expression is silenced, the loss of non-CG methylation in the promoter region of SDC F-box gene determines its overexpression27.
According to the above mentioned data27, we firstly verified that under control conditions SDC resulted silent in the WT and overexpressed in ddc also in our transcriptomic analysis (data not shown, complete raw transcriptomic data are available at NCBI SRA under the BioProject accessionPRJNA641242;https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA641242). Moreover, our data also showed that at the transcriptomic level SDC is not modulated by Cd since its expression level did not significantly change in ddc nor in WT whatever heavy metal concentration was applied.
Thereafter, we tested the involvement of SDC in the growth response of ddc mutant under Cd exposure, by monitoring primary root length of Arabidopsis thaliana WT, ddc and sdc plants grown under the following conditions: (i) on growth medium without Cd as control (Ctrl) (ii) on a medium supplemented with 25/50 µM Cd; (iii) limited to the WT and sdc mutant plants, on a medium supplemented with 25/50 µM Cd plus 15 µM 5-Azacytidine (5-Aza), an inhibitor of DNA methylation applied in order to mimic the hypomethylated state of ddc mutant.
Under control conditions, all three samples showed a similar root length. However, at 21 DAG, root was lightly shorter in ddc vs WT, while sdc displayed an intermediate length (Fig. 8A). Under both Cd treatments, roots were averagely longer in ddc than in WT. Again, sdc roots exhibited an intermediate length, more similar to WT than ddc (Fig. 8 B,C). Interestingly, WT and sdc plants treated with Cd plus 5-Aza had longer roots than the plants treated only with Cd, and quite comparable to ddc roots exposed to Cd (Fig. 8 D,E).
Primary root length of WT, sdc and ddc plants of A. thaliana, germinated and grown in long day condition (A) on growth medium without Cd as control (Ctrl), (B,C) on a medium supplemented with 25/50 µM Cd, (D,E) limited to WT and sdc plants, on a medium supplemented with 25/50 µM Cd plus 15 µM 5-Azacytidine (5-Aza). Root length was monitored up to 21 days after germination (DAG) every two days from germination. The results represent the mean value (± SD) of three independent biological replicates (n = 45). Statistical analysis was performed between samples at the same growth stage, by using two-way ANOVA with Tukey post hoc test (P ≤ 0.05) after Shapiro–Wilk normality test. Means with the same letter are not significantly different at P ≤ 0.05.
Discussion
This study showed that, under a long-lasting Cd treatment, A. thaliana ddc mutant exhibited a better growth performance than WT plants especially at 25 µM Cd concentration, tightly related to a differential modulation of GPs highly relevant for plant growth.
Attention was mainly focused on GPs related to hormones, key molecules in the control of plant growth and stress response, whose action is regulated by interconnected epigenetic mechanisms12,28. Accordingly, all hormone-related GPs were differentially modulated in ddc vs WT under control conditions but mainly under Cd stress, although at different extent for each hormone class. That is consistent with literature data showing that many plant hormones and other signal molecules are involved in Cd sensing and downstream plant response10,29. However, the general picture is somehow controversial differing in relation to species, plant organ and age, concentration and duration of metal exposure30.
In our work, for all hormone classes the major differences between ddc and WT occurred at the lowest Cd concentration (25 μM Cd). In particular, for auxin Cd impact was prominent on hormone conjugation rather than biosynthesis. Namely, the two GPs related to auxin biosynthesis were oppositely affected by Cd in both ddc and WT, likely as a compensatory mechanism to maintain hormone level. However, at 25 μM Cd IAA amount, which is the most relevant auxin, was higher in ddc than WT, evidencing that maintenance of bioactive auxin level was more efficient in the mutant. The downregulation of genes involved in auxin conjugation detected in ddc under such treatment provides a suitable explanation for this behaviour.
Transcriptomic data also suggested an enhancement of auxin signalling in ddc vs WT at 25 μM Cd. Notably, in Arabidopsis a Cd-induced simultaneous decrease of IAA content and increase of IAA oxidase activity resulted into a downregulation of numerous auxin-responsive and growth-related genes31. Moreover, under Cd exposure high level of IAA were found to prevent growth inhibition and increase heavy metal tolerance32. Therefore, although at post-transcriptional level a different regulation can occur, the better growth performance of ddc under 25 μM Cd could be warranted by transcriptomic changes addressed to prevent hormone inactivation and enhance its signalling. Interestingly, in our previous work an organ-specific and opposite alterations of auxin translocation and homeostasis was detected in ddc root and leaf as compared to WT, tightly related to mutant phenotypic alterations33. Furthermore, such hormone pathway alterations were associated to organ-specific changes in the expression level of auxin-related genes, which in some cases were in turn associated to gene-specific defective methylation level33. Altogether, these results highlight that a relationship between methylation status and auxin metabolism and translocation play a relevant role in modulating growth and development of ddc under both control conditions and Cd stress.
CKs also resulted differentially impacted by Cd in ddc vs WT both at transcriptomic and biochemical level. As for all hormones, CKs homeostasis relies on the balance between biosynthesis, catabolism and/or inactivation34. Transcriptomic major differences dealt with GPs related to CKs catabolism and inactivation rather than biosynthesis, at least at the lowest Cd concentration. Literature data showed an increase of CKs catabolic oxidation in Triticum durum exposed to 0.04 mM Cd35. A more complex picture was detected in our samples under a long-lasting treatment at a lower concentration (25 μM Cd). Namely, a Cd-induced downregulation of cytokinin-oxidase genes was observed only in ddc. Simultaneously, GPs related to CKs inactivation through N-glycosylation were upregulated in both ddc and WT, while O-glycosylation was upregulated only in ddc. Accordingly, hormone quantification showed that CKs O-glycosylated forms increased in Cd-treated ddc and were more abundant than in WT. Notably, while N-glycosylation causes irreversible CKs inactivation, O-glycosylated CKs represent the hormone fraction available for storage and transport, that can be reconverted in active CKs by β-glucosidases16. Therefore, the genetic modulation implemented by ddc under 25 µM Cd concentration was addressed to preserve CKs pool. That is consistent with the high CKs level detected in ddc vs WT at 25 μM Cd, despite the GP related to hormone biosynthesis exhibited a comparable EP. Incidentally, CKs are essential for counteracting leaf senescence, protecting photosystems and enhancing photosynthesis36. Accordingly, under Cd treatment leaf number and area was higher in ddc vs WT, evidencing that leaf growth was less impaired in ddc. Therefore, the genetic modulation underway in ddc could represent an important strategy to assure resistance to Cd stress.
On the other hand, also GAs play a relevant role in leaf development37. A downregulation of pathways related to the biosynthesis of active GAs occurred in ddc at 25 μM Cd. Likely as a compensatory defence mechanism, a strong downregulation of the GPs related to GAs inactivation and an enhancement of hormone signalling also occurred in ddc at the lowest Cd concentration.
A rather complex picture emerged for JA, SA and ABA, which represent the most relevant hormones for the perception and downstream response of plants to stresses, including heavy metals38. Namely, under control conditions, at transcriptomic level JA biosynthesis was downregulated and a lower hormone amount was detected in ddc vs WT. Whereas, for SA and ABA a post-transcriptional regulation can be envisaged, as already demonstrated for ABA39, since GPs related to their biosynthesis showed similar EP in ddc vs WT, despite of significantly higher and lower hormone level detected in ddc, respectively.
At 25 μM Cd, a downregulation of biosynthesis-related GPs and hormone level, was observed for all three hormones. However, for SA and ABA this effect was more pronounced in ddc than WT, while JA was more affected in WT than ddc. likely explaining the comparable JA level in Cd-treated ddc and WT. 25 μM Cd also induced a downregulation of GP related to ABA degradation only in ddc vs WT, probably as a compensatory mechanism for maintaining adequate hormone level. An opposite effect was observed for GP related to ABA signalling, which appeared upregulated in the WT and downregulated in ddc whatever concentration was used.
This scenario was unexpected, since it is largely documented that level and activity of these stress-related hormones, mainly of ABA, usually increase following abiotic stress, including heavy metal40. However, the exact mechanisms of hormone action and their crosstalk with the whole signalling network of plant under stress are yet to be fully clarified. In addition, hormone dynamic under stress largely depends on the species, the plant organ and growth stage, the stress intensity and duration40. For example, Cd treatment was found to induce a differential JA accumulation in several plant species, including A. thaliana which exhibited a biphasic model, with an early hormone accumulation, followed by cyclic decreases and increases41. Notably, low JA concentrations act as protectant against Cd stress, while higher concentrations induce toxic effects, such as ROS accumulation, root growth inhibition and lipid peroxidation42. Similar toxicity is displayed by high levels of SA, which has been proposed as the ‘life or death switch’ of cells43. Moreover, negative effects on plant growth can be exerted also by high levels of ABA, which acts as an antagonist of GAs action39. Therefore, it is likely that under the long-lasting Cd treatment that we applied, plant activity was directed to avoid toxic effects related to a prolonged activation of all these three hormone classes, by decreasing their level and/or downregulating their signalling. Interestingly, in ddc which exhibited the best growth performance, these adaptive responses were more pronounced.
In summary, our results clearly showed that, under a prolonged metal exposure and within a specific threshold concentration (i.e. 25 μM Cd), a differential transcriptional modulation of hormone pathways is a key mechanism for the capacity of A. thaliana ddc mutant, defective in methylation, to better counteract Cd toxicity compared to WT. Moreover, ddc prompt response at the lowest Cd concentration (i.e. 25 μM Cd) also evidenced that such aptitude was associated to its greater capacity to sense heavy metal stress and put in place an early modulation of gene expression. We propose that this behaviour is related to the higher genome plasticity conferred to ddc by DNA hypomethylated status.
In this context it is relevant to recall that the overexpression of the SDC F-box gene in ddc, due to the loss of non-CG methylation in its promoter region, is reported to be ultimately responsible of the developmental phenotypes of this mutant, such as curled leaves and reduced growth27.
In line with this evidence, also our transcriptomic analysis evidenced that, under control conditions, SDC is silent in the WT and overexpressed in ddc. Furthermore, since SDC expression level did not significantly change in ddc nor in WT whatever Cd concentration was applied, our data also evidenced that, at the transcriptomic level, SDC expression is not modulated by Cd. Moreover and very interestingly, the analysis of root growth allowed us to verify that under Cd treatment the roots of WT and sdc plants where shorter than ddc ones whereas, following treatment with both Cd and the DNA methylation inhibitor 5-Aza, both displayed longer roots, quite comparable to ddc ones. Thus, under Cd exposure a root length comparable to ddc was reported only in 5-Aza-treated plants mimicking ddc global DNA hypomethylation status, independently by the activation or the silencing of SDC which occurs in ddc and sdc, respectively. These results pointed out two important evidences: (i) a global DNA hypomethylation is certainly involved in a better plant response to Cd stress; (ii) such response is independent by SDC gene.
Therefore, it appears that SDC activity is not directly related to the response to Cd. Future analysis of methylome status of ddc and WT under Cd treatment could give further insight into the different and interacting loci with a direct and or indirect role in the transduction pathways and response mechanisms to Cd stress of ddc mutant.
At present, on the basis of our results, the following scheme is proposed to link transcriptomic and hormonal differences to the major Cd tolerance exhibited by ddc (Fig. 9). The emerging picture is that plant activity is directed to enhance and/or maintain the level and signalling of hormones which are relevant in sustaining the growth, such as auxins, CKs and GAs more than those of hormones specifically related to stress response such as JA, ABA and SA. This could represent the plant strategy, more effective in ddc than in WT, to avoid the negative effects of long-lasting activity of stress-related hormones. In view of the emerging relationship between the phytohormone action and epigenetic mechanisms12, this supposed role of methylation status in modulating plant strategy for ‘life or death switch’ under stress condition appears relevant at both theorical and applicative level.
Methods
Plant Lines and growth conditions
Plants of Arabidopsis thaliana (L.) Heynh ecotype Columbia-0 (Col-0), drm1 drm2 cmt3.11 (ddc) DNA methylation mutant and suppressor of drm1 drm2 cmt3 (sdc) silencing mutant, both in Col-0 background, were used. Arabidopsis thaliana seeds and plants were handled according to45 and by following the methods recommended by Arabidopsis Biological Resource Center (ABRC; https://abrc.osu.edu/) available at https://www.arabidopsis.org/download_files/Protocols/abrc_plant_growth.pdf.
Seeds were surface sterilized and sown on Petri dishes containing half-strength MS medium45. Regarding the different treatments, medium was supplemented with 25 µM and 50 μM Cd, as described by10, and/or 15 µM 5-Azacytidine (5-Aza), according to46. Detailed information on plant lines and selected treatment conditions are reported in Supplementary Methods S1 online. We will refer to the different samples as follows: WT Ctrl, WT 25 µM Cd, WT 50 µM Cd, WT 25 µM Cd + 15 µM 5-Aza, WT 50 µM Cd + 15 µM 5-Aza, ddc Ctrl, ddc 25 µM Cd, ddc 50 µM Cd, sdc Ctrl, sdc 25 µM Cd, sdc 50 µM Cd, sdc 25 µM Cd + 15 µM 5-Aza, sdc 50 µM Cd + 15 µM 5-Aza.
Cd quantification
Cd quantification was performed according to47 on samples collected at 21 DAG. Three independent replicates were carried out. For statistical analysis, two-way ANOVA with Tukey post hoc test (P ≤ 0.05) was applied after Shapiro–Wilk normality test. The results of such analysis are provided in Supplementary Fig. S10 online.
Growth parameters analysis
Root length was monitored in plants grown in vitro in a vertical position every two days until 21 DAG. Rosette leaf number and area were evaluated in plants grown in round Petri dishes for 21 DAG. Leaf series was obtained as described by48. Measurements were performed by scanning the plates and analysing the resulting images by using ImageJ software (https://imagej.nih.gov/ij/). Three independent replicates were performed (n = 45). Statistical analysis was carried out by using Student’s t-test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001) between ddc vs WT grown in the same conditions.
Total RNA extraction
Total RNA was isolated from 100 mg of plant tissue by using the RNeasy Plant Mini kit (Qiagen, Hilden, Germany) and DNA contamination was eliminated through on column DNase digestion (RQ1 RNase-Free Dnase, Cat. Nr. M6101). Extracted RNA was quantified by using the Qubit RNA BR (Broad-Range) Assay Kit, while its integrity was checked by using an Agilent 2100 Bioanalyzer (Agilent Technologies). Only RNA samples with an RNA integrity number ≥ 8 were subsequently used.
RNA-seq
cDNA libraries were constructed from 1 µg of total RNA, using the Illumina TruSeq Stranded Total RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). Quality of the obtained libraries and the fragments length were verified on the Bioanalyzer 2100 by using an Agilent 2100 DNA 1000 Kit and quantified by fluorimetry using the Qubit dsDNA HS (High sensitivity) Assay kit (Q232854). The sequencing of the cDNA libraries was carried out on Illumina Genome Analyzer IIx (SCS v2.10) platform.
Preprocessing and analysis of RNA-seq data
RNA-Seq reads in FASTQ format were inspected using FASTQC program (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Only reads with Phred quality score Q > 30 (Q30 Quality Score) were used (from 90 to 95%, for a total of 29.4 Giga reads of 50 bp paired-end reads). Reads were cleaned by using Trim Galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and matched to Arabidopsis TAIR10 gene sequences database, allowing for two mismatched bases. Only the alignments unique and concordant in SAM format were converted in binary BAM format by SAMtools. Basic statistics were calculated using Picard tools (CollectRnaSeq Metrics.jar) (http://picard.sourceforge.net/). Transcriptome quantification and RNA differential expression were performed using CuffDiff2 (http://cufflinks.cbcb.umd.edu/) software version 2.1.1, as described by49.
Gene expression levels were determined by FPKM calculation, using the Cufflinks method50. Bioinformatic analysis was performed by multiple pairwise comparisons of gene expression levels. Differentially expressed genes (DEGs) were selected on the basis of fold change (FC) (5 ≥|log2 FC|≥ 2 and FDR < 0.05).
Gene enrichment analysis
A functional annotation analysis of DEGs was performed by using Gene Ontology (GO) annotations51 (http://www.geneontology.org/). Gene Enrichment analysis, based on biological process ontology and KEGG database, was performed by selecting the over-represented GO terms through the ClueGO plug-in of the Cytoscape software52,53. The significance of each term and group was determined by the calculation of a Bonferroni-corrected P-value using the hypergeometric distribution. Only the GO terms with P ≤ 0.05 were selected.
Analysis of hormone-related pathways
The analysis of hormone-related pathways was performed by using the KEGG Mapper tool54 (https://www.genome.jp/kegg/mapper.html) for hormones signalling pathways and the online tool PlantMetGenMAP55 (http://bioinfo.bti.cornell.edu/cgibin/MetGenMAP/home.cgi), which allows a large-scale exploration of gene expression data-set and the identification of the significantly altered biochemical pathways and biological processes through robust statistical tests.
Libraries results validation through quantitative Real-Time PCR (qRT-PCR)
Transcriptomic analysis was validated by estimating the expression level of 14 hormone-related key genes. Primers sequences and additional information regarding the selected genes are shown in Supplementary Table S1 online. Detailed information is reported on Supplementary Methods S1 online.
Hormone level quantification
Hormone level quantification was performed on WT and ddc mutant plants grown in control conditions and under 25 µM Cd treatment, collected at 21 DAG. Detailed information on hormone level quantification methods is reported on Supplementary Methods S1 online.
Data availability
Raw transcriptomic data generated during and/or analysed during the current study are available at NCBI SRA under the BioProject accession PRJNA641242 (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA641242).
References
Chiappetta, A. et al. A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 6, 392 (2015).
Bitonti, M. B. et al. Distinct nuclear organization, DNA methylation pattern and cytokinin distribution mark juvenile, juvenile-like and adult vegetative apical meristems in peach (Prunus persica (L.) Batsch). J. Exp. Bot. 53, 1047–1054 (2002).
Pecinka, A. & Mittelsten Scheid, O. Stress-induced chromatin changes: A critical view on their heritability. Plant Cell Physiol. 53, 801–808 (2012).
Finnegan, E. J., Peacock, W. J. & Dennis, E. S. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc. Natl. Acad. Sci. U. S. A. 93, 8449–8454 (1996).
Zhang, H., Lang, Z. & Zhu, J.-K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 19, 489–506 (2018).
Wendte, J. M. et al. Epimutations are associated with CHROMOMETHYLASE 3-induced de novo DNA methylation. Elife 8, e47891 (2019).
Cao, X. & Jacobsen, S. E. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. U. S. A. 99, 16491–16498 (2002).
Pinto, A. P. et al. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 326, 239–247 (2004).
Greco, M., Chiappetta, A., Bruno, L. & Bitonti, M. B. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot. 63, 695–709 (2012).
Bruno, L. et al. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 8, 1323 (2017).
Boyes, D. C. et al. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 13, 1499–1510 (2001).
Yamamuro, C., Zhu, J.-K. & Yang, Z. Epigenetic modifications and plant hormone action. Mol. Plant 9, 57–70 (2016).
Jiang, Z., Li, J. & Qu, L.J. Auxins in Hormone Metabolism and Signaling in Plants (eds. Li, J., Li, C., Smith, S.M.) 39–76 (Academic Press, 2017). https://doi.org/10.1016/B978-0-12-811562-6.00002-5.
Ren, H. & Gray, W. M. M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 8, 1153–1164 (2015).
Brumos, J., Alonso, J. M. & Stepanova, A. N. Genetic aspects of auxin biosynthesis and its regulation. Physiol. Plant. 151, 3–12 (2013).
Feng, J., Shi, Y., Yang, S. & Zuo, J. Cytokinins in Hormone Metabolism and Signaling in Plants (eds. Li, J., Li, C., Smith, S.M.) 77–106 (Academic Press, 2017). https://doi.org/10.1016/B978-0-12-811562-6.00003-7.
Hwang, I. & Sheen, J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383 (2001).
Imamura, A., Yoshino, Y. & Mizuno, T. Cellular localization of the signaling components of Arabidopsis His-to-Asp phosphorelay. Biosci. Biotechnol. Biochem. 65, 2113–2117 (2001).
Gao, X., Zhang, Y., He, Z. & Fu, X. Gibberellins in Hormone Metabolism and Signaling in Plants (eds. Li, J., Li, C., Smith, S.M.) 107–160 (Academic Press, 2017). https://doi.org/10.1016/B978-0-12-811562-6.00004-9.
Yoshida, H. & Ueguchi-Tanaka, M. DELLA and SCL3 balance gibberellin feedback regulation by utilizing INDETERMINATE DOMAIN proteins as transcriptional scaffolds. Plant Signal. Behav. 9, e29726 (2014).
Chini, A. et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666 (2007).
Kushiro, T. et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 23, 1647–1656 (2004).
Park, S.-Y. et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science (80-.) 324, 1068–1071 (2009).
Brocard, I. M., Lynch, T. J. & Finkelstein, R. R. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol. 129, 1533–1543 (2002).
Hua, J. & Meyerowitz, E. M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271 (1998).
Chern, M., Canlas, P. E. & Ronald, P. C. Strong suppression of systemic acquired resistance in Arabidopsis by NRR is dependent on its ability to interact with NPR1and its putative repression domain. Mol. Plant 1, 552–559 (2008).
Henderson, I. R. & Jacobsen, S. E. Tandem repeats upstream of the Arabidopsis endogene SDC recruit non-CG DNA methylation and initiate siRNA spreading. Genes Dev. 22, 1597–1606 (2008).
Davies, P. J. Plant Hormones: Biosynthesis, Signal Transduction, Action! (Kluwer Academic, New York, 2004).
Fattorini, L. et al. Cadmium and arsenic affect quiescent centre formation and maintenance in Arabidopsis thaliana post-embryonic roots disrupting auxin biosynthesis and transport. Environ. Exp. Bot. 144, 37–48 (2017).
Chmielowska-Bąk, J., Lefèvre, I., Lutts, S. & Deckert, J. Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J. Plant Physiol. 170, 1585–1594 (2013).
Hu, Y. F. et al. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 170, 965–975 (2013).
Srivastava, R. K., Pandey, P., Rajpoot, R., Rani, A. & Dubey, R. S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251, 1047–1065 (2014).
Forgione, I. et al. Hypomethylated drm1 drm2 cmt3 mutant phenotype of Arabidopsis thaliana is related to auxin pathway impairment. Plant Sci. 280, 383–396 (2018).
Wang, J., Ma, X.-M., Kojima, M., Sakakibara, H. & Hou, B.-K. Glucosyltransferase UGT76C1 finely modulates cytokinin responses via cytokinin N-glucosylation in Arabidopsis thaliana. Plant Physiol. Biochem. 65, 9–16 (2013).
Veselov, D., Veselov, D., Kudoyarova, G., Symonyan, M. & Veselov, S. Effect of cadmium on ion uptake, transpiration and cytokinin content in wheat seedlings. Bulg. J. Plant Physiol. 29, 353–359 (2003) (Special Issue).
Piotrowska-Niczyporuk, A., Bajguz, A., Zambrzycka, E. & Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 52, 52–65 (2012).
Mansour, M. & Kamel, A.-R. Interactive effect of heavy metals and gibberellic acid on mitotic activity and some metabolic changes of Vicia faba L. plants. Cytol. Int. J. Cytol. 70, 275–282 (2005).
Verma, V., Ravindran, P. & Kumar, P. P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16, 86 (2016).
Shu, K., Zhou, W., Chen, F., Luo, X. & Yang, W. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front. Plant Sci. 9, 416 (2018).
Asgher, M., Khan, M. I. R., Anjum, N. A. & Khan, N. A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 252, 399–413 (2015).
Maksymiec, W., Wójcik, M. & Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66, 421–427 (2007).
Soares, A. M. D. S., Souza, T. F. D., Jacinto, T. & Machado, O. L. T. Effect of Methyl Jasmonate on antioxidative enzyme activities and on the contents of ROS and H2O2 in Ricinus communis leaves. Braz. J. Plant Physiol. 22, 151–158 (2010).
Gust, A. A. & Nürnberger, T. A life or death switch. Nature 486, 198 (2012).
Rivero, L. et al. Handling Arabidopsis plants: Growth, preservation of seeds, transformation, and genetic crosses. Methods Mol Biol. 1062, 3–25 (2014).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962).
Zhao, Q. et al. 5-Azacytidine promotes shoot regeneration during Agrobacterium-mediated soybean transformation. Plant Physiol. Biochem. 141, 40–50 (2019).
Liu, K. et al. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol. Environ. Saf. 113, 207–213 (2015).
Cookson, S. J., Turc, O., Massonnet, C. & Granier, C. Phenotyping the Development of Leaf Area in Arabidopsis thaliana. In Plant Developmental Biology. Methods in Molecular Biology (Methods and Protocols) Vol. 655 (eds Hennig, L. & Köhler, C.) 89–103 (Humana Press, Totowa, NJ, 2010).
Annese, A. et al. Whole transcriptome profiling of Late-Onset Alzheimer’s Disease patients provides insights into the molecular changes involved in the disease. Sci. Rep. 8, 4282 (2018).
Roberts, A., Pimentel, H., Trapnell, C. & Pachter, L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 27, 2325–2329 (2011).
Ashburner, et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Bindea, G. et al. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Joung, J.-G. et al. Plant MetGenMAP: An integrative analysis system for plant systems biology. Plant Physiol. 151, 1758–1768 (2009).
Acknowledgements
We acknowledge Maria Greco (Francis Crick Institute, London UK) for her pioneer work on ddc response to Cd. This work was supported by grants from the University of Calabria-Italy (ex 60%). Illumina Genome Analyzer IIx (SCS v2.10) platform for this research was supplied by PON Ricerca e Competitività 2007–2013, Sistema Integrato di Laboratori per L’Ambiente – (SILA) PONa300341, Piattaforma Omica.
Author information
Authors and Affiliations
Contributions
M.P., L.B., A.C., M.B.B. designed research; M.P., A.M., L.B., L.M., E.T. performed research; M.P., E.P. performed bioinformatic analysis; M.P., A.M., L.B., A.C., L.M., P.P., M.B.B. analysed data and discussed results; L.B., M.B.B. supervised the research; M.P. and M.B.B. wrote the paper. All authors contributed to improving the paper and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pacenza, M., Muto, A., Chiappetta, A. et al. In Arabidopsis thaliana Cd differentially impacts on hormone genetic pathways in the methylation defective ddc mutant compared to wild type. Sci Rep 11, 10965 (2021). https://doi.org/10.1038/s41598-021-90528-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90528-5
- Springer Nature Limited
This article is cited by
-
Transcriptome analysis of Pennisetum americanum × Pennisetum purpureum and Pennisetum americanum leaves in response to high-phosphorus stress
BMC Plant Biology (2024)
-
The roles of abscisic acid and ethylene in cadmium accumulation and tolerance in plants
Plant and Soil (2023)
-
Exogenous methyl jasmonate (MeJA) enhances the tolerance to cadmium (Cd) stress of okra (Abelmoschus esculentus L.) plants
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)