Abstract
Hypertension in end-stage renal disease patients is highly prevalent and poorly controlled. Data on the ambulatory blood pressure (BP) profile and BP variability (BPV) in peritoneal dialysis (PD) patients are absent. This study examined the BP profile and BPV of patients undergoing PD in comparison with hemodialysis (HD) and predialysis chronic kidney disease CKD patients. Thirty-eight PD patients were matched for age, sex, and dialysis vintage with 76 HD patients and for age and sex with 38 patients with CKD stage 2–4. Patients under PD or HD underwent 48-h and CKD patients 24-h ambulatory BP monitoring. BP levels and BPV indices were compared for the 48-h, first and second 24 h, daytime and nighttime periods. Two-way mixed ANOVA for repeated measurements was used to evaluate the effects of dialysis modality and time on ambulatory BP in PD and HD. During all periods studied, SBP and DBP were numerically higher but not significantly different in PD than in HD patients. Systolic BP was significantly higher in PD or HD than in predialysis CKD (PD: 138.38 ± 20.97 mmHg; HD: 133.75 ± 15.5 mmHg; CKD: 125.52 ± 13.4 mmHg, p = 0.003), a difference evident also during daytime and nighttime periods. Repeated-measurements ANOVA showed no effect of dialysis modality on ambulatory BP during any period studied. All BPV indices studied were similar between PD and HD patients, in whom they were higher than in CKD individuals (first 24-h systolic-ARV: PD: 11.86 ± 3.19 mmHg; HD: 11.23 ± 3.45 mmHg; CKD: 9.81 ± 2.49 mmHg, p = 0.016). Average BP levels and BPV indices are similar between PD and HD patients, in whom they are higher than in their CKD counterparts. The dialysis modality has no effect on the ambulatory BP profile. These results suggest that PD is no better than HD with regard to overall BP control or BP fluctuations over time.
Similar content being viewed by others
Introduction
Hypertension is the most common modifiable risk factor in patients with chronic kidney disease (CKD), with a prevalence that progressively increases with advancing CKD stage [1]. In patients with end-stage renal disease (ESRD) undergoing hemodialysis (HD) or peritoneal dialysis (PD), the prevalence of hypertension is estimated at 80–90% [2,3,4].
The use of ambulatory BP monitoring (ABPM) is currently recommended for diagnosis and treatment decisions in all ESRD patients due to a number of advantages, including higher prognostic value [2]. However, the majority of existing evidence in the field originates from HD studies, as only a few older works evaluated blood pressure (BP) profile patients undergoing PD [4], and even fewer did so in comparison with HD [5, 6]. These studies compared only average BP values obtained from ABPM, so differences in BP profile between patients on different dialysis modalities over time have not been investigated. Furthermore, no study so far has examined the ambulatory BP profile in PD or HD patients in comparison with that of patients with predialysis CKD.
Fluctuations of BP over time are described by the term “BP variability” (BPV) and are categorized into very-short-term (beat-by-beat), short-term (within 24 h), mid-term (day-by-day) and long-term (visit-to-visit) BPV based on the time interval of measurement [7]. Long-term BPV has been associated with cardiovascular events and mortality independently of BP in hypertensive individuals [8, 9], as well as in HD patients [10]. In patients with CKD, short-term BPV increases with advancing CKD stage [11]. In HD, we recently showed that BPV is increased from day 1 to day 2 of the interdialytic interval [12] and is associated with a higher risk of cardiovascular events and death [13]. Both acute increases and acute decreases in BP may be responsible for target-organ dysfunction and cardiovascular events in HD [14, 15]. In theory, PD individuals would have lower short-term BPV than HD patients due to the continuous nature of the modality and the absence of rapid volume removal in HD sessions. However, there are currently no data comparing short-term BPV between these methods.
The aim of this study was to evaluate the BP profile and BPV of patients undergoing PD by 48-h ABPM in comparison with HD patients and patients with predialysis CKD.
Materials and methods
Study design
Patients undergoing PD or HD and those with CKD were recruited from two tertiary nephrology departments of northern Greece. We included as cases adult patients (>18 years) with ESRD who had been treated with PD for at least 3 months. A blinded member of our team matched PD patients (cases) with potential controls who were on standard thrice-weekly HD treatment in our HD units as well as patients with CKD stage 2–4 followed in the general nephrology outpatient clinics of our departments. Potential HD and CKD controls were then invited to participate in the study. PD and HD patients were matched in a 1:2 ratio for age, sex, and dialysis vintage, and PD and CKD controls were matched in a 1:1 ratio for age and sex. The 1:2:1 ratio was selected following the concept of flexible matching with varying proportions to increase the power and efficiency of the study [16]. The protocol was approved by the ethics committee of the School of Medicine, Aristotle University of Thessaloniki, and all participants provided informed written consent to participate in the study.
Data collection
Demographics, anthropometric characteristics, comorbidities, concomitant medications, and dialysis-related parameters were recorded for every participant. Subjects were instructed to visit their unit 1 h before their programmed follow-up visit (PD patients) or 1 h before the second or the third dialysis session of the week (HD patients). Patients underwent a physical examination and venous blood sampling for routine laboratory tests. Patients with CKD were evaluated before a programmed visit at the outpatient clinic. ABPM was planned to last 48 h for PD and HD patients and 24 h for CKD patients. For patients receiving HD, the 48-h period started with the start of the dialysis session and included the dialysis session and the subsequent interdialytic period. During ABPM, participants were instructed to continue their regular medication and carry out their usual activities.
Ambulatory BP was measured with the Mobil-O-Graph NG (IEM, Stolberg, Germany), an oscillometric device whose brachial BP detection unit was validated according to standard protocols and was shown to provide practically identical values with a widely used ABPM monitor [17]. The device was programmed to measure BP every 20 min between 07:00 a.m. and 10:59 p.m. (daytime) and every 30 min between 11:00 p.m. and 06:59 a.m. (nighttime) in all patients. Measurements were included in the analysis only if >80% of the recordings were valid, there were ≤2 nonconsecutive daytime hours with fewer than two valid measurements, and there was ≤1 nighttime hour without any valid recording [18]. Patients with inaccurate measurements were called in to undergo the ABPM again in another week. In order to minimize the possible effect of manual BP measurements, only measurements recorded at the prespecified time intervals at which the device was set to take measurements (not manual readings performed by the patients) were used in this analysis.
For the analyses of average BP levels and BPV, the daytime and nighttime periods were defined as previously reported [12] to enable comparisons. The daytime periods were those between 07:00 a.m. and 10:59 p.m. and the nighttime those between 11:00 p.m. and 06:59 a.m. of each day. For patients dialyzing in the first shift, the start of the 48-h period coincided with the start of the daytime, and the daytime periods included consecutive hours. However, for patients dialyzing in the second or third dialysis shifts, the daytime periods did not include consecutive time (e.g., for a patient starting dialysis in the second shift at 12:00 noon, the daytime of the first 24 h was considered the time between 12:00 noon and 10:59 p.m. followed by the next morning between 07:00 a.m. and 11:59 a.m.). The nighttime periods of the first and second 24 h were constant and were defined as the period between 11:00 p.m. and 06:59 a.m. of each day. Similarly, as PD patients were evaluated during their regular monthly visit, the ABPM may have started in the morning, noon, or afternoon hours, and therefore, the daytime 1 and daytime 2 periods were defined in a similar way as for HD patients.
BPV parameters
Standard deviation (SD), weighted SD (wSD), coefficient of variation (CV), average real variability (ARV), and variation independent of mean (VIM) were the BPV indices evaluated on the basis of data obtained from recordings of the Mobil-O-Graph device. The formulas used to calculate these parameters are described previously and are presented in the Supplementary Information (Supplementary Table 1) [12, 19, 20]. All BPV parameters were calculated separately for the first and second 24-h periods of the 48-h monitoring, as well as for the two daytime (daytime 1, daytime 2) periods and the two nighttime (nighttime 1, nighttime 2) periods of the 48-h monitoring, with the exception of wSD, which by definition cannot be calculated separately for daytime and nighttime.
The dipping pattern of nocturnal BP was calculated with the following formula: 1 − mean night/mean day ratio of SBP (%). Patients were divided into four categories: extreme dippers, with a nocturnal BP fall of >20%; dippers, with a fall of >10% and ≤20%; nondippers, with a fall of ≥0% and ≤10%; and reverse dippers, with a nocturnal increase in SBP. A simpler definition was also employed for purposes of comparison with earlier studies, in which patients were divided into two categories according to the presence (dippers and extreme dippers) or absence (nondippers and reverse dippers) of dipping.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 22.0 (SPSS Inc, Chicago, IL, USA). Continuous variables are presented as mean ± SD or as median (IQR) according to the normality of the distribution, which was determined with the Kolmogorov–Smirnov test for sample sizes ≥ 50 and the Shapiro–Wilk test for sample sizes < 50. Categorical variables are presented as absolute frequencies and percentages (n, %). One-way ANOVA or relevant nonparametric tests, where applicable, were used for between-group comparisons of continuous variables, and the Bonferroni post hoc test was used for pairwise comparisons. The chi-square test was used for comparisons of categorical variables. To evaluate the effect of dialysis modality and time on the trends of ambulatory BP levels and to determine whether an interaction between the two existed, we compared the mean differences between PD and HD of the mean hourly values in a 40-h period from 3:00 p.m. (first 24 h) to 7:00 a.m. (end of second 24 h), as well as daytime and nighttime SBP and DBP individual measurements, using two-way mixed ANOVA for repeated measurements. Greenhouse–Geiser correction was applied to overcome the violation of the sphericity assumption. When including cases and controls in a 1:2 ratio, a sample size of 35 PD patients and 70 HD patients was found to have 80% statistical power, for values of α = 0.05, to detect a mean difference of 7 mmHg between the groups regarding the main endpoint of the study (mean 48 h ambulatory systolic BP), assuming an SD value of 12 mmHg. Based on the above, we aimed to include 38 PD patients, 76 HD patients, and 38 CKD stage 2–4 patients. Probability values of p < 0.05 (two-tailed) were considered statistically significant for all comparisons.
Results
Baseline characteristics
Table 1 presents the demographic characteristics, dialysis vintage, comorbidities, antihypertensive medication, and visit laboratory data of all study participants. As expected, no differences were noted between the 38 PD and 76 HD patients for age, sex, or dialysis vintage or between the 38 PD patients and 38 CKD controls for age or sex. Moreover, there were no differences between the three groups with regard to any major comorbidity, except for coronary heart disease and heart failure, which were less frequent in CKD patients. As expected, there were significant differences in urine volume. Notably, significant differences between groups were observed in the total number of prescribed antihypertensive drugs (PD: 2.32 ± 1.34; HD: 1.72 ± 1.3; CKD: 2.66 ± 1.38, p = 0.001) and the use of ACEis/ARBs (52.6% vs 19.7% vs 65.8%, p < 0.001), CCBs (39.5% vs 31.6% vs 63.2%, p = 0.005), and diuretics (57.9% vs 23.7% vs 50%, p = 0.001).
BP levels in patients undergoing PD, HD, and patients with CKD
Significant differences were noted in SBP values between patients undergoing PD, patients undergoing HD, and their CKD controls (when the 48-h period or either of the 24-h periods of monitoring were compared with the 24-h recording in CKD patients), as shown in Table 2 (48-h period PD: 138.38 ± 20.97 mmHg; HD: 133.75 ± 15.5 mmHg; CKD: 125.52 ± 13.4 mmHg, p = 0.003; first 24-h period: 138.17 ± 21.96 vs 131.46 ± 15.87 vs 125.52 ± 13.4 mmHg, p = 0.006; second 24-h period: 138.53 ± 20.52 vs 136.17 ± 16.3 vs 125.52 ± 13.4 mmHg, p = 0.001). Pairwise comparisons for the 48-h period confirmed significantly higher SBP in patients under PD than in CKD controls and in patients under HD than in CKD controls (p = 0.003 and p = 0.041, respectively). However, there were no differences between PD and HD patients. Pairwise comparisons for the second 24-h period revealed similar results. Of note, ANOVA indicated significant differences between the three groups in SBP values during all daytime and nighttime periods of both 24 h. Concerning DBP, ANOVA showed significant differences between the three study groups only during nighttime 2 (79.82 ± 15.33 vs 79.33 ± 11.48 vs 72.82 ± 10.29 mmHg, p = 0.016), with higher nighttime 2 DBP values for patients under PD or HD than for CKD patients (p = 0.042 and p = 0.025, respectively).
Trajectories of BP levels by dialysis modality and time
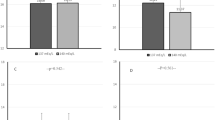
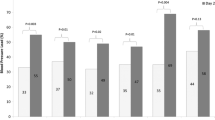
Figure 1 presents the estimated marginal means for the 40-h SBP and DBP levels in the PD and HD groups using two-way mixed ANOVA for repeated measurements. Figure 2 depicts the differences between PD and HD in estimated marginal mean ambulatory BP levels during daytime and nighttime periods of the first and second 24 h.
Estimated marginal mean levels of (a) daytime 1 SBP, (b) nighttime 1 SBP, (c) daytime 2 SBP, (d) nighttime 2 SBP, (e) daytime 1 DBP, (f) nighttime 1 DBP, (g) daytime 2 DBP and (h) nighttime 2 DBP during the first and the second days of the ambulatory recordings in patients on peritoneal dialysis or hemodialysis
Dialysis modality had no significant effect on SBP at the different time points of the 40-h period [F(1,67) = 0.713, p = 0.401, partial η2 = 0.011] or in the various time intervals studied (Figs. 1a and 2a–d). Similarly, dialysis modality had no significant effect on DBP during the 40-h period [F(1,67) = 0.382, p = 0.539, partial η2 = 0.006] or in the respective time intervals (Figs. 1b and 2e–h).
In contrast to dialysis modality, the effect of time on SBP levels was significant during the 40-h period [F(15.284,1023.999) = 2.12, p = 0.007, partial η2 = 0.031], daytime 1 [F(4.783,401.761) = 2.461, p = 0.035, partial η2 = 0.028], and daytime 2 [F(9.654,878.54) = 3.028, p = 0.001, partial η2 = 0.032] but was not significant for the rest of the study periods. Time significantly affected DBP levels during the 40-h period [F(16.925,1133.97) = 3.593, p < 0.001, partial η2 = 0.051] and all time intervals studied. There was no significant interaction between dialysis modality and time on SBP or DBP level over the 40-h period or the various intervals studied.
BPV indices in patients undergoing PD, HD and patients with CKD
All BPV indices of brachial SBP and DBP in the 48-h and two separate 24-h periods are presented in Table 3. There were no significant differences in BPV indices studied between PD and HD patients, but all BPV indices in these patients were numerically higher than those in CKD patients. ΑNOVA testing indicated significant differences with regard to second 24-h SBP-SD (p = 0.023), first 24-h SBP-wSD (p = 0.031), second 24-h SBP-wSD (p = 0.015), second 24-h SBP-VIM (p = 0.007), and both 24-h SBP-ARVs (first 24 h: 11.86 ± 3.19 vs 11.23 ± 3.45 vs 9.81 ± 2.49 mmHg, p = 0.016; second 24 h: 12.18 ± 4.11 vs 12.96 ± 4.57 vs 9.81 ± 2.49 mmHg, p = 0.001). Pairwise comparisons confirmed significantly higher SBP-wSD during the first 24 h, SBP-VIM during the second 24 h, and SBP-ARV during both 24 h in patients under PD than in CKD controls (p = 0.043, p = 0.027, p = 0.016, and p = 0.035, respectively) and higher second 24-h SBP-SD, SBP-wSD, SBP-VIM, and SBP-ARV in patients under HD than in CKD controls (p = 0.028, p = 0.017, p = 0.009, and p < 0.001, respectively). With regard to DBP-BPV indices, ANOVA revealed differences in DBP-wSD and DBP-ARV between the three groups during both 24 h, and similarly, pairwise comparisons showed higher first 24-h DBP-wSD and both 24-h DBP-ARV in patients under PD than in CKD controls and higher DBP-wSD and DBP-ARV during the second 24 h in patients under HD than in CKD controls.
BPV indices during daytime and nighttime periods
Differences in daytime and nighttime DBP-BPV indices are presented in Supplementary Table 2. ANOVA testing revealed significant differences between the three groups with regard to SBP-SD, SBP-VIM, and SBP-ARV during the daytime in both 24 h and during the nighttime only in the second 24 h (data and pairwise comparisons presented in Supplementary Table 2 in Supplementary Information).
Dipping pattern
Table 4 presents the dipping patterns (reverse dipper, nondipper, dipper, extreme dipper) during the first and second 24-h periods for the three study groups. It also includes dipping categories by only the presence or absence of dipping during the same study periods for purposes of comparison with previous studies. No significant differences were revealed in the dipping pattern between cases and controls.
Discussion
This is the first study to compare the BP profile and short-term BPV indices in patients undergoing PD with those of patients undergoing HD and patients with CKD. We observed that during all periods studied, SBP and DBP levels were not significantly different between PD and HD patients. SBP and DBP levels were numerically higher on all occasions in PD than in HD; during the first 24-h period, this difference between the two groups was broader in the pairwise comparison (first 24-h SBP 138.17 ± 21.96 vs 131.46 ± 15.87 mmHg). All BPV indices studied as well as the dipping profile were similar between PD and HD patients. Further, repeated-measurements ANOVA showed no effect of dialysis modality and no interaction between modality and time on ambulatory BP during any period studied. By ANOVA, SBP levels during most periods studied and DBP levels during nighttime 2 were found to be higher in PD and HD individuals than in CKD controls. Similarly, most BPV indices were significantly higher in dialysis patients than in CKD individuals.
To the best of our knowledge, only two older studies have compared ambulatory BP profiles in patients undergoing HD and patients undergoing PD. Rodby et al. examined 33 HD and 27 PD patients over a 48-h period and found that HD patients had higher average SBP (142.1 ± 16.3 vs 130.4 ± 17.1 mmHg, p < 0.01) but similar diurnal patterns compared with PD [6]. Tonbul et al. observed that the mean 44-h, daytime and nighttime BP values did not differ between 22 patients on HD and 24 patients on continuous ambulatory PD (CAPD), but BP values in HD were lower during the first day and higher during the second night compared with PD [5]. They also found that a significantly higher proportion of patients on CAPD were dippers than those on HD (88% vs 18%, p < 0.001). Our results are in the same direction as the study of Tonbul et al. but indicate a trend toward lower BP levels in HD individuals over both the first and second 24-h periods. We also observed similar dipping profiles, with the proportions of reverse dippers and nondippers adding up to 85–90% in both dialysis modalities. This could be related to the fact that our study had a more careful design, i.e., it included a larger number of dialysis individuals (n = 114) and followed a blinded matching procedure, which was not true for the two aforementioned studies. However, this could also be due to changes in BP control in HD patients over time, following increasing international emphasis on the need to attain better interdialytic control [21]. Another difference from previous studies may be that almost two-thirds of our PD patients were treated with automated PD (APD) during the night, and only one-third were treated with CAPD; this reflects the current situation of PD therapies in many countries [22].
As of this writing, no study has evaluated ambulatory BP levels in PD and HD patients in comparison with CKD individuals. Large observational studies have documented that office and ambulatory BP levels progressively increase and BP control deteriorates as CKD advances from stage 1 to stage 5, just before initiation of renal replacement therapies [1, 23]. In this study, we expand on previous observations showing that despite starting dialysis, which is a powerful tool for sodium and water removal, ambulatory BP levels in both PD and HD patients remain significantly higher than in their CKD counterparts. Importantly, the average number of prescribed antihypertensive drugs and the use of major classes such as ACEis/ARBs and CCBs were lower in PD and HD patients than in CKD controls, despite higher ambulatory BP, highlighting again a false impression on the part of treating physicians of adequate ambulatory BP control in dialysis.
In recent years, BPV has been recognized as an independent cardiovascular risk factor in the general population, as well as in patients with diabetes or hypertension [7]. Following this evidence, several studies in HD patients showed that long-term BPV, calculated from a few peridialytic BP readings, is independently associated with cardiovascular events and mortality [24, 25], which was an extremely striking observation, given that peridialytic BP levels per se show no association with outcomes [2, 26]. Similarly, high intradialytic BPV has been associated with all-cause and cardiovascular mortality in HD patients [27]. Data on short-term BPV derived from ABPM recordings in CKD patients are less common. A cross-sectional study in 16,546 hypertensive patients from the Spanish ABPM registry, of whom 6276 (38.9%) had CKD stage 1–5, showed that all SBP-BPV indices increased with advancing CKD stage, resulting in very high levels in predialysis CKD stage 5 [11]. In the first study in HD patients, we showed that systolic and diastolic SD, wSD, and ARV were even higher and further increased from the first to the second interdialytic day [12]. In addition, in a prospective cohort study of 227 HD patients, we showed that all systolic SD, wSD, CV, and ARV indices measured over the interdialytic interval were associated with the risk of cardiovascular events, independent of ambulatory BP levels [13].
As of this writing, no study has examined short-term BPV in patients undergoing PD. Thus, this study adds important information on the PD population by showing that all BPV indices were considerably higher than in individuals with CKD. In addition, during all periods studied, BPV indices were similar between PD and HD patients. This is an undoubtedly surprising finding, as conventional thinking would suggest that the continuous nature of PD would protect patients from the large BP fluctuations imposed by the intermittent nature of HD treatment [28, 29]. Thus, other factors, including volume overload and uncontrolled BP, may underlie this increased BPV in PD patients. Early, small studies on the dipping profile indirectly support our observations and point out that such factors may affect normal BP regulation in PD. Luik et al. compared day-night variation differences between a 3-day interdialytic interval in HD patients, a 24-h period in PD patients, and in subjects with normal kidney function, showing that the circadian pattern did not differ between the two dialytic modalities [30]. Disappearance of dipping is associated with volume overload as assessed with bioimpedance analysis in PD patients [31].
Among the strengths of this study is the careful design, including blinded matching for a set of crucial parameters; this resulted in the absence of differences between the two dialysis groups for a majority of important variables, including age, sex, dialysis vintage, and comorbidities. This is also the first study in the field including a group of carefully matched CKD patients. We used 48-h ABPM, which is a demanding procedure, in both PD and HD patients, which has not been done before in PD. We also used all modern indices of BPV, allowing us to capture different components of it. SD and CV are influenced by the mean BP level [32, 33] and the magnitude of the nocturnal BP fall [34]. The wSD index, by weighting the daytime and nighttime BP SD for the duration of these periods, selectively removes the contribution of the nocturnal BP fall [35]; this is of utmost importance, as 80–90% of ESRD patients have abnormal dipping profiles. The ARV index is sensitive to the individual BP measurement order, is not affected by the mean BP level, and, by taking into account the sequential order of BP changes, better captures intermittent BP fluctuations [33]. The main limitation of the study is its observational nature, which precludes drawing conclusions about cause-and-effect associations. The group of CKD patients underwent 24-h ABPM; this could have affected our findings, as daytime BP levels in the second day have been shown to be lower than in the first day in hypertensive patients undergoing 48-h monitoring [36]. The number of subjects may be considered small, and a larger sample may have rendered some borderline differences significant; however, this is by far the largest study in the field.
In conclusion, ambulatory BP levels were not different between PD and HD patients. If anything, BP was numerically higher in patients undergoing PD, a phenomenon sustained during both the first and second 24-h periods, despite BP rise during the interdialytic period in HD. During all periods studied, patients undergoing PD and HD exhibited higher SBP levels than CKD controls. Furthermore, all BPV indices were similar between PD and HD patients, in whom they were significantly higher than in CKD controls. These results clearly suggest that PD is no better than HD with regard to overall BP control or short-term BP fluctuations. Longitudinal studies evaluating the associations of ambulatory BP and BPV with future cardiovascular events in PD patients are needed to shed more light on the complex BP-related effects in this heavily diseased population.
References
Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121:332–40.
Sarafidis PA, Persu A, Agarwal R, Burnier M, de Leeuw P, Ferro CJ, et al. Hypertension in dialysis patients: a consensus document by the European Renal and Cardiovascular Medicine (EURECA-m) working group of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and the Hypertension and the Kidney working group of the European Society of Hypertension (ESH). Nephrol Dial Transpl. 2017;32:620–40.
Sarafidis PA, Mallamaci F, Loutradis C, Ekart R, Torino C, Karpetas A, et al. Prevalence and control of hypertension by 48-h ambulatory blood pressure monitoring in haemodialysis patients: a study by the European Cardiovascular and Renal Medicine (EURECA-m) working group of the ERA-EDTA. Nephrol Dial Transpl. 2019;34:1542–8.
Cocchi R, Degli Esposti E, Fabbri A, Lucatello A, Sturani A, Quarello F, et al. Prevalence of hypertension in patients on peritoneal dialysis: results of an Italian multicentre study. Nephrol Dial Transpl. 1999;14:1536–40.
Tonbul Z, Altintepe L, Sozlu C, Yeksan M, Yildiz A, Turk S. Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens. 2002;16:585–9.
Rodby RA, Vonesh EF, Korbet SM. Blood pressures in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis. 1994;23:401–11.
Parati G, Ochoa JE, Bilo G, Agarwal R, Covic A, Dekker FW, et al. Hypertension in chronic kidney disease part 2: role of ambulatory and home blood pressure monitoring for assessing alterations in blood pressure variability and blood pressure profiles. Hypertension. 2016;67:1102–10.
Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–57.
Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212–8.
Amari Y, Morimoto S, Iida T, Yurugi T, Oyama Y, Aoyama N, et al. Characteristics of visit-to-visit blood pressure variability in hemodialysis patients. Hypertens Res. 2019;42:1036–48.
Sarafidis PA, Ruilope LM, Loutradis C, Gorostidi M, de la Sierra A, de la Cruz JJ, et al. Blood pressure variability increases with advancing chronic kidney disease stage: a cross-sectional analysis of 16 546 hypertensive patients. J Hypertens. 2018;36:1076–85.
Karpetas A, Loutradis C, Bikos A, Tzanis G, Koutroumpas G, Lazaridis AA, et al. Blood pressure variability is increasing from the first to the second day of the interdialytic interval in hemodialysis patients. J Hypertens. 2017;35:2517–26.
Sarafidis PA, Loutradis C, Karpetas A, Tzanis G, Bikos A, Raptis V, et al. The association of interdialytic blood pressure variability with cardiovascular events and all-cause mortality in haemodialysis patients. Nephrol Dial Transpl. 2019;34:515–23.
Liu W, Wang L, Huang X, Yuan C, Li H, Yang J. Orthostatic blood pressure reduction as a possible explanation for memory deficits in dialysis patients. Hypertens Res. 2019;42:1049–56.
Loutradis C, Bikos A, Raptis V, Afkou Z, Tzanis G, Pyrgidis N, et al. Nebivolol reduces short-term blood pressure variability more potently than irbesartan in patients with intradialytic hypertension. Hypertens Res. 2019;42:1001–10.
Stürmer T, Brenner H. Degree of matching and gain in power and efficiency in case-control studies. Epidemiology. 2001;12:101–8.
Sarafidis PA, Lazaridis AA, Imprialos KP, Georgianos PI, Avranas KA, Protogerou AD, et al. A comparison study of brachial blood pressure recorded with Spacelabs 90217A and Mobil-O-Graph NG devices under static and ambulatory conditions. J Hum Hypertens. 2016;30:742–9.
Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–66.
Bikos A, Angeloudi E, Memmos E, Loutradis C, Karpetas A, Ginikopoulou E, et al. A comparative study of short-term blood pressure variability in hemodialysis patients with and without intradialytic hypertension. Am J Nephrol. 2018;48:295–305.
de la Sierra A, Banegas J, Bursztyn M, Parati G, Stergiou G, Mateu A, et al. Prognostic relevance of short-term blood pressure variability. Hypertension. https://doi.org/10.1161/HYPERTENSIONAHA.119.14508.
Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25:1630–46.
Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–44.
Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, et al. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013;62:285–94.
Brunelli SM, Thadhani RI, Lynch KE, Ankers ED, Joffe MM, Boston R, et al. Association between long-term blood pressure variability and mortality among incident hemodialysis patients. Am J Kidney Dis. 2008;52:716–26.
Shafi T, Sozio SM, Bandeen-Roche KJ, Ephraim PL, Luly JR, St Peter WL, et al. Predialysis systolic BP variability and outcomes in hemodialysis patients. J Am Soc Nephrol. 2014;25:799–809.
Parati G, Ochoa JE, Bilo G, Agarwal R, Covic A, Dekker FW, et al. Hypertension in chronic kidney disease part 1: out-of-office blood pressure monitoring: methods, thresholds, and patterns. Hypertension. 2016;67:1093–101.
Flythe JE, Inrig JK, Shafi T, Chang TI, Cape K, Dinesh K, et al. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61:966–74.
Inrig JK, Patel UD, Gillespie BS, Hasselblad V, Himmelfarb J, Reddan D, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50:108–18.
Georgianos PI, Sarafidis PA. Pro: should we move to more frequent haemodialysis schedules? Nephrol Dial Transpl. 2015;30:18–22.
Luik AJ, Struijk DG, Gladziwa U, von Olden RW, von Hooff JP, de Leeuw PW, et al. Diurnal blood-pressure variations in haemodialysis and CAPD patients. Nephrol Dial Transpl. 1994;9:1616–21.
Yang JH, Cheng LT, Gu Y, Tang LJ, Wang T, Lindholm MB, et al. Volume overload in patients treated with continuous ambulatory peritoneal dialysis associated with reduced circadian blood pressure variation. Blood Purif. 2008;26:399–403.
Parati G, Rizzoni D. Assessing the prognostic relevance of blood pressure variability: discrepant information from different indices. J Hypertens. 2005;23:483–6.
Pierdomenico SD, Di Nicola M, Esposito AL, Di Mascio R, Ballone E, Lapenna D, et al. Prognostic value of different indices of blood pressure variability in hypertensive patients. Am J Hypertens. 2009;22:842–7.
Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, et al. Factors that affect blood pressure variability. A community-based study in Ohasama, Japan. Am J Hypertens. 1997;10:1281–9.
Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–66.
Hermida RC, Calvo C, Ayala DE, Fernández JR, Ruilope LM, López JE. Evaluation of the extent and duration of the “ABPM effect” in hypertensive patients. J Am Coll Cardiol. 2002;40:710–7.
Acknowledgements
The authors would like to express their gratitude to the doctors and nurses of the two departments of nephrology where the study was undertaken for their help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alexandrou, M.E., Loutradis, C., Schoina, M. et al. Ambulatory blood pressure profile and blood pressure variability in peritoneal dialysis compared with hemodialysis and chronic kidney disease patients. Hypertens Res 43, 903–913 (2020). https://doi.org/10.1038/s41440-020-0442-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0442-0
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Accuracy of 24 h ambulatory blood pressure recordings for diagnosing high 44 h blood pressure in hemodialysis: a diagnostic test study
Hypertension Research (2024)
-
Dialysate sodium and short-term blood pressure variability in patients with intradialytic hypertension: a randomized crossover study
Journal of Human Hypertension (2024)
-
Diagnosis and treatment of hypertension in dialysis patients: a systematic review
Clinical Hypertension (2023)
-
Electrocardiographic measurements in children with pre-dialysis chronic kidney disease and undergoing kidney replacement therapy
European Journal of Pediatrics (2023)
-
Effect of patient gender on short-term blood pressure variability in hemodialysis patients
Journal of Human Hypertension (2022)