Abstract
The bone marrow has been widely recognised to host a unique microenvironment that facilitates tumour colonisation. Bone metastasis frequently occurs in the late stages of malignant diseases such as breast, prostate and lung cancers. The biology of bone metastasis is determined by tumour-cell-intrinsic traits as well as their interaction with the microenvironment. The bone marrow is a dynamic organ in which various stages of haematopoiesis, osteogenesis, osteolysis and different kinds of immune response are precisely regulated. These different cellular components constitute specialised tissue microenvironments—niches—that play critical roles in controlling tumour cell colonisation, including initial seeding, dormancy and outgrowth. In this review, we will dissect the dynamic nature of the interactions between tumour cells and bone niches. By targeting certain steps of tumour progression and crosstalk with the bone niches, the development of potential therapeutic approaches for the clinical treatment of bone metastasis might be feasible.
Similar content being viewed by others
Background
The dissemination of cancer cells from the primary growth site is a necessary first step in metastasis. However, very few disseminated tumour cells (DTCs) manage to establish secondary tumours at distant organs.1 In addition to evolving to acquire intrinsic invasive properties to leave the primary tumour, when these DTCs reach their destination, they need to interact productively with new organ microenvironments to facilitate survival and colonisation.2 Despite a steady increase in the survival rate of cancer patients owing to advances in early detection and better treatment options, an effective treatment for metastatic disease —the major cause of death among cancer patients—is still lacking.3,4 One of the most common sites for metastasis is the skeleton, and bone metastasis occurs in 65–80% of patients with advanced breast or prostate cancers,5 as well as being frequent in patients with lung and many other cancers. Bone metastases can be classified as osteoblastic or osteolytic, depending on whether bone deposition or bone destruction, respectively, predominates, although in many cases the metastases are a mix of these phenotypes. Osteolytic metastases, which tend to be more aggressive, are more often derived from breast cancer, multiple myeloma, melanoma, and non-small cell lung cancer, whereas osteoblastic lesions tend to occur more frequently as a result of metastasising prostate cancer and small cell lung cancer.6 The most frequent sites of the lesions include the vertebrae, sacrum, and the proximal femur and sporadically include the bones distal to the knee and elbow.7,8 Complications from bone metastasis, such as bone pain, fracture, hypercalcaemia and spinal cord compression, constitute some of most devastating cancer-related morbidities.3,9
Bone metastasis is a complex multistep process in which circulating (disseminated) tumour cells extravasate, enter the bone marrow compartment and occupy one of two specialised microenvironments or ‘niches’—the perivascular niche, which is consist of perivascular cells and endothelial cells of the sinusoids in the bone marrow and the endosteal niche, which is composed of key bone cells including the osteoblast lineage cells and osteoclast lineage cells on the bone surface. This step is followed by a period of dormancy in which the DTCs adapt, survive and reside in the bone for a long period of time—possibly years or even decades.10,11 The third step is reactivation of cancer cells that have acquired the ability to escape from dormancy, followed by their outgrowth to form micrometastasis, eventually leading to the development of overt bone metastasis.12
Our knowledge of these earlier steps of bone metastasis formation, including early seeding, survival, dormancy and reactivation, remains very limited. Yet, this window during early bone metastasis is when tumour cells are likely to be most vulnerable to therapeutic intervention, and represents the best opportunity for a cure. In this review, we will describe the properties of bone marrow niche cells that make bone a fertile ‘soil’ to host metastatic tumour growth. Both the endosteal niches and the perivascular niches have been reported to impact the three essential properties of DTCs: survival, temporary growth arrest, and therapeutic resistance.13 We will summarise the current understanding of the molecular mechanisms that are involved in early seeding, dormancy and outgrowth and therapeutic resistance of DTCs during bone metastasis. Finally, we will discuss how these molecular insights might facilitate the development of new clinical strategies to prevent or treat bone metastasis.
An overview of the bone in normal physiology
The balance of osteoclast and osteoblast activity is critical for the maintenance and remodelling of the skeleton system. During postnatal bone remodelling, the formation of osteoblasts, which are derived from skeletal stem cells (SSCs) and bone-lining cells, is tightly regulated by a series of transcription factors and signalling pathways. Runx2 and osterix are key transcription factors that are responsible for promoting the commitment of bone marrow stromal cells (BMSCs) to osteoprogenitors and their continued differentiation to mature osteoblasts,14,15 which secrete bone matrix proteins for bone formation.16 Osteoblasts finally become osteocytes, which account for 95% of all bone cells.17,18 Osteoclasts are large, multinucleated cells of haematopoietic origin. Osteoclast maturation requires various cytokines, including the receptor activator of nuclear factor κB ligand (RANKL) and the macrophage colony-stimulating factor (M-CSF or CSF-1), which are produced by neighbouring osteogenic cells19; mice that lack CSF-1 developed osteopetrosis as a consequence of a lack of bone-resorbing osteoclasts.20,21 Osteoclast-mediated bone resorption and osteoblast-mediated bone formation are well balanced under physiological conditions. Furthermore, the osteogenic lineage cells and osteoclasts form an endosteal niche to maintain bone remodeling.22
As the tissue that hosts haematopoiesis and osteogenesis in the human body, bone contains a variety of resident cell types, the dynamic interactions between which regulate the highly active environment of the bone marrow itself, and are crucial in controlling blood and bone formation.23,24,25 In addition, bone marrow is an important source of developmental and self-renewal signals, such as members of the transforming growth factor β (TGF-β) family, Wnt, Notch, Hedgehog, and CXC motif chemokine 12 [CXCL12, also known as stromal cell-derived factor-1 (SDF-1)], which can facilitate tumour cell colonisation and outgrowth in the bone.26,27,28,29
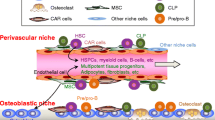
Research has focused on two niches in the bone marrow: the perivascular niche, which is close to the sinusoids in the bone marrow and the endosteal niche, which is localised on the surface of cortical and trabecular bone (Fig. 1). These two niches interact with each other to maintain normal bone homoeostasis,30,31,32 as well as each hosting two adult stem cell populations—haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). Disruption of the established bone niches and homoeostasis often results in bone diseases such as osteoporosis, osteopetrosis, osteoarthritis and bone malignancies.33,34 Extensive studies have elucidated both the location and cellular compartments of HSC and MSC niches. Using new imaging methods for niches, lineage-tracing analyses of the sources of key HSC-niche-supporting factors such as stem cell factor (SCF)35 and CXCL1236 in genetically modified mice have observed different HSC frequencies in different niche situations.
The perivascular niche and its role in bone metastases
The perivascular niche, which usually consists of BMSCs, endothelial cells and pericytes that closely line the sinusoids, has been shown to be important for maintaining the survival and full potential of HSCs30,37,38,39,40 (Fig. 2a). CXCL12 is mainly expressed in perivascular BMSCs, which are also known as CXCL12-abundant reticular cells (CAR cells), as well as in endothelial cells, osteoprogenitors and osteoblasts.41,42 DTCs and HSCs often occupy the same space in the bone compartment and share certain molecular similarities, such as the expression of CXC chemokine receptor type 4 (CXCR4), a known receptor for CXCL12.43,44,45
Tumour cells interact with different niche cells for metastatic colonisation in bone. a In the perivascular niche, similar to HSCs, tumour cells interact with bone-marrow stromal cells (BMSCs), which express the chemokine ligand CXCL12. Endothelial E-selectin engages in tumour cell to promote mesenchymal-to-epithelial transition, stemness, survival, and growth. b In the endosteal niche, tumour cells are thought to alter the normal balance of bone turnover by directly activating osteoclast formation by expressing the Notch ligand Jagged1 or vascular cell adhesion molecule (VCAM)1 or by inducing osteogenic cells (stromal cells, lining cells, osteoblasts and osteocytes) to produce the osteoclast-stimulating factors macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor κΒ ligand (RANKL). Osteoclast-mediated bone resorption leads to the release of transforming growth factor (TGF)-β, which facilitates a ‘vicious cycle’ to fuel the development of osteolytic bone metastasis. Osteoblasts also promote bone metastasis by secreting Wnta5a and interleukin (IL)-6 or forming gap junctions and E-cadherin/N-cadherin junctions.
BMSCs
BMSCs are one of the most important cell types in the perivascular niche. They are multipotent cells that can differentiate into osteoblasts, chondrocytes and adipocytes.46 Different subsets of BMSC populations with different combinations of characteristic markers have been identified. Cells similar to BMSCs and bone marrow skeletal cells have been identified as site-specific skeletal stem cells in other skeletal compartments such as periosteum and the growth plate, showing different differentiation potential, as these spatially restricted and local resident SSCs can form different structure of the bone.47 BMSCs have been reported not only to contribute to bone turnover by differentiating into bone-producing osteoblasts, but also to provide a perivascular compartment that maintains HSCs, known as the HSC niche.40,48,49,50
Less than 0.1% of DTCs survive during circulation and homing to secondary organs.1 Several perivascular niche cells, predominantly BMSCs, produce CXCL12, which assists DTC homing to the bone, and these bone-tropic DTCs have a relatively high expression of CXCR4 compared with primary tumour cells. The overexpression of CXCR4 in breast cancer cells increased the formation of bone metastasis,43 whereas its inhibition dramatically reduced the bone metastatic burden in other cancers such as prostate cancer and melanoma.51 The CXCR4–CXCL12 interaction can be disrupted pharmacologically by treatment with AMD3100, a CXCR4 antagonist, and this agent was used to show that inhibiting the CXCR4–CXCL12 interaction between cancer cells and the stromal cells sensitises the metastatic cells to standard chemotherapy.52 Furthermore, BMSCs express interleukin (IL)-6, a potent osteoclast-activating factor that contributes to osteolysis in both myeloma53 and neuroblastoma.54 IL-6 also acts as a pro-tumorigenic cytokine to stimulate cancer cell proliferation and survival.55 BMSCs also generate exosomes, which contain certain miRNAs, such miR-23b, that are capable of inducing a dormant phenotype in exosome-receiving cancer cells by inhibiting myristoylated alanine-rich C-kinase substrate (MARCKS), which encodes a protein that promotes cell cycling and motility. This phenomenon was confirmed by the observation of increased miR-23b levels and decreased MARCKS expression in metastatic breast cancer cells in the bone marrow of patients.56
Endothelial cells
The blood vessels in different type and location of the bone are heterogeneous in structure and function, which enables optimal delivery of oxygen and nutrients.57 Blood vessels in the bone are not just transport conduits as the units of blood vessel tubules –endothelial cells are important in regulating osteogenesis and bone haematopoiesis.58 The sinusoidal endothelium in the bone marrow is discontinuous, with flattened and irregular shapes and inadequate coverage by thinner basal lamina. Such characteristics allow the free exchange of large molecules and the passage of haematopoietic and endothelial precursors, in contrast to the tight cell–cell junctions that are present in barrier-forming continuous capillaries.59,60 The sinusoidal vasculature also enables the free exchange of DTCs from the blood circulation to the bone marrow, and the detection of DTCs in the bone marrow in breast ductal carcinoma in situ (DCIS)61 and in patients with localised prostate cancer62 supports this concept. However, results from other studies indicate that endothelial cells present in the sinusoids act as gatekeepers against extravasation and initial seeding.
E-selectin is widely expressed in the bone vasculature and assists in the recruitment of immune cells from the blood during inflammation63; it is also an important component of HSC vascular niches. E-selectin has been demonstrated to be hijacked by cancer cells to trigger mesenchymal-to-epithelial transition (MET), the reverse process of epithelial-to-mesenchymal transition (EMT), which is thought to be a crucial step in early colonisation in distal organs.64 E-selectin binds to Golgi glycoprotein 1 (Glg1; E-selectin ligand 1) or other E-selectin ligands that are glycosylated by the α1-3 fucosyltransferases Fut3 or Fut6 on the surface of cancer cells. This binding promotes MET while simultaneously sustaining cancer stem cell traits by activating Wnt signalling.65
Studies have also shown that DTCs can be protected from chemotherapy by the vascular endothelium as inhibition of integrin-mediated interaction between DTCs and the perivascular niche, which is mediated in part by endothelial-derived von Willebrand factor and vascular cell adhesion molecule 1 (VCAM1), sensitises DTCs to chemotherapy.66
The endosteal niche and its role in metastases
The endosteal niche principally consists of different bone cells derived from BMSCs (bone-lining cells, osteoblasts and osteocytes) and from HSCs (osteoclasts).22 This niche plays a key role in promoting both osteolytic and osteoblastic bone metastasis (Fig. 2b).
Osteoclasts and osteolytic metastasis
In a mouse mammary tumour bone metastasis model, CSF-1 produced by tumour cells contributes to osteoclast development and survival in the bone microenvironment.67 Nutrients and growth factors can be rate-limiting for tumour growth after tumour cells have colonised the bone microenvironment, but the bone matrix represents a fertile ‘soil’ for supporting the growth of metastatic cancer cells, acting as a reservoir of various growth factors, minerals and ample nutrients. A classic model of osteolytic bone metastasis is the so-called ‘vicious cycle’. In this model, tumour invasion into the bone is associated with increased osteoclast activation and their recruitment to the site. This results in increased release of growth factors from the bone matrix, which, in turn, can positively feed back to fuel the development of osteolytic metastasis and enhance metastatic tumour growth in bone in certain types of cancer.3,68,69,70,71 A key component of this cycle is TGF-β, which is released from the bone matrix as a consequence of bone resorption by osteoclasts. TGF-β stimulates tumour cells to produce parathyroid hormone-related protein (PTHrP)72 and the Notch ligand Jagged1,73 which further facilitate osteoclast maturation. Jagged1 promotes osteoclastogenesis by directly promoting Notch signalling in pre-osteoclasts, while PTHrP signals to osteoblasts and induces the production of RANKL.74 By binding to its receptor RANK, RANKL triggers downstream NF-κB signalling75 and induces osteoclast maturation from haematopoietic precursors.76 Activated osteoclasts degrade the bone matrix on cortical and trabecular surfaces, leading to the release of numerous growth factors, including TGF-β, from the bone matrix. In addition, TGF-β-induced Jagged1 further enhances the vicious cycle by stimulating the expression of the tumour-growth-promoting cytokine IL-6 from stromal cells and osteoblasts.73 VCAM1 is another vital tumour cell product that plays a role in the outgrowth of indolent micrometastasis through the recruitment of osteoclast progenitors that express integrin α4β1.77 Several therapies such as bisphosphonates, antibodies against RANKL, Jagged1 and CSF-1, or small molecule inhibitors have already been approved for clinical use or are under development to treat osteolytic bone metastasis by preventing progression of the vicious cycle.78,79
Osteoblasts and osteoblastic bone metastasis
Following osteoclast-mediated bone resorption, cells of osteogenic lineage—osteoblasts—rebuild the bone. The continuous process of bone remodelling is achieved by communication between bone cells and other cells in the microenvironment. Osteoblasts regulate the formation and function of osteoclasts, but also respond to signals from osteoclasts and other cells including tumour cells.16 Wnt-family proteins are known to promote osteoblast differentiation and bone formation,80 and prostate cancer cells are able to regulate osteoblastic activity by expressing and secreting Wnts as well as the endogenous Wnt inhibitor dickkopf-1 (DKK-1) to modulate Wnt signalling.81 In addition, Wnt5a from osteoblastic cells induces prostate cancer cell dormancy via the Wnt5a–ROR2–SIAH2 signalling axis, suggesting a potential therapeutic utility to induce and maintain prostate cancer cell dormancy and to prevent the metastatic tumour formation in bone82 In breast cancer, DKK-1 promotes bone metastasis by regulating canonical Wnt signalling in osteoblasts but inhibits lung metastasis through non-canonical Wnt signaling.83
Osteogenic niches also play a role in the early outgrowth of bone metastasis prior to the engagement of the osteolytic cycle. Heterotypic cadherin interactions between tumour-cell-derived E-cadherin and osteoblast-derived N-cadherin activate mammalian target of rapamycin (mTOR) signalling in tumour cells to promote their outgrowth.84 Breast cancer cells also benefit from calcium obtained through connexin 43-containing gap junctions made with osteogenic cells, as the cancer cells cannot obtain calcium directly from the microenvironment.85 The activation of hypoxia-inducible factor (HIF) signalling in osteoprogenitor cells under the generally hypoxic condition in bone metastasis not only increases the ability of breast cancer cells to metastasise to the bone, but also promotes primary tumour growth and remote dissemination to the lungs and other organs by regulating blood levels of CXCL12, which binds to CXCR4 in breast cancer cells. These findings suggest that cells of osteoblast lineage might serve as systemic regulators of the tumour environment.86 A precise definition of the different stages of osteogenic cells, including skeletal stem cells, osteoblast progenitors and mature osteoblasts, by using diverse surface markers or specific transcription factor expression levels, might be helpful to study their interactions with cancer cells.
Osteocytes and lining cells
Other bone cells, such as osteocytes and lining cells, are also important components of the endosteal niche. Osteocytes are embedded in the bone matrix in a comprehensive dendritic structure, which forms bone canaliculi through gap junctions.87 This network allows osteocytes to sense other osteocytes and to communicate local and distant signals, including biomechanical stress and biological paracrine/endocrine signals.88 Recent evidence has shown that prostate tumour growth in bone can increase pressure within the medullary cavity, which leads to the upregulation of pro-metastatic factors CC motif chemokine ligand 5 (CCL5) and matrix metalloproteinases in osteocytes—the main mechanotransducing cells in bone. The fact that osteocytes can sense physical forces induced by tumour growth, and respond to tumours by secreting factors associated with cancer growth and mobility suggests osteocytes are critical mediators in the bone metastasis process.89 Osteocytes can also produce RANKL to induce osteoclast formation and activation, which provides an amplifying loop to promote osteolytic metastasis.90
In normal physiology, lining cells regulate calcium balance and collagen deposition,91 but the function of lining cells in bone metastasis is not well understood. However, given the fact that lining cells interact with other bone cells, they might help tumour cells sense the stiffness of the bone matrix, or possibly transduce mechanotransduction signals.92 As lining cells can serve as osteoprogenitors, and can respond to signals that induce or inhibit osteoblast differentiation, the interaction between lining cells and tumour cells requires deeper investigation.
Immune cells in the bone niches
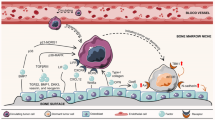
Immune cells are another major cell population in the bone marrow. The pro- and anti-tumour effects of the immune system at primary sites have been widely studied, but the crosstalk between bone metastatic cancer cells and bone marrow immune cells remains largely unexplored (Fig. 3).
Other cells in the bone niches include immune cells in the bone marrow (dendritic cells, macrophages, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, T cells and megakaryocytes), adipocytes and sympathetic nerve cells. These bone stromal cells regulate the immune escape, dormancy and proliferation abilities of tumour cells.
T cells
Active CD4+ T cells, CD8+ T cells and natural killer (NK) cells might all contribute to the immune surveillance and elimination of bone metastasis93,94,95 and, indeed, the depletion of T cells and NK cells accelerated bone metastasis in a murine breast cancer model.96 However, active CD4+ T cells might also have pro-metastatic roles, as they have been shown to promote osteoclastogenesis and induce premetastatic osteolytic lesions, which might facilitate the colonisation of myeloma and breast cancer cells in bone.97,98 Considerably more CD4+/Foxp3+ regulatory T (Treg) cells were found in the bone marrow of prostate cancer patients with bone metastases than in the bone marrow of patients without bone metastases, where they might confer immunosuppressive functions.99 Treg cells obtained from bone metastatic prostate cancer patients showed greater migratory and proliferative capacities and were shown to be responsible for the inhibition of osteoclasts, resulting in the generation of osteoblastic bone lesions. In contrast, RANKL levels in CD3+ T cells within the marrow of BALB/c mice with transplanted 4T1 mammary tumours were elevated compared with those of mice injected with non-metastatic 67NR cells,98 consequently enhancing osteoclastic activities in the bone and thereby leading to bone metastatic colonisation of 4T1 cells.
Macrophages and myeloid cells
In addition to T cells, macrophages also contribute to the development of bone metastasis. In primary tumours, the presence of tumour-associated macrophages (TAMs) is associated with poor prognosis and the development of metastases.100,101 A cell population named metastasis-associated macrophages (MAMs), which are essential to promoting tumour metastasis, has been found in different metastatic tissues.101 A 2020 study found abundant macrophages in human and mouse breast cancer bone metastases. These bone MAMs originate from inflammatory monocytes and express high levels of CD204, which specifically labels macrophages infiltrated inside bone metastasised tumour well as the IL-4 receptor. Ablation of the IL-4 receptor in monocytes/macrophages can effectively inhibit the growth of bone metastasis, suggesting that MAMs can serve as a promising target in bone metastasis.102
Myeloid-derived suppressor cells (MDSCs) comprise a heterogeneous group of immunosuppressive cells, including immature monocytic cells, neutrophils and dendritic cells, and constitute up to 20–30% of all bone marrow cells.103 Besides their immunosuppressive function, MDSCs have the potential to differentiate into osteoclasts, which might subsequently promote bone loss in bone metastasis.104 Plasmacytoid dendritic cells were found to be increased with bone metastasis in a breast cancer model, and depletion of this population reduced bone loss and suppressed tumour growth in the bone.105
The CCL2–CC motif chemokine receptor 2 (CCL2–CCR2) signalling pathway and the CSF-1–CSF-1 receptor (CSF-1R) signalling pathway are important for the differentiation of macrophages and myeloid cells from their progenitors or precursors and for their recruitment to the tumour microenvironment. CCL2 expressed by both tumour cells and stromal cells can be recognised by CCR2-expressing myeloid cells in the pre-metastatic niche, and this interaction facilitates tumour cell extravasation and colonisation.106 As a potent chemoattractant, CCL2 expressed by the tumour and stromal cells then promotes metastatic progression by recruiting TAMs,107 MAMs,108 monocytes109 and MDSCs.110 Inhibition of the CCL2–CCR2 interaction by anti-human CCL2 antibodies has been shown to decrease prostate cancer cell growth in bone.111 CSF-1–CSF-1R signalling promotes the differentiation of myeloid progenitors into monocytes, macrophages, dendritic cells and osteoclasts,112 as well as regulating the recruitment, polarisation and differentiation of TAMs.79 A CSF-1R kinase inhibitor, JNJ-28312141, showed a potential inhibitory effect on solid tumour growth and bone metastases by reducing the number of TAMs through suppressing their recruitment and differentiation.113 In summary, the diversity of immune niches is just beginning to be recognised, and further investigation of the link between the immune system and bone homoeostasis as well as bone metastasis is required.
Additional cell types in the bone niches
Other cell types have also been studied in the bone niches (Fig. 3). The sympathetic nervous system has been suggested to stimulate BMSCs and promote breast cancer bone metastasis in mice through activation of the sympathetic nervous system mediated through β-adrenergic signalling.114 This finding indicates that patients’ systemic physiological conditions, especially the sympathetic nervous system, can be involved in regulating the tumour microenvironment and metastasis to bone and other organs. Moreover, this concept highlights the possibility to develop new therapeutic approaches by targeting sympathetic nervous system. Increasing evidence also shows that bone marrow adipocytes can attract and interact with metastatic tumour cells, and provide an alternative source of growth factors and energy to support metastatic growth.115 Mature polyploid megakaryocytes are responsible for the production of platelets in response to thrombopoietin (THPO). Previous research looking at the role of megakaryocytes in regulating bone metastasis has been controversial—although tumour growth increased megakaryocyte numbers, both pro-metastatic and anti-metastatic roles of megakaryocytes have been observed.116,117 Mice lacking megakaryocytes developed more aggressive bone metastasis compared with wild-type animals,118 and thrombopoietin-induced megakaryocyte expansion in the marrow leads to decreased prostate cancer bone metastasis formation,119 suggesting that the increase of megakaryocytes in bone marrow could confer a protective mechanism against bone metastasis in breast cancer. On the other hand, however, silencing THPO, which reduces the number of platelets and density of mature megakaryocytes, inhibits tumour progression and metastasis in a mouse mammary tumour model (MMTV-PyMT).120 Furthermore, megakaryocytes can produce factors such as RANKL, VEGF and TGF-β, which have been shown to promote bone metastasis.121 More research is therefore needed to clarify the context-dependent role of platelets and megakaryocytes in different stages and different types of cancer bone metastasis.
Current therapeutic approaches in bone metastases
Current strategies for the treatment of bone metastases depend on three principles. First, cancer cells might be eliminated by systemically/locally targeting them, thereby suppressing their proliferation and any metastatic traits. Second, stromal cells can also be therapeutic targets by disrupting the interaction between the altered bone niche and cancer cells. This strategy can be achieved based on the studies revealing the nature of niche and cancer cells during bone metastasis. And, third, additional lines of treatment comprise palliative therapies, with the purpose of alleviating symptoms or morbidity associated with bone metastasis. This approach can be very helpful in improving the quality of life for cancer patients, as bone metastasis can be extremely debilitating and painful.
In general, systemic therapy is often required for the treatment of advanced bone metastasis, and chemotherapy, hormonal therapy, targeted therapies or a combination of these options can be used.
Hormonal therapy
In advanced, hormone-driven tumours, such as prostate and breast cancers, the first-line approach very often involves hormone deprivation to repress proliferative signalling in the cancer cells. However, although patients have benefited from hormone-deprivation therapies, the need for improved treatment exists when considering the impact that hormone deprivation has on the bone niche. Oestrogen and androgen can promote osteoclast survival and enhance osteoblast activity directly or indirectly, which leads to an imbalance in bone remodelling.122,123,124 Hormone deprivation therapy could further induce a loss of bone mineral density and enhance the formation of osteolytic lesions in bone metastatic cancer patients.125
Osteoclast-targeted therapy
Drugs that inhibit osteoclast-mediated resorption, including bisphosphonates (such as zoledronic acid) and denosumab (RANKL antibody), are currently used to treat tumour-induced bone disease. Bisphosphonates have been used to prevent bone loss in multiple clinical indications, such as osteoporosis and Paget’s disease, as well as bone metastatic cancer.126 However, the role of bisphosphonates in treating breast cancer bone metastasis remains controversial. Bisphosphonate treatment has been reported to only be effective in improving the survival of patients who were postmenopausal when treatment started.127 This result has been validated in mice, as zoledronic acid only works in mice with low oestrogen levels.128 These findings can be explained by the observation that oestrogen binding to the oestrogen receptor induces osteoclast apoptosis in females to prevent bone loss,124 suggesting that oestrogen and bisphosphonates might act redundantly. It is therefore crucial to monitor bone structure while administering hormonal therapies. Denosumab was approved by the FDA based on a study showing that this agent prolonged time to a skeletal-related event (pathological fracture, radiation therapy, surgery to bone, or spinal cord compression) when compared with zoledronic acid treatment.129,130 Denosumab is a human monoclonal antibody that has the ability to bind to both membrane-bound and soluble RANKL with high affinity.131 As mentioned before, RANKL is secreted abundantly by BMSCs, osteoblasts and osteocytes, and when it is bound to RANK, which is located on the surface of osteoclasts, it promotes osteoclastic activity and bone destruction. By disrupting the RANKL–RANK interaction, denosumab therefore inhibits osteoclast activation and bone resorption. Denosumab has been shown to be clinically effective in alleviating pain and hypercalcaemia associated with malignancy.132 Further approaches used to target osteoclasts in bone metastatic cancer include the cathepsin-K inhibitor odanacatib and the c-Src inhibitor dasatinib. Odanacatib inhibits bone resorption activity of osteoclasts while does not reduce osteoclast number.133 In preclinical studies, osteoclast-specific cathepsin K ablation inhibits bone resorption while maintaining the number of osteoclasts that are sufficient to maintain bone homoeostasis.134 Dasatinib can accelerate osteoblast differentiation through inhibition of both Src and Abl, but inhibit osteoclast activity through inhibition of M-CSF receptor C-FMS.135,136,137 Synergistic benefits could potentially be achieved by combining inhibitors of bone resorption with traditional treatments such as chemotherapy, radiation and hormonal therapy.
Chemotherapy
Chemotherapy is commonly used in the control of systemic diseases. However, recent studies indicate that chemotherapy-induced change in bone nice may have unintended consequence in affecting metastatic progression. Chemotherapy agents induce the expression of Jagged1 in cells of osteoblastic lineage through the generation of reactive oxygen species, and subsequently promote the seeding of cancer cells to bone as well as their resistance to chemotherapy.78 Importantly, combining chemotherapy with Jagged1 neutralising antibody treatment achieved synergistic benefit in reducing the risk of metastatic relapse in bone, indicating a potential avenue to increase the efficacy of adjuvant chemotherapy.
Conclusions and perspectives
Bone niche consists of multicellular components with intricate interactions.
The sinusoidal endothelium and BMSCs that closely lining the sinusoids in the bone marrow provide a perivascular niche to support HSCs, as well as to support DTCs homing to the bone and their resistance to chemotherapy. Osteoclasts, osteoblasts, osteocytes and lining cells, by remodelling the endosteal niche, not only can maintain normal bone homoeostasis but also can foster tumour cells during dormancy/micrometastasis as well as during the development of overt lesions. Immune cells and other components in the bone marrow display both pro-tumour and anti-tumour effects and participate in the regulation of bone metastasis. Tumour cells take advantage of the bone niche and, at the same time, remodel the bone microenvironment to facilitate the development and treatment resistance of bone metastasis.
Although significant progress has been made in understanding the nature of bone metastasis, the disease remains mostly incurable once tumour cells gain a foothold in bone and start proliferating. The steps of metastasis—from initial seeding in bone to dormancy and finally outgrowth—need to be investigated in further detail in order to prevent the occurrence and progression of bone metastasis. Different animal models, each with their own advantages and disadvantages, have been used in studies that focus on elucidating the mechanisms governing each step. Spontaneous metastasis models are generated by using transgenic mice or mice with orthotopically transplanted tumours. These models provide a comprehensive overview of the whole process from primary tumour growth to spontaneous metastasis. However, they often exhibit long latency and a limited incidence of metastases with a high degree of variation. Experimental models involve injecting tumour cells into the circulation via arterial or venous routes, or directly into different organs. These methods are useful to study metastatic mechanisms in the secondary sites but are not able to trace the initial process of metastasis.138 Owing to the lack of good models, our knowledge about some of the crucial early phases of bone metastasis, such as how tumour cells survive through dormancy, is still very limited. Substantial evidence suggests that cancer cells interact with cells in the bone niches to modify normal physiological processes and/or hijack the niche factors in favour of promoting tumour cell seeding, survival via dormancy, and outgrowth in the bone. Understanding the molecular basis of such interactions depends on the development of mouse models that closely mimic the natural process of dormancy in human cancer development and that allow single or small nodules of dormant cells to be visualised and their progression monitored during metastasis development.
Studies of the interactions between cancer cells and the bone microenvironment, including the perivascular niche and endosteal niche, are revealing promising therapeutic targets. However, there are still many unknown areas that need to be explored: the nature of other niche factors that participate in the crosstalk between cancer cells and niches; how cancer cells influence normal bone-resident stem cells such as HSCs, and the differentiation and mobilisation of HSC-derived immune cells; how these interactions affect the immune regulation in the whole body to favour metastatic colonisation into other organs; and the development of immune evasion of cancer. Continued progress in these research areas will help shape the future of cancer therapeutics.
References
Wan, L., Pantel, K. & Kang, Y. Tumor metastasis: moving new biological insights into the clinic. Nat. Med. 19, 1450–1464 (2013).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Coleman, R. E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 27, 165–176 (2001).
Macedo, F., Ladeira, K., Pinho, F., Saraiva, N., Bonito, N., Pinto, L. et al. Bone metastases: an overview. Oncol. Rev. 11, 321–321 (2017).
Kimura, T. Multidisciplinary approach for bone metastasis: a review. Cancers (Basel) 10, 156 (2018).
Guzik, G. Results of the treatment of bone metastases with modular prosthetic replacement—analysis of 67 patients. J. Orthop. Surg. Res. 11, 20 (2016).
Guise, T. A., Kozlow, W. M., Heras-Herzig, A., Padalecki, S. S., Yin, J. J. & Chirgwin, J. M. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 5(Suppl), S46–S53 (2005).
Pan, H., Gray, R., Braybrooke, J., Davies, C., Taylor, C., McGale, P. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Croucher, P. I., McDonald, M. M. & Martin, T. J. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16, 373–386 (2016).
Obenauf, A. C. & Massague, J. Surviving at a distance: organ-specific metastasis. Trends Cancer 1, 76–91 (2015).
Ghajar, C. M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247 (2015).
Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007).
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002).
Harada, S. & Rodan, G. A. Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 (2003).
Franz-Odendaal, T. A., Hall, B. K. & Witten, P. E. Buried alive: how osteoblasts become osteocytes. Dev. Dyn. 235, 176–190 (2006).
Frost, H. M. In vivo osteocyte death. J. Bone Joint Surg. Am. 42, 138–143 (1960).
Ikeda, K. & Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 159, 1–8 (2016).
Chitu, V. & Stanley, E. R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006).
Gow, D. J., Sester, D. P. & Hume, D. A. CSF-1, IGF-1, and the control of postnatal growth and development. J. Leukoc. Biol. 88, 475–481 (2010).
Haider, M. T., Smit, D. J. & Taipaleenmaki, H. The endosteal niche in breast cancer bone metastasis. Front. Oncol. 10, 335 (2020).
Shen, Y. & Nilsson, S. K. Bone, microenvironment and hematopoiesis. Curr. Opin. Hematol. 19, 250–255 (2012).
Frenette, P. S., Pinho, S., Lucas, D. & Scheiermann, C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 31, 285–316 (2013).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Hsu, Y. C. & Fuchs, E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 13, 103–114 (2012).
Moore, K. A. & Lemischka, I. R. Stem cells and their niches. Science 311, 1880–1885 (2006).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Mendez-Ferrer, S., Michurina, T. V., Ferraro, F., Mazloom, A. R., Macarthur, B. D., Lira, S. A. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Yu, V. W. & Scadden, D. T. Heterogeneity of the bone marrow niche. Curr. Opin. Hematol. 23, 331–338 (2016).
Birbrair, A. & Frenette, P. S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 1370, 82–96 (2016).
Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care (Engl.) 26, e12740 (2017).
Feng, X. & McDonald, J. M. Disorders of bone remodeling. Annu. Rev. Pathol. 6, 121–145 (2011).
Pinho, S., Lacombe, J., Hanoun, M., Mizoguchi, T., Bruns, I., Kunisaki, Y. et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Greenbaum, A., Hsu, Y. M., Day, R. B., Schuettpelz, L. G., Christopher, M. J., Borgerding, J. N. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Park, D., Spencer, J. A., Koh, B. I., Kobayashi, T., Fujisaki, J., Clemens, T. L. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Sacchetti, B., Funari, A., Michienzi, S., Di Cesare, S., Piersanti, S., Saggio, I. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Agarwal, P., Isringhausen, S., Li, H., Paterson, A. J., He, J., Gomariz, A. et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell 24, 769–784 e766 (2019).
Asada, N., Kunisaki, Y., Pierce, H., Wang, Z., Fernandez, N. F., Birbrair, A. et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223 (2017).
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Chatterjee, S., Behnam Azad, B. & Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 124, 31–82 (2014).
Shiozawa, Y., Havens, A. M., Pienta, K. J. & Taichman, R. S. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 22, 941–950 (2008).
Chen, F., Lin, X., Xu, P., Zhang, Z., Chen, Y., Wang, C. et al. Nuclear export of smads by RanBP3L regulates bone morphogenetic protein signaling and mesenchymal stem cell differentiation. Mol. Cell Biol. 35, 1700–1711 (2015).
Ambrosi, T. H., Longaker, M. T. & Chan, C. K. F. A revised perspective of skeletal stem cell biology. Front. Cell Dev. Biol. 7, 189 (2019).
Acar, M., Kocherlakota, K. S., Murphy, M. M., Peyer, J. G., Oguro, H., Inra, C. N. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Asada, N., Takeishi, S. & Frenette, P. S. Complexity of bone marrow hematopoietic stem cell niche. Int. J. Hematol. 106, 45–54 (2017).
Sun, Y. X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J. et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 20, 318–329 (2005).
Nervi, B., Ramirez, P., Rettig, M. P., Uy, G. L., Holt, M. S., Ritchey, J. K. et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113, 6206–6214 (2009).
Shain, K. H., Yarde, D. N., Meads, M. B., Huang, M., Jove, R., Hazlehurst, L. A. et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 69, 1009–1015 (2009).
Ara, T., Song, L., Shimada, H., Keshelava, N., Russell, H. V., Metelitsa, L. S. et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 69, 329–337 (2009).
Brocke-Heidrich, K., Kretzschmar, A. K., Pfeifer, G., Henze, C., Loffler, D., Koczan, D. et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood 103, 242–251 (2004).
Ono, M., Kosaka, N., Tominaga, N., Yoshioka, Y., Takeshita, F., Takahashi, R. U. et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal 7, ra63 (2014).
Ramasamy, S. K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017, 5046953 (2017).
Hendriks, M. & Ramasamy, S. K. Blood vessels and vascular niches in bone development and physiological remodeling. Front. Cell Dev. Biol. 8, 602278–602278 (2020).
Lafage-Proust, M. H., Roche, B., Langer, M., Cleret, D., Vanden Bossche, A., Olivier, T. et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep 4, 662 (2015).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357, eaal2379 (2017).
Sanger, N., Effenberger, K. E., Riethdorf, S., Van Haasteren, V., Gauwerky, J., Wiegratz, I. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Melchior, S. W., Corey, E., Ellis, W. J., Ross, A. A., Layton, T. J., Oswin, M. M. et al. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin. Cancer Res. 3, 249–256 (1997).
Walz, G., Aruffo, A., Kolanus, W., Bevilacqua, M. & Seed, B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science 250, 1132–1135 (1990).
Gunasinghe, N. P., Wells, A., Thompson, E. W. & Hugo, H. J. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 31, 469–478 (2012).
Esposito, M., Mondal, N., Greco, T. M., Wei, Y., Spadazzi, C., Lin, S. C. et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 (2019).
Carlson, P., Dasgupta, A., Grzelak, C. A., Kim, J., Barrett, A., Coleman, I. M. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Yagiz, K. & Rittling, S. R. Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp Cell Res. 315, 2442–2452 (2009).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11, 411–425 (2011).
Kang, Y. Dissecting tumor-stromal interactions in breast cancer bone metastasis. Endocrinol. Metab. (Seoul) 31, 206–212 (2016).
Ell, B. & Kang, Y. SnapShot: Bone Metastasis. Cell 151, 690–690 e691 (2012).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R. et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Iotsova, V., Caamano, J., Loy, J., Yang, Y., Lewin, A. & Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 3, 1285–1289 (1997).
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Zheng, H., Bae, Y., Kasimir-Bauer, S., Tang, R., Chen, J., Ren, G. et al. Therapeutic antibody targeting tumor- and osteoblastic niche-derived jagged1 sensitizes bone metastasis to chemotherapy. Cancer Cell 32, 731–747 e736 (2017).
Hume, D. A. & MacDonald, K. P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 (2012).
Westendorf, J. J., Kahler, R. A. & Schroeder, T. M. Wnt signaling in osteoblasts and bone diseases. Gene 341, 19–39 (2004).
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A. & Keller, E. T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 65, 7554–7560 (2005).
Ren, D., Dai, Y., Yang, Q., Zhang, X., Guo, W., Ye, L. et al. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 216, 428–449 (2019).
Zhuang, X., Zhang, H., Li, X., Li, X., Cong, M., Peng, F. et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 19, 1274–1285 (2017).
Wang, H., Yu, C., Gao, X., Welte, T., Muscarella, A. M., Tian, L. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Wang, H., Tian, L., Liu, J., Goldstein, A., Bado, I., Zhang, W. et al. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34, 823–839 e827 (2018).
Devignes, C. S., Aslan, Y., Brenot, A., Devillers, A., Schepers, K., Fabre, S. et al. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc. Natl Acad. Sci. USA 115, E992–E1001 (2018).
Delgado-Calle, J. & Bellido, T. Osteocytes and skeletal pathophysiology. Curr. Mol. Biol. Rep. 1, 157–167 (2015).
Robling, A. G. & Bonewald, L. F. The Osteocyte: New Insights. Annu. Rev. Physiol 82, 485–506 (2020).
Sottnik, J. L., Dai, J., Zhang, H., Campbell, B. & Keller, E. T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 75, 2151–2158 (2015).
Delgado-Calle, J., Anderson, J., Cregor, M. D., Hiasa, M., Chirgwin, J. M., Carlesso, N. et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 76, 1089–1100 (2016).
Dierkes, C., Kreisel, M., Schulz, A., Steinmeyer, J., Wolff, J. C. & Fink, L. Catabolic properties of microdissected human endosteal bone lining cells. Calcif. Tissue Int. 84, 146–155 (2009).
Johnson, R. W. & Suva, L. J. Hallmarks of Bone Metastasis. Calcif. Tissue Int. 102, 141–151 (2018).
Feuerer, M., Rocha, M., Bai, L., Umansky, V., Solomayer, E. F., Bastert, G. et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer 92, 96–105 (2001).
Zhang, K., Kim, S., Cremasco, V., Hirbe, A. C., Collins, L., Piwnica-Worms, D. et al. CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer Res. 71, 4799–4808 (2011).
Lode, H. N., Xiang, R., Dreier, T., Varki, N. M., Gillies, S. D. & Reisfeld, R. A. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 91, 1706–1715 (1998).
Bidwell, B. N., Slaney, C. Y., Withana, N. P., Forster, S., Cao, Y., Loi, S. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Colucci, S., Brunetti, G., Rizzi, R., Zonno, A., Mori, G., Colaianni, G. et al. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood 104, 3722–3730 (2004).
Monteiro, A. C., Leal, A. C., Goncalves-Silva, T., Mercadante, A. C., Kestelman, F., Chaves, S. B. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8, e68171 (2013).
Zhao, E., Wang, L., Dai, J., Kryczek, I., Wei, S., Vatan, L. et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 1, 152–161 (2012).
Wyckoff, J. B., Wang, Y., Lin, E. Y., Li, J. F., Goswami, S., Stanley, E. R. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Mendoza-Reinoso, V., McCauley, L. K. & Fournier, P. G. J. Contribution of macrophages and T cells in skeletal metastasis. Cancers (Basel) 12, 1014 (2020).
Ma, R. Y., Zhang, H., Li, X. F., Zhang, C. B., Selli, C., Tagliavini, G. et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 217, e20191820 (2020).
Zhao, E., Xu, H., Wang, L., Kryczek, I., Wu, K., Hu, Y. et al. Bone marrow and the control of immunity. Cell Mol. Immunol. 9, 11–19 (2012).
Zhuang, J., Zhang, J., Lwin, S. T., Edwards, J. R., Edwards, C. M., Mundy, G. R. et al. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS ONE 7, e48871 (2012).
Sawant, A., Hensel, J. A., Chanda, D., Harris, B. A., Siegal, G. P., Maheshwari, A. et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 189, 4258–4265 (2012).
Qian, B., Deng, Y., Im, J. H., Muschel, R. J., Zou, Y., Li, J. et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4, e6562 (2009).
Saji, H., Koike, M., Yamori, T., Saji, S., Seiki, M., Matsushima, K. et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92, 1085–1091 (2001).
Kitamura, T., Qian, B. Z., Soong, D., Cassetta, L., Noy, R., Sugano, G. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Silzle, T., Kreutz, M., Dobler, M. A., Brockhoff, G., Knuechel, R. & Kunz-Schughart, L. A. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur. J. Immunol. 33, 1311–1320 (2003).
Huang, B., Lei, Z., Zhao, J., Gong, W., Liu, J., Chen, Z. et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 252, 86–92 (2007).
Kirk, P. S., Koreckij, T., Nguyen, H. M., Brown, L. G., Snyder, L. A., Vessella, R. L. et al. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in bone. Int. J. Mol. Sci. 14, 10483–10496 (2013).
Ell, B., Mercatali, L., Ibrahim, T., Campbell, N., Schwarzenbach, H., Pantel, K. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–556 (2013).
Manthey, C. L., Johnson, D. L., Illig, C. R., Tuman, R. W., Zhou, Z., Baker, J. F. et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol. Cancer Ther. 8, 3151–3161 (2009).
Sloan, E. K., Priceman, S. J., Cox, B. F., Yu, S., Pimentel, M. A., Tangkanangnukul, V. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Morris, E. V. & Edwards, C. M. The role of bone marrow adipocytes in bone metastasis. J. Bone Oncol. 5, 121–123 (2016).
Lucotti, S. & Muschel, R. J. Platelets and Metastasis: New Implications of an Old Interplay. Front. Oncol. 10, 1350 (2020).
Maroni, P. Megakaryocytes in bone metastasis: protection or progression? Cells 8, 134 (2019).
Jackson, W. 3rd, Sosnoski, D. M., Ohanessian, S. E., Chandler, P., Mobley, A., Meisel, K. D. et al. Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res. 77, 1942–1954 (2017).
Li, X., Koh, A. J., Wang, Z., Soki, F. N., Park, S. I., Pienta, K. J. et al. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner. Res. 26, 125–134 (2011).
Shirai, T., Revenko, A. S., Tibbitts, J., Ngo, A. T. P., Mitrugno, A., Healy, L. D. et al. Hepatic thrombopoietin gene silencing reduces platelet count and breast cancer progression in transgenic MMTV-PyMT mice. Blood Adv. 3, 3080–3091 (2019).
Psaila, B., Lyden, D. & Roberts, I. Megakaryocytes, malignancy and bone marrow vascular niches. J. Thromb. Haemost. 10, 177–188 (2012).
Smith, E. P., Specker, B. & Korach, K. S. Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J. Steroid Biochem. Mol. Biol. 118, 264–272 (2010).
Morrissey, C., Roudier, M. P., Dowell, A., True, L. D., Ketchanji, M., Welty, C. et al. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: results from the University of Washington Rapid Autopsy Series. J Bone Miner. Res. 28, 333–340 (2013).
Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K. et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007).
Ren, G., Esposito, M. & Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. (Berl.) 93, 1203–1212 (2015).
Drake, M. T., Clarke, B. L. & Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045 (2008).
Early Breast Cancer Trialists’ Collaborative, G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361 (2015).
Ottewell, P. D., Wang, N., Brown, H. K., Reeves, K. J., Fowles, C. A., Croucher, P. I. et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin. Cancer Res. 20, 2922–2932 (2014).
Lacey, D. L., Boyle, W. J., Simonet, W. S., Kostenuik, P. J., Dougall, W. C., Sullivan, J. K. et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11, 401–419 (2012).
Fizazi, K., Carducci, M., Smith, M., Damiao, R., Brown, J., Karsh, L. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Kostenuik, P. J., Nguyen, H. Q., McCabe, J., Warmington, K. S., Kurahara, C., Sun, N. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 24, 182–195 (2009).
Oyajobi, B. O., Anderson, D. M., Traianedes, K., Williams, P. J., Yoneda, T. & Mundy, G. R. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 61, 2572–2578 (2001).
Bone, H. G., McClung, M. R., Roux, C., Recker, R. R., Eisman, J. A., Verbruggen, N. et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J. Bone Miner. Res. 25, 937–947 (2010).
Lotinun, S., Kiviranta, R., Matsubara, T., Alzate, J. A., Neff, L., Luth, A. et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 123, 666–681 (2013).
Id Boufker, H., Lagneaux, L., Najar, M., Piccart, M., Ghanem, G., Body, J. J. et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer 10, 298 (2010).
Vandyke, K., Dewar, A. L., Diamond, P., Fitter, S., Schultz, C. G., Sims, N. A. et al. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J. Bone Miner. Res. 25, 1759–1770 (2010).
Lee, Y. C., Huang, C. F., Murshed, M., Chu, K., Araujo, J. C., Ye, X. et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene 29, 3196–3207 (2010).
Eckhardt, B. L., Francis, P. A., Parker, B. S. & Anderson, R. L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat. Rev. Drug Discov. 11, 479–497 (2012).
Acknowledgements
We thank the members of our laboratory for helpful discussions. We also apologise to the many investigators whose important studies could not be cited directly here owing to space limitations. Illustrations created with Biorender.com.
Author information
Authors and Affiliations
Contributions
F.C. and Y.H. co-wrote and revised the manuscript. Y.K. co-wrote and revised the manuscript and provided overall guidance.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
Y.K. holds equity interest in KayoThera and Firebrand Therapeutics.
Funding information
The work in the authors’ laboratory is supported by grants from the Brewster Foundation, American Cancer Society, Susan G. Komen Foundation, Breast Cancer Research Foundation, the NIH and the U.S. Department of Defense to Y.K.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, F., Han, Y. & Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br J Cancer 124, 1912–1920 (2021). https://doi.org/10.1038/s41416-021-01329-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01329-6
- Springer Nature Limited
This article is cited by
-
Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer
Nature Reviews Urology (2024)
-
Unraveling the metastatic niche in breast cancer bone metastasis through single-cell RNA sequencing
Clinical and Translational Oncology (2024)
-
State of the Art Modelling of the Breast Cancer Metastatic Microenvironment: Where Are We?
Journal of Mammary Gland Biology and Neoplasia (2024)
-
Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy
Cellular & Molecular Immunology (2022)