Abstract
Killer cell immunoglobulin-like receptors (KIR) consists of activating and inhibitory genes are essential for natural killer cell education. To determine the association of KIRs with susceptibility to invasive Breast cancer (BC), genotyping of 16 KIRs was performed by sequence-specific primers-polymerase chain reaction in 226 confirmed cases of BC with defined estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) status and 226 healthy controls (CNs). We observed a lower frequency of 2DL1 and 2DS4del along with increased frequency of 2DS4fl in cases compared to CNs. Further analysis revealed a higher frequency of KIR2DL2, 2DS1, 2DS2,3DS1 in ER+ cases, 2DL2, 2DL5 in PR+ and 2DL1 in HER2+ cases compared to CNs. The detrimental role of KIR2DS4fl was observed in ER+ and PR+ cases whereas 2DS4del confers protection against ER+, PR+, and HER2+ cases. We noted the predisposing role of Bx genotype, KIR2DS1, 2DS2, 2DS5, 2DL2, 2DL5 for lymphatic invasion in ER+ cases along with a higher rate of lymph node metastasis (LNM) in carriers of Bx genotype and KIR2DS1 in ER+ cases. We suggest a link between B haplotype associated genes with the increased risk of lymphatic invasion and LNM, particularly in ER+ cases of BC.
Similar content being viewed by others
Introduction
According to the World Health Organization report, “Breast cancer (BC) is the most commonly diagnosed cancer affecting 2.1 million of females annually, and it causes the largest number of cancer-related deaths among women” [1]. There are many factors which can influence the risk of breast cancer in women and can be categorized as intrinsic factor like age, gender, race, genetic, family history of cancer (FHC), benign breast conditions and extrinsic factors including lifestyle, dietary habits, medical interventions such as oral contraceptives and hormone replacement therapy over a long period of time [2].
The most frequent diagnosed cases of breast cancers among women are invasive/infiltrating ductal carcinoma (IDC) invading into glands, surrounding tissues and distant areas as well while the second type is in situ breast cancer also known as lobular neoplasia which is classified as lobular carcinoma in situ and ductal carcinoma in situ [3].
BC is heterogeneous tumor with several molecular characteristics which are defined based on immunohistochemistry (IHC) profile of hormone receptors for either estrogen receptor (ER), progesterone receptor (PR) aside from human epidermal growth factor 2 (HER2) which is considered as an important prognostic biomarker for breast cancer [4]. In addition to ER/PR/HER2 status, the tumor size, lymphatic and vascular invasion, lymph node involvement along with tumor grade and stage can affect treatment strategies and overall survival in patients with breast tumors [5]. Breast tumor microenvironment contains of cancer associated fibroblasts, myeloid derived stromal cells macrophages lymphocytes and NK cells in cooperation with soluble factors and extracellular matrix [6].
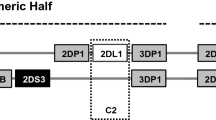
Natural killer (NK) cells are an essential part of innate immunity and play a key role in the immune surveillance and elimination of malignant/virally infected cells [7]. They act directly against tumors through cytolytic activity by releasing perforin, granzymes, or inflammatory cytokines. Indirectly, they can modulate the adaptive immune response using different mechanisms [8]. The activity of NK cell is influenced by a balance of the inhibitory and activating signals derived from a variety of cell surface receptors [9]. Among them, Killer cell Immunoglobulin-like Receptors (KIRs) which distinguish and bind to certain HLA-I ligands, have essential role in NK cell development, education and activation. KIR gene family consists of activating and inhibitory genes that encoded cell surface KIRs on mature NK cells and certain subset of T lymphocytes [10,11,12]. The exceptional diversity of KIR genes and number is the result of nucleotide sequence polymorphisms and different number of KIRs in individuals [13]. Based on individual gene content, two groups of haplotype A (fixed gene content; predominantly inhibitory) and B (variable gene content; predominantly activating) has been reported in previous researches [14]. Individuals can be grouped into AA genotype (homozygous for KIR A haplotype) or Bx genotype (homozygous for KIR BB and heterozygous for A/B) [15]. There are also framework genes (KIR3DL3, 3DP1, 2DL4, 3DL2) exist in all haplotypes which divide each haplotype into two regions: 3DL3 to 3DP1 delimit centromeric region and 2DL4 to 3DL2 delimit telomeric half. The KIR locus can also be classified as centromeric (Cen) and telomeric (Tel) gene motifs including Cen-A/A,/BB, AB or Tel-A/A,B/B,A/B [15, 16].
NK cell expresses inhibitory KIRs which recognize the Class I HLA ligands by a process known as education or licensing in order to distinguish unhealthy cells which contribute to missing-self recognition of tumoral cells and self-tolerance of NK cells [17]. The lacking of inhibitory KIR receptor or absence of its cognate ligand, leads to hyporesponsiveness of NK cell and failure in the elimination of target cells [17, 18]. Various expression of inhibitory and activating KIRs create different kind of educated NK cells with distinct ability to react against target cells with diverse expression levels of HLA, which plays a prominent role in the pathogenesis of various diseases [19]. Several studies previously reported associations of KIR genes with risk of BC [20,21,22].
This study aimed to investigate the genetic diversity of 16 KIR genes and determine the haplotypes, genotypes, clusters and Cen/Tel gene motifs in 226 confirmed cases of invasive breast cancer with determined ER, PR, and HER2 status along with 226 healthy controls (CNs). The specific objective of this research was to identify the KIRs impact on genetic predisposition or resistance to invasive breast cancer in Iranians and assess the clinical significance of KIRs in ER/PR/HER2 + breast cancer.
Results
Predisposing role of KIR2DS4fl and protecting role of KIR2DL1 and 2DS4del
The frequency of 16 KIR genes was compared between 226 BC patients and 226 CNs. Framework genes (KIR3DL3/3DL2/3DP1/2DL4) were presented in 100% of cases and CNs. A significantly lower frequency of 2DL1 (p = 0.0406, OR = 0.193, CI = 0.04–0.89) was observed in BC patients compared to CNs. Regarding KIR2DS4 variants, we found highly significant decrease of 2DS4del (p = 0.0001, OR = 0.39, CI = 0.24–0.61) and increased frequency of 2DS4fl (p = 0.0041, OR = 1.8, CI = 1.22–2.68) in BC group compared to CNs. These comparisons imply that KIR2DL1 and 2DS4del variant were associated with protection against BC, while 2DS4fl showed an increased risk of BC. In spite of the higher frequency of B haplotype associated genes in BC group and A haplotype associated genes in CNs, we did not observe any significant differences in other KIRs frequencies between cases with breast cancer and CNs (Table 1).
Increased frequency of Bx genotype and C4T4 subset in patients with breast cancer
According to the presence or absence of certain KIR genes, we defined AA and Bx genotypes. Bx genotypes were segregated into 4 genotypes based on C4/T4 gene clusters. To determine association of KIR haplotypes and genotypes with BC development, we compared their frequencies between cases and controls. We observed a significant decrease in carriers of AA genotype (p = 0.0464, OR = 0.62, CI = 0.4–0.97) and increased frequency of Bx genotype (p = 0.0464, OR = 1.6, CI = 1.03–2.48) in BC patients compared to controls. Moreover, BC patients had a significantly higher frequency of C4T4 genotype (p = 0.044, OR = 1.89, CI = 1.05–3.38) compared to CNs. Our results showed protective effect of AA genotype is associated with protection against BC whereas Bx and C4T4 genotypes is associated with increased susceptibility to BC (Table 2).
Overall, 48 different genotypes were identified in 452 participants (Supplementary Table 1) based on data from allele frequency database (http://www.allelefrequencies.net). 31 common genotypes were found in both BC patients and CNs and 17 genotypes were observed only in one individual.
Susceptibility of Cen-Bx and resistance of Cen-A/A carrier to breast cancer
Comparing the distribution of Cen/Tel gene motifs in cases with breast cancer and controls (Table 3), displayed a lower frequency of Cen-A/A carrier in cases than controls (p = 0.041, OR = 0.66, CI = 0.45–0.96) which conferred protection against breast cancer. On the contrary, we found that having Cen-Bx motif (Cen-A/B and Cen-B/B together) was associated with BC risk (p = 0.041, OR = 1.52, CI = 1.03–2.22). We found no significant association between Cen-A/B or Cen-B/B and telomeric motifs with BC risk.
Expression of ER/PR/HER2 alter the association levels of KIRs with breast carcinoma
To examine the role of KIRs in breast tumor development, we assessed the distribution of histological and clinical features such as ER/PR/HER2 status, invasion (lymphatic/vascular/perineural), LNM, histological grade and clinical stage among 226 breast cancer cases with Invasive Ductal Carcinoma (IDC) (Supplementary Table 2). For the purpose of assessing the impact of ER/PR/HER2 status on the association levels between KIRs and the risk of breast cancer, the cases were classified to several groups considering the IHC-based classification of ER/PR/HER2 expression (Table 4).
In addition to the predisposing and protecting role of KIR2DS4fl and 2DS4del respectively which was observed in comparison of whole cases with controls, we found a higher frequency of KIR2DL2, 2DS1, 2DS2, and 3DS1 in cases with ER-positive and 2DL2, 2DL5 in PR-positive and 2DL1 in HER2 positive breast cancer compared to controls. Whilst the associations of certain KIRs varied among other groups (ER+ PR+, ER+ PR+ HER2 + , ER + HER2 + , PR + HER2 +), there was one thing common to all which was the protective role of 2DS4del and AA genotype against all subtypes of breast carcinoma (Table 4). In contrast some KIRs were unique to each of ER, PR or HER2 positive groups. For example, the KIR2DS1 (p = 0.036, OR = 1.89, CI = 1.2–2.9), 2DS2 (p = 0.036, OR = 2.12, CI = 1.3–3.3) and 3DS1 (p = 0.046, OR = 1.55, CI = 1.1–2.3) occurred more frequently in ER+ and the 2DL5 (p = 0.036, OR = 1.64, CI = 1.05–2.5) in PR+ cases while 2DL1 carriers (p = 0.038, OR = 0.15, CI = 0.03–0.8) displayed lower frequency in HER2+ patients with breast cancer compared to controls. Furthermore, comparing the carrier frequencies of KIRs in ER-PR-HER2- group (triple-negative breast cancer), we found that 2DL5 occurred less frequently in this group compared to others (p = 0.015, OR = 0.14, CI = 0.03–0.7), however, considering the low sample size of this group (n = 10), the observed finding may not be generalized and more sample size is needed for validation of this result.
The detrimental role of B haplotype associated genes (KIR2DS1, 2DS2, 2DS5, 2DL2, 2DL5) in lymphatic invasion and lymph node metastasis
To assess the clinical significance of KIR genes and genotypes in pathogenesis of breast cancer, we identified their association with histological features as lymphatic, vascular and perineural invasion as well as lymph node metastasis (LNM) in patients with breast cancer. We found that lymphatic invasion occurred more frequently in carriers of Bx genotype (p = 0.0056, OR = 2.93, CI = 1.4–6.1) along with KIR2DS1 (p = 0.032, OR = 1.9, CI = 1.09–3.32), 2DS2 (p = 0.032, OR = 1.95, CI = 1.09–3.32), 2DS5 (p = 0.049, OR = 1.84, CI = 1.04–3.26), 2DL2 (p = 0.023, OR = 2.03, CI = 1.4–3.62), and 2DL5 (p = 0.022, OR = 2.03, CI = 1.14–3.6); besides, the carriers of KIR2DS1 (p = 0.0118, OR = 2.2, CI = 1.22–3.9) had higher rate of LNM compared to cases lacking these certain KIRs.
To explore the impact of ER, PR, and HER2 expression on the occurrence of lymphatic/vascular invasion and LNM, we compared these histopathologic features among ER+ , PR+ and HER2+ cases with breast cancer as displayed in Table 5. The most striking observation to emerge from the data comparison was that possessing Bx genotype increased the risk of lymphatic invasion for 16.1 times (p < 0.0001, OR = 16.1, CI = 3.6–71.6) in ER+ cases and for 9.36 (p = 0.0002, OR = 9.36, CI = 2.6–33.5) in PR+ breast cancer while there was no observed difference in HER2 cases with breast tumor.
Interestingly, the similar results were revealed among ER+ cases and whole patients with BC as well which was a positive association of carrying KIR2DS1, 2DS2, 2DS5, 2DL2, 2DL5 with lymphatic invasion occurrence while in the case of PR expression, these associations were limited to KIR2DS2, 2DL2, 2DL5, and once again there was no significant difference in the expression of HER2+ cases. Consequently, we noted a higher rate of LNM in carriers of Bx genotype and particularly KIR2DS1 in just ER+ cases as can be seen from the Table 5.
The results obtained from the further analysis on vascular and perineural invasion indicated the protecting role of Bx genotype against vascular invasion among ER+ cases whilst no significant association were found between KIRs and invasions.
Discussion
In the present study, we examined the association of KIR genes, genotypes and the centromeric/telomeric gene motifs on the susceptibility or resistance to breast cancer. According to our results, it is assumed that KIR2DL1 was associated with protection against breast cancer, which is in line with decreased frequency of KIR2DL1 in BC patients in Turkish population [20] and protecting role of KIR2DL1 combined with HLA-C2 in Saudi Arabian patients with BC [21]. These data suggest that 2DL1 gene may protect individuals against breast cancer development. In another study conducted by Alomar et al., a lower BC risk was shown in the presence of KIR2DL5A, 2DS2, 2DS3, and combination of KIR2DL1 and HLA-C2 as well [21]. Jobim et al. reported an association between KIR2DL2 with increased susceptibility to BC in Brazilian population [22]. The beneficial and harmful effects of some inhibitory KIRs have been revealed in different tumor types in the Fars population. In contrast to the potential protective role of KIR2DL2 in bladder cancer [23], the detrimental role of KIR3DL1 in basal cell carcinoma (BCC) [24], KIR2DL5 in meningioma [25], colorectal adenocarcinoma (CRC) [26], and head and neck squamous cell carcinoma (HNSCC) [27] have been reported in Fars Province of Iran. Regarding the clinical aspects of cancers, the association of KIR2DL2 with increased LNM and advanced clinical stage of HNSCC [28] as well as decreased rate of metastasis in CRC cases who had more inhibitory KIRs compared with activating genes [26] have been reported in the same population in our previous studies.
Considering KIR2DS4 variants, we observed a significant increase in 2DS4fl and a higher decrease in 2DS4del variant in BC patients compared to controls which is compatible with our previous results in BCC [24], meningioma [25], CRC [26] and HNSCC [27] in Fars population. In other studies, 2DS4fl was associated with higher viral load and HIV-1 transmission rate [29], susceptibility to chronic myeloid leukemia (CML) [30] and occurrence of acute Graft Versus Host Disease [31]. Although, the function of KIR2DS4del as a soluble form of 2DS4fl receptor is currently unknown, it is presumable that it can neutralize the deleterious effect of 2DS4fl receptor noticed in this study.
Next, we found that AA genotype showed protection against BC which was similar to the findings of previous studies on Iranian patients with meningioma [25] and HNSCC [27]. In a study in Chinese Southern Han population, AA genotype was associated with protection against acute lymphoblastic leukemia, acute myeloid leukemia, and CML and beyond that, NK cells from carriers of AA genotype have shown more cytolytic activity against leukemic cells than other genotypes [32]. Moreover, the favorable role of AA genotype has been reported in advanced-stage of classic Hodgkin lymphoma [33]. The inhibitory KIRs are predominant in AA genotype which can bind to HLA-C and mediate in NK cell education process which is necessary to generate mature and functional NK cells. Many studied on breast cancer have been reported the downregulation of HLA-I on tumor cells in order to evade from T cell responses which leads to damping inhibitory signals of KIR/HLA-I ligand and sensitize tumor cells to NK cell-mediated antibody-dependent cell-mediated cytotoxicity [34,35,36]. On the other hand, in consistent with our data, prior studies have been reported that Bx genotype conferred susceptibility to diseases which is associated with chronic inflammation or infectious agents including gastric cancer [37], meningioma [25], and HNSCC [27]. Bx genotype contain mostly activating KIRs, and exhibit highly divergent NK cell phenotypes due to stochastic expression of KIRs on NK cell surface. NK cells from Bx genotype carriers have a high activation threshold, and most likely to become hyporesponsive and lose the ability of eliminating tumor cells [38]. It has been shown that NK cell dysfunction contribute in breast cancer progression because of immunosuppressive microenvironment of breast tumor which may induce overexpression of inhibitory KIRs and downregulation of activating KIRs, along with the presence of factors like transforming growth factor beta which may diminish NK cell antitumor function [39].
To investigate the effect of centromeric or telomeric motifs on BC risk, we compared their frequencies between cases and controls. It seems that the protective effect of AA genotype in BC patients is due to the presence of Cen-AA motif in which 2DL1 is in strong linkage disequilibrium with 2DL3 and both are inhibitory KIRs which are involved in NK cell education. Gooneratne et al. suggested that NK cells educated by 2DL1/HLA-C2 were associated with enhanced anti-HIV-I-antibody-mediated NK cell response against HLA-I deficient target cells [40]. In contrast, in the Chinese Han population presence of Cen-B in donors had improved survival after unrelated donor hematopoietic stem cell transplantation [41]. In another study, it has been reported that Cen-B motif was negatively associated with Vogt-Koyanagi-Harada disease in Japanese [42]. It has been revealed that NK cell overactivation in Tel-B motif carriers increased risk of syphilis [43] and it confers susceptibility to gastric cancer [37] while it protect the carriers from CMV infection following kidney transplantation [44]. A study on meningioma patients in Fars population showed the predisposing role of Cen AB and Tel AB in developing meningioma along with protective role of Cen AA, Tel AA, and Tel BB against it when there was no association between presence of Tel-B motif and BC risk in our study. NK cells with Cen-Bx motif maybe incapable of developing educated NK cells to react against breast cancer cells.
The inconsistency observed in previous studies on association of KIRs and breast cancer may be due to the fact that they did not take account the histologic type of the breast tumor and most importantly the expression of estrogen, progesterone and HER2 receptors which may influence physiological functions of the immune cells. It is known that sex and reproductive status of individual as biological factors can affect the function of both innate and acquired immune system [45]. To control for bias, categorization of patients was carried out according to their ER/PR/HER2 status of tumor. The most striking result to emerge from our data was different associations of KIRs with the risk of breast cancer according to the ER/PR/HER2 status of tumor and these associations were more pronounced among ER+ cases with IDC with the predisposing role of KIR2DL2, 2DS1, 2DS2, 3DS1, 2DS4fl, and the protecting role of 2DS4del. There were some similarities among these groups, particularly the higher frequency of Bx genotype carriers and lower frequency of 2DS4del in all patient groups compared to controls. Further to this, some associations were unique to each of ER, PR, or HER2 positive groups. For example, the KIR2DS1, 2DS2 and 3DS1 in ER+, the 2DL5 in PR+ and 2DL1 in HER2+ cased with invasive ductal carcinoma. There is some evidence in the literature for hormone-biased immune response and several studies investigated the suppressive effect of estradiol on NK cells activity [46], it has been shown that estrogen has an immunoenhancing effect on immune cells such as altering the expression of HLA-I on both tumor and normal breast cells [47]. Hence, it is probable that it can alter the threshold of NK cell activation via KIR/HLA interactions as well which may somewhat describe different results of KIRs association with breast cancer development based on ER/PR/HER2 status. Furthermore, in clinical aspects, the lymphatic invasion as a typical channel for tumor metastasis was associated with the presence of Bx genotype along with possessing of KIR2DS1, 2DS2, 2DS5, 2DL2, and 2DL5 in whole patients with BC and in ER+ cases as well; besides, the carriers of KIR2DS1 had higher rate of LNM in mentioned groups compared to cases lacking 2DS1.
These findings provide further support for the hypothesis that the expression of ER, PR, and HER2 receptors on breast tumor cells may alter physiological condition in tumor microenvironment leading to behavioral differences of activating and inhibitory KIRs which regulate tumor cell recognition, NK cell activation and their effector functions. However, more investigations on this issue needs to be undertaken before the association of KIRs with breast cancer development and histopathological characteristics is more clearly understood. This study provides the first comprehensive assessment of the association between KIR gene/genotypes and breast cancer risk and relevant clinical significance as well. We found that Bx genotype carriers may be more susceptible to BC development and progression that could be due to acquisition of hyporesponsive NK cell repertoire in an immunosuppressive microenvironment which is unable to mount a robust NK cell response against tumor. The current research has only examined the KIRs system and has not been able to determine their cognate HLA-I ligands, hence, larger cohorts of ER/PR/HER2 subtypes of invasive breast cancer along with defining both KIRs and HLA-I ligands are required to justify this hypothesis. It is also important to consider the allelic polymorphism of KIR genes which can alter the ligand affinity and signal transduction which relates to various educational levels in NK cells. Regarding the complex role of KIRs on NK cell response, further investigations on KIRs polymorphism at allelic level along with functional analysis is required to reveal their association with developing BC which may have potential effect in the treatment of breast cancer. We hope that these findings will serve as a framework for future explorations of KIRs role in different subtypes of breast cancer which can make several noteworthy contributions to cancer immunotherapy and personalized medicine.
Materials and methods
Study population
We conducted a case control study on 452 individuals in Fars province of Iran. Pathologically confirmed cases of 226 patients with IDC breast cancer were recruited from Faghihi hospital, along with 226 age-sex matched CNs who did not have an FHC from Motahari clinic. The mean age of cases was 47.64 ± 13.64 and 47.81 ± 13.88 for CNs. The expression of ER, PR, HER2 status was previously determined by using IHC detection system. The demographic data along with histological and clinical presentations were gathered in datasheets. The histopathological assessments, KIRs genotyping and statistical analysis were carried out blind.
The research has been performed in accordance with the Declaration of Helsinki and was ethically approved by Medical Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1397.573) and informed consent was obtained from all research participants.
KIR genotyping
In total, 5 ml blood samples were collected in EDTA containing tubes. Genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen, Germany) as stated by manufacturer’s instructions. We applied a set of specific KIR primers designed by Vilches et al. [48] and Ashouri et al. [49] (Supplementary Table 3.1) using in-house sequence-specific primers-polymerase chain reaction (PCR-SSP) method was used for typing of 16 KIR genes and determining KIR2DS4 full (2DS4fl) and deleted (2DS4del). 100 ng of genomic DNA along with 5 μl master mix (Ampliqon A/S, Denmark) and certain amounts of primers were used for each reaction mix as displayed in Supplementary Table 3.2. PCR was performed in TECHNE Thermal cycler (USA) according to thermal conditions in Supplementary Table 3.3. In total, 7 μl of each PCR product was mixed with KBC power load (Kawsar Biotech Company, Iran) and then loaded in 2.5% agarose gel and in 4% gel for segregation of the KIR2DS4 variants. A UV transilluminator (SMART, UK) was used for detecting target DNA by comparing with the mobility of pUC19 and 50 bp DNA Ladder (Fermentas, Lithuania). We applied the reference samples from DNA exchange program provided by Prof. Raja Rajalingam, Ph.D. at the University of California, San Francisco to check the accuracy of typing [27]. Furthermore, KIR genotypes, haplotypes, clusters and motifs (Cen/Tel) were assigned according to previous studies [15, 25].
Statistical analysis
Sample size were chosen for ensuring high power of study according to our previous researches on investigating KIRs role in certain tumors and considering the adequate sample size for performing these type of research studies in the existing literature. Our data was analyzed by SPSS (IBM, US) Version 16.0. The frequency differences of KIR genes, haplotypes, genotypes, clusters, centromeric and telomeric halves between BC patients and CNs were tested by two-tail chi-square test and considering Yatesʼ continuity correction. P values ≤ 0.05 were considered statistically significant. OR with 95% CI were estimated to assess the precision of associations between cases and CNs. Whereas, we had multiple associations/outcomes, each of them had different biologically relevant effect sizes, hence, the statistical powers were different between outcomes for specified sample size.
References
Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–30.
Kamińska M, Ciszewski T, Łopacka-Szatan K, Miotła P, Starosławska E. Breast cancer risk factors. Prz menopauzalny= Menopause Rev. 2015;14:196.
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66.
Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ. 2015;351:h4901.
Dias AS, Almeida CR, Helguero LA, Duarte IF. Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer. 2019;121:154–71.
Pfeifer C, Highton AJ, Peine S, Sauter J, Schmidt AH, Bunders MJ, et al. Natural killer cell education is associated with a distinct metabolic profile. Front Immunol. 2018;9:3020.
Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9.
Sternberg-Simon M, Brodin P, Pickman Y, Önfelt B, Kärre K, Malmberg K-J, et al. Natural killer cell inhibitory receptor expression in humans and mice: a closer look. Front Immunol. 2013;4:65.
Augusto DG. The impact of KIR polymorphism on the risk of developing cancer: not as strong as imagined? Front Genet. 2016;7:121.
Dorak MT. Role of natural killer cells and killer immunoglobulin-like receptor polymorphisms. Methods Mol. Med. 2007;134:123–44.
Pende D, Falco M, Vitale M, Cantoni C, Vitale C, Munari E et al. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. 2019;10.
Rajalingam R. Diversity of killer cell immunoglobulin-like receptors and disease. Clin Lab Med. 2018;38:637–53.
Uhrberg M. The KIR gene family: life in the fast lane of evolution. Eur J Immunol. 2005;35:10–5.
Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–9.
Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010;129:8–19.
Ivarsson MA, Michaëlsson J, Fauriat C. Activating killer cell Ig-like receptors in health and disease. Front Immunol. 2014;5:184.
Rajagopalan S, Long EO. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med. 1997;185:1523–8.
Boudreau JE, Hsu KC. Natural killer cell education in human health and disease. Curr Opin Immunol. 2018;50:102–11.
Ozturk OG, Gun FD, Polat G. Killer cell immunoglobulin-like receptor genes in patients with breast cancer. Med Oncol. 2012;29:511–5.
Alomar SY, Alkhuriji A, Trayhyrn P, Alhetheel A, Al-jurayyan A, Mansour L. Association of the genetic diversity of killer cell immunoglobulin-like receptor genes and HLA-C ligand in Saudi women with breast cancer. Immunogenetics. 2017;69:69–76.
Jobim MR, Jobim M, Salim PH, Portela P, Jobim LF, Leistner-Segal S, et al. Analysis of KIR gene frequencies and HLA class I genotypes in breast cancer and control group. Hum Immunol. 2013;74:1130–3.
Jamali E, Barani S, Yousefinejad F, Ariafar A, Talei GR, Ghaderi A. KIRs gene content diversity in Iranians with urothelial bladder cancer. Mol Biol Rep. 2018;45:713–9.
Yousefinejad F, Jowkar F, Barani S, Jamali E, Mahmoudi E, Ramezani A, et al. Killer cell immunoglobulin-like receptors (KIRs) genotype and haplotype analysis in iranians with non-melanoma skin cancers. Iran Biomed J. 2019;23:330.
Barani S, Taghipour M, Ghaderi AJI. Positive association of Bx genotype, KIR2L5, KIR2DS5 and full-length KIR2DS4 with the risk of meningioma. Immunobiology. 2020;225:151900.
Barani S, Hosseini SV, Ghaderi A. Activating and inhibitory killer cell immunoglobulin like receptors (KIR) genes are involved in an increased susceptibility to colorectal adenocarcinoma and protection against invasion and metastasis. Immunobiology. 2019;224:681–6.
Barani S, Khademi B, Ashouri E, Ghaderi A. KIR2DS1, 2DS5, 3DS1 and KIR2DL5 are associated with the risk of head and neck squamous cell carcinoma in Iranians. Hum Immunol. 2018;79:218–23.
Barani S, Khademi B, Ghaderi A. KIR2DS4, KIR2DL2, and KIR2DS4del are linked with basaloid tumors, lymph node metastasis, advanced stage and metastatic risk in head and neck squamous cell carcinoma. Exp Mol Pathol. 2020;112:104345.
Chavan VR, Chaudhari D, Ahir S, Ansari Z, Mehta P, Mania-Pramanik JJBri. Variations in KIR genes: a study in HIV-1 serodiscordant couples. Biomed Res Int. 2014;2014:891402.
Giebel S, Nowak I, Wojnar J, Krawczyk-Kulis M, Holowiecki J, Kyrcz-Krzemien S, et al. Association of KIR2DS4 and its variant KIR1D with leukemia. Leukemia. 2008;22:2129.
Bao X, Hou L, Sun A, Qiu Q, Yuan X, Chen M, et al. The impact of KIR2DS4 alleles and the expression of KIR in the development of acute GVHD after unrelated allogeneic hematopoietic SCT. Bone Marrow Transpl. 2010;45:1435.
Deng Z, Zhao J, Cai S, Qi Y, Yu Q, Martin MP, et al. Natural killer cells offer differential protection from three kinds of leukemia in southern Chinese Han. Front Immunol. 2019;10:1646.
La Nasa G, Greco M, Littera R, Oppi S, Celeghini I, Caria R, et al. The favorable role of homozygosity for killer immunoglobulin-like receptor (KIR) A haplotype in patients with advanced-stage classic Hodgkin lymphoma. J Hematol. 2016;9:26.
Perez M, Cabrera T, Lopez Nevot M, Gomez M, Peran F, Ruiz‐Cabello F, et al. Heterogeneity of the expression of class I and II HLA antigens in human breast carcinoma. Int J Immunogenetics. 1986;13:247–54.
Wintzer H, Benzing M, Von Kleist S. Lacking prognostic significance of β 2-microglobulin, MHC class I and class II antigen expression in breast carcinomas. Br J cancer. 1990;62:289.
Fleming K, McMichael A, Morton J, Woods J, McGee JOD. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981;34:779–84.
Hernandez EG, Partida-Rodriguez O, Camorlinga-Ponce M, Nieves-Ramirez M, Ramos-Vega I, Torres J, et al. Genotype B of killer cell immunoglobulin-like receptor is related with gastric cancer lesions. Sci Rep. 2018;8:6104.
Rajalingam R. Human diversity of killer cell immunoglobulin-like receptors and disease. Korean J Hematol. 2011;46:216–28.
Mamessier E, Sylvain A, Thibult M-L, Houvenaeghel G, Jacquemier J, Castellano R, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Investig. 2011;121:3609–22.
Kristensen AB, Kent SJ, Parsons MS. Contribution of NK cell education to both direct and anti-HIV-1 antibody-dependent NK cell functions. J Virol. 2018;92:e02146–17.
Bao X, Wang M, Zhou H, Zhang H, Wu X, Yuan X, et al. Donor killer immunoglobulin-like receptor profile Bx1 imparts a negative effect and centromeric B-specific gene motifs render a positive effect on standard-risk acute myeloid leukemia/myelodysplastic syndrome patient survival after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2016;22:232–9.
Levinson RD, Yung M, Meguro A, Ashouri E, Yu F, Mizuki N, et al. KIR and HLA genotypes implicated in reduced killer lymphocytes immunity are associated with Vogt-Koyanagi-Harada disease. PloS ONE. 2016;11:e0160392.
Zhuang Y, Song Y, Zhu C, Zhang Y, Wang D, Nie X, et al. Association of KIR genotypes and haplotypes with syphilis in a Chinese Han population. Scand J Immunol. 2012;75:361–7.
Stern M, Hadaya K, Hönger G, Martin PY, Steiger J, Hess C, et al. Telomeric rather than centromeric activating KIR genes protect from cytomegalovirus infection after kidney transplantation. Am J Transplant. 2011;11:1302–7.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626.
Segovia-Mendoza M, Morales-Montor J. Immune tumor microenvironment in breast cancer and the participation of estrogens and its receptors into cancer physiopathology. Front Immunol. 2019;10:348.
Lee HJ, Song IH, Park IA, Heo S-H, Kim Y-A, Ahn J-H, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016;7:30119.
Vilches C, Castano J, Gomez‐Lozano N, Estefania E. Facilitation of KIR genotyping by a PCR‐SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70:415–22.
Ashouri E, Ghaderi A, Reed E, Rajalingam R. A novel duplex SSP–PCR typing method for KIR gene profiling. Tissue Antigens. 2009;74:62–7.
Acknowledgements
The study was financially supported by Shiraz University of Medical Sciences [grant number: 1396-01-01-16590] and partly by Shiraz Institute for Cancer Research [grant number: ICR-100-509]. Ethical approval of the research was confirmed by Medical Ethics Committee of Shiraz University of Medical Sciences [IR.SUMS.REC.1397.573]. The present study was part of a MSc thesis written by Marjan Hematian Larki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hematian Larki, M., Barani, S., Talei, AR. et al. Diversity of KIRs in invasive breast cancer patients and healthy controls along with the clinical significance in ER/PR/HER2+ patients. Genes Immun 21, 380–389 (2020). https://doi.org/10.1038/s41435-020-00117-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-020-00117-1
- Springer Nature Limited
This article is cited by
-
KIR-HLA gene diversities and susceptibility to lung cancer
Scientific Reports (2022)
-
Coexistence of inhibitory and activating killer-cell immunoglobulin-like receptors to the same cognate HLA-C2 and Bw4 ligands confer breast cancer risk
Scientific Reports (2021)