Abstract
Neutrophils and lymphocytes are key regulators of breast cancer (BC) development and progression. Neutrophil to lymphocyte ratio (NLR) values have been found to offer clear prognostic utility when evaluating BC patients. In this study, we sought to determine whether BC patient baseline NLR values are correlated with pathological complete response (pCR) following neoadjuvant chemotherapy (NCT) treatment. In total, 346 BC patients underwent NCT at our hospital from January 1, 2014 to October 31, 2019, and data pertaining to these patients were retrospectively analyzed. Correlations between clinicopathological characteristics and pCR rates were assessed via multivariate logistic regression analyses. A predictive scoring model was used to gauge the likelihood of pCR based upon regression coefficient (β) values for each significant variable identified through these analyses. NLR cut-off values suitable for identifying patients likely to achieve pCR following NCT treatment were calculated using receiver operating characteristic (ROC) curves. All patients in the present study were females with a median age of 48 years old (range 22–77). An optimal NLR cut-off value of 1.695 was identified and was associated with respective sensitivity and specificity values of 63.6% and 45.5%. We found that higher NLR values were significantly associated with younger age, premenopausal status, and non-pCR status. Logistic regression analyses indicated that NLR, tumor size, hormone receptor (HR) status, and Ki-67 expression were all independent predictors of pCR. The area under the curve (AUC) for the resultant predictive scoring model was 0.705, and this model was assessed via K-fold cross-validation (k = 10) and bootstrapping validation, yielding respective AUC values of 0.68 and 0.694. Moreover, the incorporation of NLR into this predictive model incrementally improved its overall prognostic value relative to that of a model not incorporating NLR (AUC = 0.674). BC patients with a lower baseline NLR are more likely to exhibit pCR following NCT treatment, indicating that NLR may be a valuable biomarker for BC patient prognostic evaluation and treatment planning. Overall, our results demonstrate that this NLR-based predictive model can efficiently predict NCT efficacy in early BC patients with a high degree of accuracy.
Similar content being viewed by others
Introduction
Neoadjuvant chemotherapy (NCT) is commonly used to treat breast cancer (BC) patients with locally advanced or inflammatory disease in order to permit surgical treatment or to decrease tumor size and thereby better allow for breast-conserving surgery to be performed. Pathological complete response (pCR) rates following treatment are higher in BC patients with human epidermal growth factor receptor-2 positive (HER2+) or triple-negative (TN) relative to patients with HER2 negative (HER2−) or hormone receptor positive (HR+) disease. Some studies have suggested that pCR following NCT treatment can be used as a reliable indicator of patient disease-free survival (DFS) and overall survival (OS), particularly in patients with aggressive TNBC or HER2− BC1. As such, many studies have sought to identify clinicopathological variables associated with pCR in BC patients following NCT, with tumor size and grade being among the primary factors known to be predictive of this treatment outcome2,3. Additional studies have also demonstrated that particular genetic and molecular phenotypes can be used to predict NCT treatment outcomes4. However, the high costs associated with these genetic and molecular tests limit their utility in regular clinical practice. It is therefore vital that more readily accessible pathologic or laboratory biomarkers associated with NCT treatment outcomes be identified.
Inflammation is a key driver of cancer development and progression, and is closely linked to metastasis5. Neutrophils form an important component of the tumor-induced inflammatory response, whereas lymphocytes are most often related to anti-tumor immune responses such that neutrophil to lymphocyte ratio (NLR) values can reflect the dynamic balance between inflammatory and anti-inflammatory responses in cancer patients. As they serve as a readout for inflammatory status, NLR values have been employed to evaluate treatment efficacy and patient prognosis in cancer patients undergoing chemotherapy6. In BC, pretreatment NLR values have been found to be associated with patient prognosis. One meta-analysis found higher pretreatment NLR values to be associated with reduced DFS7. While BC tumors do not generate large quantities of neoantigens8, they are commonly subjected to lymphocyte infiltration, with variations in the degree of this infiltration across BC subtypes9,10. The presence of tumor-infiltrating lymphocytes (TILs) in BC patients is closely associated with pCR following treatment, with regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) serving to suppress immune functionality and to reduce NCT efficacy in BC11. However, few studies to date have assessed whether pretreatment NLR values can predict prognosis and treatment efficacy in BC patients undergoing NCT treatment. While some studies have found a higher NLR to be associated with lower pCR rates in BC6,12, this finding is not universal and thus remains controversial13,14. The present study was therefore designed to explore the prognostic utility of NLR values as a tool for predicting NCT treatment outcomes in BC patients.
Materials and methods
Patients
In total, we retrospectively analyzed outcomes from 1435 primary BC patients that underwent NCT treatment at Henan Cancer Hospital from January 1, 2014 to October 31, 2019. Patients eligible for inclusion in this study were those that met the following criteria: (1) female; (2) had not undergone any invasive procedures or biopsies within one week prior to blood sample collection; (3) had a confirmed diagnosis of invasive BC as determined by core needle biopsy, with tumor estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67 status being known; (4) had stage II-III BC, with T staging have been conducted via clinical examination, and with N staging having been conducted via needle aspiration biopsy or core needle biopsy in response to positive axillary lymph node (ALN) palpation or suspicious imaging findings (AJCC 7th edition); (5) patients did not have any contraindications precluding chemotherapy upon general examination; (6) underwent six standard NCT cycles; (7) surgery was conducted after NCT; and (8) breast and ALN pathological findings were available. Patients were excluded from this study if they: (1) were male; (2) had undergone invasive examinations within 1-week prior to blood sample collection; (3) had bilateral BC; (4) had evidence of active infection within the past week; (5) had inflammatory BC; (6) suffered from any chronic autoimmune or inflammatory disease or hematological disorders; or (7) underwent fewer than 6 NCT cycles. After screening patients using these criteria, 346 were included in our final analysis.
All HER2− patients received a standardized NCT regimen composed of six courses of TEC (75 mg/m2 docetaxel, 75 mg/m2 epirubicin, and 500 mg/m2 cyclophosphamide) every 3 weeks. Of the 140 patients in this study with HER2+ BC, 89 were administered tri-weekly TCH (docetaxel 75 mg/m2, carboplatin AUC 6, and trastuzumab 8 mg/kg loading dose, 6 mg/kg maintenance dose) treatment, while the remaining 51 HER2+ patients underwent TEC treatment. All patients underwent mastectomy or breast-conserving surgery and ALN dissection following NCT.
This study was approved by the Ethical Review Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2019001). As this study had a retrospective design, the requirement for informed consent was waived by the hospital. We present this article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Pathology
Tissue samples from BC patients were fixed using 10% formaldehyde, paraffin-embedded, and cut into serial 4 μm-thick sections that were subjected to routine hematoxylin and eosin (H&E) staining. Immunohistochemical (IHC) staining was then used to assess tumor ER, PR, HER2, and Ki-67 status. Tumors were considered to be ER or PR positive when at least 1% of tumor nuclei were positive for these hormone receptors, as per the corresponding American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline15. Tumors were considered to be HER2-positive when they were scored either a 3+ via IHC or a 2+ with subsequent fluorescence in situ hybridization (FISH) confirmation, as per the corresponding ASCO/CAP HER2 guideline16. While Ki-67 cut-off criteria vary between centers, we defined a sample to be Ki-67-high if the proliferation index was > 30%17. Tumors were separated into four subtypes based upon HR and HER2 status: HR+HER2−, HR+HER2+, HR−HER2+, HR−HER2−. PCR was defined as the absence of any detectable tumor cell residue (ypT0ypN0) in primary breast tumors and axillary lymph node samples collected during surgery following NCT1.
Blood data collection
Peripheral complete blood counts were performed at baseline in our hospital laboratory according to the standardized operative procedures. Blood data were collected by reviewing medical records. NLR was calculated as the ratio between the absolute neutrophil and lymphocyte counts.
Statistical analysis
SPSS 17.0 and SAS 9.4 were used for all statistical analyses. Categorical variables were analyzed via chi-squared tests or Mann–Whitney U tests, with P < 0.05 as the significance threshold. Factors yielding a P-value < 0.1 in univariate analyses were incorporated into subsequent multivariate binary logistic regression analysis. Factors yielding a P-value < 0.05 in this multivariate analysis were considered to be independent related factors. The probability of pCR in this study was assessed by developing a predictive scoring model. This model was constructed by incorporating regression coefficient (β) values for each independent related variable identified in the above multivariate analyses. Numbers were rounded to the nearest whole number and were converted into scores, while negative numbers were added to a 1 or 2 in order to yield a positive number. The total risk score was then generated by summing together the individual scores for each variable. The NLR and risk score cut-off values were calculated based upon maximum Youden's index of the ROC curve. We additionally assessed the AUC, sensitivity, and specificity values associated with the chosen ROC curve cut-off values. The risk score discriminative abilities were assessed via K-fold cross-validation (k = 10) and bootstrapping validation.
Result
The relationship between NLR and patient clinicopathological parameters
In total, we retrospectively analyzed outcomes from 346 female BC patients (Fig. 1). These patients had a median age of 48 years-old (range 22–77), and 227 (66%) were premenopausal, 258 (75%) had T1-T2 tumors, 236 (68.2%) had HR+ tumors, 140 (40.5%) had Her2+ tumors, and 255 (73.7%) exhibited tumors with high Ki-67 expression (Table 1).
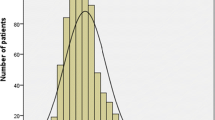
NLR values were individually determined for each patient, and ranged from 0.45–9.36 (mean 2.01 ± 0.05; median 1.84; standard deviation 0.91). An ROC curve was constructed to examine the relationship between NLR and pCR following NCT. The AUC for this curve was 0.580 (95% CI 0.508–0.652; P = 0.032). An optimal cut-off value of 1.695 was selected according to the Youden's index, yielding respective sensitivity and specificity values of 63.6% and 45.5%. Of these patients, 149 (43.1%) were considered to have a low baseline NLR (< 1.695) and 197 (56.9%) were considered to have a high baseline NLR (≥ 1.695). We found that patients with a high NLR were more likely to younger (P = 0.001), premenopausal (P = 0.001), and to experience non-pCR (P = 0.005) (Table 1, Fig. 2).
Univariate and multivariate analyses of the relationship between NLR and pCR
In total, 77 patients (22.3%) achieved pCR following NCT, with respective pCR rates of 16.8% and 29.5% in the high and low NLR groups (P = 0.005). Patients with small, HR−, HER2+, Ki-67-high tumors were more likely to achieve pCR following NCT (all P < 0.1). In addition, pCR was not found to be associated with patient age, menstrual status, tumor location, or lymph node status in these univariate analyses (all P > 0.1) (Table 2). A subsequent multivariate logistic regression analysis revealed NLR (OR 2.034, 95% CI 1.178–3.512, P = 0.011), tumor size, HR (OR 0.386, 95% CI 0.219–0.679, P = 0.001), and Ki-67 status (OR 2.318, 95% CI 1.104–4.868, P = 0.026) to all be independent predictors of patient pCR rates (Table 3).
Development of a predictive scoring model
We next sought to develop a predictive scoring model capable of estimating the odds of pCR following NCT in BC patients by utilizing the regression coefficient (β) values for each variable identified in the above analysis, with whole numbers being converted into corresponding scores. For example, β coefficients values for tumor of sizes T1, T2, T3, and T4 were 0, − 1.23, − 1.836, and − 1.227, respectively, and these values were rounded up and added to the number two to yield scores of 2, 1, 0, and 1, respectively. Total scores ranged from 0–5, and full details regarding this scoring system can be found in Table 4.
Predictive scoring model validation
We next constructed an ROC curve evaluating the relationship between patient risk scores and pCR rates. This curve yielded an AUC of 0.705, whereas the AUC value for the model that did not include NLR was 0.674, consistent with the ability of our scoring system to readily differentiate between patients that would and would not achieve pCR following NCT (Fig. 3). A Hosmer–Lemeshow Goodness-of-Fit test confirmed that this model had a good fit (P = 0.891). We selected a score of 3 points as a cut-off value for estimating patient pCR status based upon the largest Youden's index value. Patients having a score of ≥ 3 were more likely to achieve pCR. This cut-off value yielded respective sensitivity, specificity, positive predictive, and negative predictive values of 74.0%, 61.0%, 35.2%, and 89.1% in this patient cohort (Table 5). In addition, the discriminative ability of these risk scores was assessed via K-fold cross-validation (k = 10) and bootstrapping validation, yielding respective AUC values of 0.68 and 0.694.
Discussion
Many studies to date have evaluated the predictive and prognostic potential of baseline NLR values as a means of assessing BC patients18. Herein, we expanded upon these prior studies by analyzing a cohort of 346 BC patients that had undergone NCT. Through these analyses, we determined that a low NLR was an independent predictor of pCR following NCT treatment.
We selected an NLR cut-off value of 1.695 in order to differentiate between patients that were and were not likely to achieve pCR following NCT. This cut-off was selected based upon ROC curve analyses. There is currently no unified consensus regarding optimal NLR cut-off values for the evaluation of BC patients. Prior studies of BC patients undergoing NCT have utilized NLR cut-off values ranging from 1.7–3.33, and a recent meta-analysis incorporating 39 studies and 17,079 total BC patients undergoing adjuvant chemotherapy similarly confirmed this lack of cut-off consistency (range 1.7–4.0)7,19,20. The cut-off value selected in the present study was lower than the values reported in prior studies. This may be due to site-specific differences or may suggest that inclusion/exclusion criteria in our study were more stringent. No patients that had undergone any invasive treatments or evaluations prior to blood sample collection were included in our study, as our inclusion/exclusion criteria were more stringent than prior studies.
When we analyzed the relationship between NLR and other clinicopathological characteristics in these BC patients, we found that younger patients and premenopausal patients were more likely to have higher NLR values. This was consistent with the results of a prior study conducted in Mexico which determined that patients with an NLR > 2 tended to be younger13. Together, these results indicate that NLR values may be associated with differential effects in younger and older patients, although further research will be necessary to confirm these findings.
Multiple studies to date have reported on the prognostic value of NLR as a means of assessing outcomes in patients undergoing NCT6,21,22. In a 2019 meta-analysis of 11 studies incorporating 2107 BC patients, lower NLR values were found to be associated with significantly better NCT responses23. However, a large single study incorporating 1519 BC patients did not detect any relationship between NLR and pCR rates13. As such, the prognostic relevance of NLR values remains controversial in BC patients undergoing NCT. In the present study, 77 patients (22.3%) achieved pCR following NCT, with significantly better pCR rates in the low NLR group relative to the high NLR group (29.5% vs. 16.8%). In a multivariate analysis, baseline NLR, tumor size, HR status, and Ki-67 status were all confirmed to be independent predictors of pCR. There may be two primary factors explaining the inconsistency between our findings and those of certain prior studies. For one, we may have selected patients more rigorously by specifically excluding individuals that had undergone invasive procedures such as core needle biopsy prior to blood sample collection. Second, NCT regimens in the abovementioned prior study were not uniform, whereas relatively uniform chemotherapy regimens were applied to all patents in our study. We excluded patients that had undergone alternative treatment regimens or an inadequate number of NCT cycles. Based on our findings and those of prior meta-analyses, we can conclude that NLR values may serve as valuable and effective predictors of pCR following NCT treatment in BC patients.
Further research is required in order to understand why patients with low NLR values are more sensitive to NCT treatment. BC development is closely associated with inflammation and with the overall immune status of affected patients, and tumor cells are capable of releasing many non-specific inflammatory cytokines and other factors that can activate or recruit other inflammatory cells capable of promoting angiogenesis and disease progression24. Inflammatory responses are characterized by the recruitment and activation of neutrophils, lymphocytes, and other cell types, and by the production of C-reactive protein. Neutrophils in particular can secrete inflammatory cytokines and can promote tumor metastasis and angiogenesis25. Reduced lymphocyte counts may be indicative of impaired anti-tumor immune responses, enabling cancer cells to more readily grow and spread26. As such, NLR values serve as a dynamic indicator of the overall immune status in a given patient, making them of key relevance in the field of oncology. Oncogenesis and cancer progression result in a loss of homeostatic balance between pro- and anti-inflammatory responses, such that lymphocyte counts tend to decrease and neutrophil/platelet counts tend to rise, leading to higher NLR and PLR values24. Ozyalvacli et al. assessed NLR distributions in BC patients and in 50 controls with benign breast diseases, confirming that BC patients exhibited significantly higher average NLR values27. Three other studies have also suggested that the risk of BC increased with rising NLR levels28,29,30. In addition, cancer stem cells make up a small subset of overall tumor cells, and possess the ability to self-renew and differentiate indefinitely. These stem cells are important mediators of tumor aggression, chemoresistance, and metastasis31. Tumor-associated neutrophils produce large quantities of bone morphogenic protein (BMP)-2 and TGF-β2, which confer stem-like properties to these cancer cells32.
Following a multivariate analysis identifying independent predictors of pCR, we developed a scoring system capable of more reliably predicting pCR rates in BC patients undergoing NCT. Several prior studies have reported similar models incorporating variables such as tumor-infiltrating lymphocyte scores or tumor cellularity on day 15 of treatment, with these models having been found to predict patient outcomes after completion of neoadjuvant anti-HER2-based therapy33. Our model, however, is more accessible than these prior models, as it incorporates NLR values which can be readily obtained. Our model yielded an AUC of 0.705, indicating that it can effectively differentiate between patients that do and do not achieve pCR. Moreover, the addition of NLR also provided an incremental improvement in the prognostic value of our model relative to one not incorporating NLR (AUC = 0.674). Internal verification of this model via K-fold cross-validation (k = 10) and bootstrapping validation yielded respective AUC values of 0.68 and 0.694, indicating it to be stable and satisfactory. As such, our model represents a valuable clinical tool for the priori assessment of which BC patients are most likely to achieve pCR following NCT. By accurately predicting the odds of pCR prior to surgery, clinicians can more effectively weigh the relative costs and benefits of such treatment so as to ensure that they act in the best interest of the patient.
There are multiple strengths to this study. For one, we provided a detailed overview of the NCT regimens used to treat patients, consistent with international recommendations. Second, a unified NCT regimen was used for all patients, thus precluding the possibility that regimen-specific differences may have influenced patient pCR rates. Third, we only enrolled patients that had not undergone any invasive procedures prior to peripheral blood collection. However, there are also certain limitations to this study. Specifically, this was a retrospective study without any external validation and without any assessment of patient survival outcomes. Future studies will thus be needed to confirm and expand upon our findings.
In conclusion, our findings indicate that NLR values can be reliably used to predict BC patient responses to NCT treatment. Importantly, we generated a risk scoring model incorporating several easily-measured baseline clinicopathological parameters that can effectively stratify patients based upon their likelihood of achieving pCR. Patients with higher scores derived from this model were more likely to achieve pCR following NCT treatment. Future large-scale prospective studies, however, will be needed to confirm our findings.
References
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384, 164–172. https://doi.org/10.1016/S0140-6736(13)62422-8 (2014).
DiCenzo, D. et al. Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from multi-institutional study. Cancer Med. https://doi.org/10.1002/cam4.3255 (2020).
Zhu, J. et al. Predictive factors and prognostic value of pathologic complete response of ipsilateral supraclavicular lymph nodes in breast cancer after neoadjuvant chemotherapy. Ann. Transl. Med. 7, 666. https://doi.org/10.21037/atm.2019.10.22 (2019).
Swain, S. M. et al. NSABP B-41, a randomized neoadjuvant trial: Genes and signatures associated with pathologic complete response. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-0152 (2020).
Elinav, E. et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771. https://doi.org/10.1038/nrc3611 (2013).
Asano, Y. et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann. Surg. Oncol. 23, 1104–1110. https://doi.org/10.1245/s10434-015-4934-0 (2016).
Guo, W. et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 8, 4135–4148. https://doi.org/10.1002/cam4.2281 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74. https://doi.org/10.1126/science.aaa4971 (2015).
De Angelis, C. et al. Evaluation of the predictive role of tumor immune infiltrate in patients with HER2-positive breast cancer treated with neoadjuvant anti-HER2 therapy without chemotherapy. Clin. Cancer Res. 26, 738–745. https://doi.org/10.1158/1078-0432.CCR-19-1402 (2020).
Loi, S. et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 37, 559–569. https://doi.org/10.1200/JCO.18.01010 (2019).
Li, F., Zhao, Y., Wei, L., Li, S. & Liu, J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 19, 695–705. https://doi.org/10.1080/15384047.2018.1450116 (2018).
Bae, S. J. et al. Prognostic value of neutrophil-to-lymphocyte ratio in human epidermal growth factor receptor 2-negative breast cancer patients who received neoadjuvant chemotherapy. Sci. Rep. 10, 13078. https://doi.org/10.1038/s41598-020-69965-1 (2020).
Munoz-Montano, W. et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in different phenotypes of locally advanced breast cancer during neoadjuvant systemic treatment. Clin. Breast Cancer https://doi.org/10.1016/j.clbc.2019.12.011 (2020).
Graziano, V. et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 44, 33–38. https://doi.org/10.1016/j.breast.2018.12.014 (2019).
Hammond, M. E. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795. https://doi.org/10.1200/JCO.2009.25.6529 (2010).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013. https://doi.org/10.1200/JCO.2013.50.9984 (2013).
Goldhirsch, A. et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann. Oncol. 20, 1319–1329. https://doi.org/10.1093/annonc/mdp322 (2009).
Corbeau, I., Jacot, W. & Guiu, S. Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: A systematic review. Cancers (Basel) https://doi.org/10.3390/cancers12040958 (2020).
Chae, S. et al. Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr. Oncol. 25, e113–e119. https://doi.org/10.3747/co.25.3888 (2018).
Losada, B. et al. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin. Transl. Oncol. 21, 855–863. https://doi.org/10.1007/s12094-018-1999-9 (2019).
Chen, Y. et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: A retrospective study. BMC Cancer 16, 320. https://doi.org/10.1186/s12885-016-2352-8 (2016).
Qian, Y. et al. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 11, 1423–1432. https://doi.org/10.2147/OTT.S148496 (2018).
Xue, L. B. et al. Prognostic role of high neutrophil-to-lymphocyte ratio in breast cancer patients receiving neoadjuvant chemotherapy: Meta-analysis. Medicine (Baltimore) 98, e13842. https://doi.org/10.1097/MD.0000000000013842 (2019).
Baxevanis, C. N., Fortis, S. P. & Perez, S. A. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.12.018 (2019).
Mukaida, N., Sasaki, S. I. & Baba, T. Two-faced roles of tumor-associated neutrophils in cancer development and progression. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21103457 (2020).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 13, 228–241. https://doi.org/10.1038/nrclinonc.2015.215 (2016).
Ozyalvacli, G. et al. Diagnostic and prognostic importance of the neutrophil lymphocyte ratio in breast cancer. Asian Pac. J. Cancer Prev. 15, 10363–10366. https://doi.org/10.7314/apjcp.2014.15.23.10363 (2014).
Gago-Dominguez, M. et al. Neutrophil to lymphocyte ratio and breast cancer risk: Analysis by subtype and potential interactions. Sci. Rep. 10, 13203. https://doi.org/10.1038/s41598-020-70077-z (2020).
Fang, Q. et al. Neutrophil-to-lymphocyte ratio, obesity, and breast cancer risk in Chinese population. Medicine (Baltimore) 97, e11692. https://doi.org/10.1097/MD.0000000000011692 (2018).
Okuturlar, Y. et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac. J. Cancer Prev. 16, 2409–2412. https://doi.org/10.7314/apjcp.2015.16.6.2409 (2015).
Batlle, E. & Clevers, H. Cancer stem cells revisited. Nat. Med. 23, 1124–1134. https://doi.org/10.1038/nm.4409 (2017).
Zhou, S. L. et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology 70, 1214–1230. https://doi.org/10.1002/hep.30630 (2019).
Nuciforo, P. et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann. Oncol. 29, 170–177. https://doi.org/10.1093/annonc/mdx647 (2018).
Acknowledgements
We are grateful to Dr. Chuanbing Wang in Henan Cancer hospital laboratory for his help for the blood data collection.
Funding
The study was supported by the Science and Technology Development Plan of Henan Province (202102310415).
Author information
Authors and Affiliations
Contributions
J.J.Z. conceived, oversaw and carried out the epidemiological study including design, enrollment, data collection, and statistical analyses, and drafted the manuscript. D.C.J. and Y.J.Z. performed the statistical analysis and interpretation of data. X.H.G., Y.Y. and H.X. contributed to enrollment, data collection and data cleaning of epidemiological study. Z.Z.L. and J.J.Z. participated in study design and analyses and helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, J., Jiao, D., Zhao, Y. et al. Development of a predictive model utilizing the neutrophil to lymphocyte ratio to predict neoadjuvant chemotherapy efficacy in early breast cancer patients. Sci Rep 11, 1350 (2021). https://doi.org/10.1038/s41598-020-80037-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80037-2
- Springer Nature Limited