Abstract
The impact of letermovir (LTV)—an anti-cytomegalovirus (CMV) drug—on human herpesvirus-6 (HHV-6) encephalitis is unclear. We hypothesized that LTV prophylaxis may increase the incidence of HHV-6 encephalitis by reducing anti-CMV therapies after allogeneic hematopoietic stem cell transplantation (HSCT). To evaluate the association between HHV-6 encephalitis and antiviral prophylaxis, 7985 adult patients from a nationwide registry who underwent their first HSCT between January 2019 and December 2021 were analyzed. The incidence of HHV-6 encephalitis on day 100 after HSCT was 3.6%; 11.5% for the broad-spectrum antiviral group (foscarnet, ganciclovir, or valganciclovir); 2.8% for the LTV group, and 3.8% for the other antiviral group (p < 0.001). These differences persisted when cord blood transplantation (CBT) was analyzed separately (14.1%, 5.9%, and 7.4%, p < 0.001). In the multivariate analysis, CBT (hazard ratio [HR]: 2.90), broad-spectrum antiviral prophylaxis (HR: 1.91), and grade II–IV acute graft-versus-host disease requiring systemic corticosteroids (HR: 2.42) were independent risk factors for encephalitis (all p < 0.001). The findings of this large modern database study indicate that broad-spectrum antiviral prophylaxis, rather than LTV prophylaxis, is paradoxically associated with HHV-6 encephalitis in the LTV era. This paradoxical finding needs to be further explored in future studies.
Graphical abstract

Similar content being viewed by others
Introduction
Human herpesvirus-6 (HHV-6) encephalitis is a rare but fatal complication of allogeneic hematopoietic stem cell transplantation (HSCT), with an incidence rate of approximately 1% after peripheral blood stem cell transplantation (PBSCT) or bone marrow transplantation (BMT) and 7–9% after cord blood transplantation (CBT) [1,2,3]. It usually occurs within the first 2–6 weeks after HSCT, and the risk factors include transplantation from a human leukocyte antigen (HLA)-mismatched donor, CBT, non-methotrexate (MTX)-based graft-versus-host disease (GVHD) prophylaxis, pre-engraftment immune reaction (PIR), and acute GVHD [2, 4,5,6]. Patients with HHV-6 encephalitis showed worse prognosis than those without encephalitis [5]. To date, no FDA (Food and Drug Administration)-approved HHV-6-specific drugs are available, and most therapeutic/prophylactic drugs for HHV-6 encephalitis are the same as those for cytomegalovirus (CMV) disease [7]. Of note, foscarnet (FCN), the PMDA (Pharmaceutical and Medical Devices Agency)-approved drug for HHV-6 encephalitis after HSCT, is the first choice for HHV-6 encephalitis [8]. The dosage is crucial for the treatment of HHV-6 encephalitis: 180 mg/kg/day of FCN is recommended to treat patients with HHV-6 encephalitis as this higher dose is associated with a better response rate [5]. Low dose FCN (90 mg/kg/day) is not sufficient to prevent HHV-6 encephalitis [9]. Prophylactic or pre-emptive therapy is currently not recommended for the prevention of HHV-6B reactivation or encephalitis after HSCT [8].
Letermovir (LTV) is a novel and specific anti-CMV prophylactic drug that has been widely used since 2017 to reduce CMV reactivation and disease after HSCT [10,11,12,13]. However, LTV is not effective against HHV-6 [14, 15], unlike other anti-CMV drugs such as FCN and ganciclovir (GCV). Consequently, LTV prophylaxis with reduced use of anti-CMV drugs that are generally effective against HHV-6 may increase the incidence of HHV-6 encephalitis. Therefore, the risk and incidence of HHV-6 encephalitis may have changed in the LTV era. In a previous monocentric study examining the relationship between LTV prophylaxis and HHV-6 encephalitis, HHV-6 encephalitis incidence did not differ between the pre-LTV and post-LTV groups [16]. However, only 10% of patients with CBT were included, resulting in less than 1% incidence of HHV-6 encephalitis in both groups. Therefore, the clinical effect of LTV prophylaxis on HHV-6 encephalitis remains largely unknown.
Based on the above rationale, we investigated the clinical impact of LTV prophylaxis on the incidence of HHV-6 encephalitis using Japanese registry-based data in the LTV era.
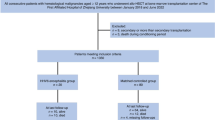
Methods
Patients and study approval
The clinical, treatment, and outcome data of HSCT recipients were obtained from the Transplant Registry Unified Management Program of the Japan Society for Transplantation and Cellular Therapy (JSTCT) and the Japanese Data Center for Hematopoietic Cell Transplantation [17, 18]. Patients who received their first allogeneic HSCT between the age of 18 and 75 years, and underwent transplantation between January 2019 and December 2021 were selected from the nationwide transplantation registry and included along with those that received single-unit CBT, PBSCT, and BMT. Patients missing survival or HHV-6 encephalitis data were excluded. HLA-matched donors were defined as having the same serologically identified HLA-A, HLA-B, and HLA-DRB1 as the recipient. Haploidentical donors were defined as related donors mismatched at 2–3 HLA antigen levels for HLA-A, HLA-B, and HLA-DRB1 with the recipient. The remaining donors were considered mismatched. Missing data are noted descriptively in each table where appropriate and are excluded from the relevant statistics when necessary. However, we considered the CMV serostatus of the cord blood (CB) unit to be “negative” if it was “blank” in the original data set, indicating that the CMV status of the donor was negative.
Endpoints and definitions
The primary endpoint was the incidence of HHV-6 encephalitis on day 100 after HSCT, diagnosed by the treating physicians using previously published diagnostic criteria [5]. Briefly, the following three factors must be present: (1) the presence of central nervous system (CNS) dysfunction, (2) a positive polymerase chain reaction (PCR) result for HHV-6 DNA in the cerebrospinal fluid (CSF), and (3) the absence of other identified causes of CNS dysfunction. Although there are 2 distinct species of HHV-6 (HHV-6A and HHV-6B), due to lack of available data, we could not distinguish between HHV-6A and HHV-6B. In addition, we cannot distinguish whether HHV-6 is a chromosomally integrated type (ciHHV-6) or not. However, in previous reports only a small proportion (0.6–1.4%) have ciHHHV-6 [19, 20], and up to 1.2–2.8% of HSCT patients could be affected by ciHHV-6 as this condition would be seen in all nucleated cells of the host as well as the graft.
To evaluate the impact of LTV prophylaxis on HHV-6 encephalitis, we divided the entire cohort into three groups according to the antiviral drugs administered prophylactically immediately after transplantation: (1) broad-spectrum antiviral drugs including prophylactic FCN, GCV, or valganciclovir (VGCV); (2) oral or intravenous LTV; and (3) other antiviral drugs that did not include FCN, GCV, VGCV, or LTV. The choice of antiviral prophylaxis medication was at the discretion of each physician. However, we were unable to determine whether the prophylactic antivirals (especially broad-spectrum antivirals) were administered to suppress CMV, HHV-6, or other viral reactivations/infections. Furthermore, although we understand that the dose of antiviral therapy is important because the efficacy of FCN for the prevention and treatment of HHV-6 encephalitis is dependent on the dosage (90 or 180 mg/kg/day) [5, 9], data regarding dosage of antiviral prophylactic drugs were unavailable in this study.
Preemptive CMV therapy was defined as anti-CMV therapy to treat CMV reactivation. CMV disease was defined as end-organ dysfunction caused by CMV. Overall survival (OS) was defined as the time from HSCT to death or the last observation. Death without relapse was defined as non-relapse mortality (NRM). Patients were classified based on disease risk during HSCT into low- and high-risk groups. The low-risk group included patients exhibiting non-malignant disease; first or second complete remission in acute myeloid leukemia and acute lymphoblastic leukemia; refractory anemia (RA); RA with ringed sideroblasts in myelodysplastic syndrome; first or second chronic phase or accelerated phase in chronic myeloid leukemia; any complete response in malignant lymphoma; completer response or very good partial response in multiple myeloma. All other statuses at HSCT were defined as high-risk. Reduced-intensity conditioning regimen was defined as (1) total body irradiation at 500 cGy as a single fraction or 800 cGy if fractionated, (2) ≤ 7.2 mg/kg intravenous busulfan, or (3) ≤ 140 mg/m2 melphalan [21]. Acute and chronic GVHD were diagnosed and graded based on previously described clinical criteria [22, 23].
Statistical analysis
Baseline patient characteristics were compared using Fisher’s Exact Test for categorical variables and the Mann–Whitney U or Kruskal–Wallis test for continuous variables. The probabilities of HHV-6 encephalitis, acute and chronic GVHD, and NRM were calculated using the cumulative incidence method (considering competing risks), and the groups were compared using Gray’s test. Death without HHV-6 encephalitis or GVHD was a competing event for HHV-6 encephalitis or GVHD. Relapse and NRM were considered mutually competing events. The OS probability was estimated using the Kaplan–Meier method, and groups were compared using the log-rank test. We used the Mantel and Byar method and the Simon and Makuch plot for survival analysis of HHV-6 encephalitis onset to avoid time-lead bias in the development of HHV-6 encephalitis on survival.
Multivariate analysis (MVA) was performed using the Fine and Gray proportional hazard model for HHV-6 encephalitis to estimate hazard ratios (HRs) with 95% confidence intervals (CIs). Initially, the MVA included stem cell sources. The following factors were included in the multivariate model: age (<50 years vs. ≥50 years), sex (female vs. male), stem cell source (BM vs. PB vs. CB), antiviral prophylaxis (broad-spectrum antiviral prophylaxis vs. LTV vs. other antiviral drugs), and grade II–IV acute GVHD requiring systemic corticosteroids (no vs. yes). We then performed a separate MVA according to the stem cell source (the CBT cohort), as CBT is a well-known risk factor for HHV-6 encephalitis. We added PIR and GVHD prophylaxis methods in the CBT cohort. We evaluated PIR and acute GVHD as time-dependent covariates. All statistical analyses were performed using RStudio and EZR version 1.61 (Saitama Medical Center, Jichi Medical University) [24], which is a graphical user interface for R (The R Foundation for Statistical Computing, version 4.2.2, Vienna, Austria). A two-sided p < 0.05 was considered statistically significant.
Results
Patient characteristics, details of antiviral prophylaxis, and CMV treatment
In total, 7985 patients with a median age of 54 years (interquartile range [IQR] 43–62, Table 1) were included in this study. Among them, 467 patients (5.8%) received broad-spectrum antiviral prophylaxis, 4911 patients (61.5%) received LTV, and 2607 patients (32.6%) received other antiviral drugs. Among the broad-spectrum antiviral prophylaxis group, 333 patients (71.3%) received FCN. The median duration between HSCT and antiviral prophylaxis initiation was 5 days (IQR day 0–7) in the broad-spectrum antiviral prophylaxis group and 1 day (IQR day 0–5) in the LTV group (p = 0.006). The median durations of antiviral prophylaxis were 46 days (IQR 24–89 days) in the broad-spectrum antiviral prophylaxis group and 91 days (IQR 55–100 days) in the LTV group (p < 0.001).
The incidence of CMV preemptive therapy on day 100 after HSCTwas 21.1% (95% CI: 20.2-22.0%, Supplemental Fig. 1A), and CMV disease on day 100 was 2.5% (95% CI: 2.1–2.8%, Supplemental Fig. 1B). The LTV prophylaxis group had the lowest incidence of CMV preemptive therapy and CMV disease on day 100 compared with the other two groups (CMV preemptive therapy: 11.9% [95% CI: 11.0–12.9%] in the LTV group, 19.9% [95% CI: 15.9–23.7%] in the broad-spectrum group, and 39.4% [95% CI: 37.4–41.3%] in the other antiviral drug group, p < 0.001, Supplemental Fig. 1C; CMV disease: 1.5% [95% CI: 1.1–1.8%] in the LTV group, 4.8% [95% CI: 2.7–6.9%] in the broad-spectrum group, and 4.0% [95% CI: 3.2–4.8%] in the other antiviral drug group, p < 0.001, Supplemental Fig. 1D). A total of 50.5% in the LTV group, 61.1% in the broad-spectrum group, and 58.1% in the other antiviral drug group received either FCN or GCV as CMV preemptive therapy. The incidence of CMV preemptive therapy with either FCN or GCV at day 100 was 13.4% (95% CI: 12.7–14.2%) in the whole cohort, comprising 8.6% (95% CI: 7.8–9.4%) in the LTV group, 14.2% (10.8–17.5%) in the broad-spectrum group, and 22.9% (21.2–24.5%) in the other antiviral drug group (p < 0.001).
These results indicated that the LTV group was less likely to receive CMV preemptive therapy or CMV drugs that were effective against HHV-6.
Outcome of HHV-6 encephalitis development and differences based on clinical factors
In total, 278 HHV-6 encephalitis cases occurred in 7985 patients, with an incidence of 3.6% (95% CI: 3.2–4.0%) on day 100 (median 24 days, range 10–196 days, Fig. 1a). Patients with HHV-6 encephalitis had significantly worse prognoses than those without HHV-6 encephalitis (median OS: 323 days vs. not reached [NR], 95% CI 245–552 vs. 1260–not available [NA], p < 0.001, Fig. 1b).
a HHV-6 encephalitis at day 100 in the entire cohort was 3.6%. b The Simon and Makuch plot reveals that the median OS was 323 days and NR in patients with and without HHV-6 encephalitis (p < 0.001). c The incidence of HHV-6 encephalitis at day 100 was 7.4% in the CBT group, 2.3% in the BMT group, and 0.9% in the PBSCT group (p < 0.001). d The incidence of HHV-6 encephalitis at day 100 was 11.5% in the broad-spectrum antiviral prophylaxis group, 2.8% in the LTV group, and 3.8% in the other antiviral drug group (p < 0.001).
The risk of developing HHV-6 encephalitis was significantly higher in patients who underwent CBT (7.4%, 95% CI 6.4–8.3%) than in those who underwent BMT (2.3%, 95% CI 1.7–2.9%) or PBSCT (0.9%, 95% CI 0.6–1.3%) (p < 0.001, Fig. 1c) as well as in patients who received broad-spectrum antiviral prophylaxis (11.5%, 95% CI 8.5–14.4%) compared to those who received LTV (2.8%, 95% CI 2.3–3.2%) or other antiviral drugs (3.8%, 95% CI 3.0–4.5%) (p < 0.001, Fig. 1d). Moreover, both HHV-6 encephalitis and CMV preemptive therapy or CMV disease occurred in 14 patients (3.0%) in the broad-spectrum antiviral group, 51 patients (1.0%) in the LTV group, and 25 patients (1.0%) in the other antiviral drugs group. Of these patients, 0/14, 9/51 (17.6%), and 5/25 (20.0%) patients had CMV preemptive therapy or CMV disease before HHV-6 encephalitis onset, respectively. The median time of onset of HHV-6 encephalitis was shorter in the LTV group (22 days) than in the broad-spectrum antiviral group (27 days). We performed MVA, including a stem cell source, to confirm the effects of antiviral prophylaxis on HHV-6 encephalitis (Table 2). CBT (hazard ratio [HR]: 2.90 [95% CI: 2.13–3.93]), broad-spectrum antiviral drugs (HR: 1.91 [95% CI: 1.35–2.69]), and grade II–IV acute GVHD requiring systemic corticosteroid (HR: 2.42 [95% CI: 1.75–3.33]) were independent risk factors (all p < 0.001), whereas PBSCT was an independent protective factor (HR, 0.41 [95% CI: 0.25–0.65]; p < 0.001).
CBT cohort
As described above, we confirmed that CBT as a stem cell source was a risk factor for HHV-6 encephalitis in the LTV era; however, we also considered that CBT could be a confounder in our analysis of antiviral prophylaxis and HHV-6 encephalitis. Therefore, to remove this potential confounding factor, we further analyzed the CBT cohort. In the CBT cohort (n = 2835, Supplemental Table 1), HHV-6 encephalitis was significantly higher in patients treated with broad-spectrum antiviral prophylaxis than in the other two groups (14.1% [95% CI: 10.3–17.8%] in the broad-spectrum antiviral group, 5.9% [95% CI: 4.7–7.0%] in the LTV group, and 7.4% [95% CI: 5.6–9.1%] in the other antiviral drug group, p < 0.001, Fig. 2a). Moreover, like the entire cohort, the median number of days for HHV-6 encephalitis onset was lesser in the LTV group (21 days) than in the broad-spectrum antiviral prophylaxis group (27 days). As non-MTX-based GVHD prophylaxis in CBT is a risk factor for HHV-6 encephalitis, we further divided the CBT cohort into two groups based on treatment (calcineurin inhibitor [CNI] and MTX or CNI and mycophenolate mofetil [MMF]). The median time of HHV-6 encephalitis onset in the two GVHD prophylaxis groups was 23 days. In both GVHD-prophylaxis groups, HHV-6 encephalitis was significantly higher in the broad-spectrum antiviral prophylaxis group than in the other two groups (CNI and MTX group: 11.8% [95% CI: 5.0–18.1%] in the broad-spectrum antiviral group, 3.2% [95% CI: 2.0–4.4%] in the LTV group, and 3.4% [95% CI: 1.4–5.3%] in the other antiviral drug group, p = 0.003, Fig. 2b; and CNI and MMF group: 15.2% [95% CI: 10.1–20.2%] in the broad-spectrum antiviral group, 8.7% [95% CI: 6.4–10.9%] in the LTV group, and 10.1% [95% CI: 7.4–12.8%] in the other antiviral drug group, p = 0.049, Fig. 2c). Broad-spectrum antiviral prophylaxis (HR: 1.95 [95% CI: 1.31–2.91]), grade II–IV acute GVHD requiring systemic corticosteroids (HR: 1.86 [95% CI: 1.23–2.83]), PIR requiring systemic corticosteroids (HR: 2.46 [95% CI: 1.77–3.41]), and CNI and MMF GVHD prophylaxis (HR: 2.01 [95% CI: 1.41–2.88]) were independent risk factors for HHV-6 encephalitis in the MVA (all p < 0.05, Table 2).
a HHV-6 encephalitis at day 100 after HSCT among CBT patients was 14.7% in the broad-spectrum antiviral prophylaxis group, 5.9% in the LTV group, and 7.4% in the other antiviral drug group (p < 0.001). b HHV-6 encephalitis at day 100 in CBT patients on CNI and MTX based GVHD prophylaxis was 11.8% in the broad-spectrum antiviral prophylaxis group, 3.2% in the LTV group, and 3.4% in the other antiviral drug group (p < 0.001). c HHV-6 encephalitis at day 100 in CBT patients on CNI and MMF based GVHD prophylaxis was 15.2% in the broad-spectrum antiviral prophylaxis group, 8.7% in the LTV group and 10.1% in the other antiviral drug group (p = 0.049).
Collectively, these results suggest that LTV prophylaxis was not associated with HHV-6 encephalitis regardless of the stem cell source. However, broad-spectrum antiviral prophylaxis was paradoxically associated with risk factors for HHV-6 encephalitis in the LTV era, especially in the CBT cohort, independent of GVHD prophylaxis.
Discussion
We re-evaluated the incidence of HHV-6 encephalitis in a modern, large-scale dataset due to the potential increase in HHV-6 encephalitis risk caused by reduction of anti-CMV drug use owing to LTV prophylaxis. This study showed that LTV prophylaxis was not associated with HHV-6 encephalitis, even in the CBT cohort; however, broad-spectrum antiviral prophylaxis was paradoxically associated with high HHV-6 encephalitis incidence in the LTV era. This suggests that broad-spectrum antiviral drugs (foscarnet, ganciclovir, valganciclovir) may not prevent HHV-6 encephalitis in high-risk patients. Overall, our results verify the risk of developing HHV-6 encephalitis in the LTV era. However, this paradoxical result needs to be further validated by an external patient cohort.
Several reports suggested that low-dose FCN (50–90 mg/kg/day) or GCV prophylaxis delayed the time to HHV-6 reactivation compared to the control group [9, 25], without showing the prophylactic efficacy on HHV-6 encephalitis onset [9, 25,26,27,28]. In addition, a previous study reported a higher incidence of HHV-6 encephalitis in the FCN group than in the standard treatment group (12.4% vs. 4.9% on day 60, p = 0.14) [9]. Our data are consistent with these previous reports showing a delay in the onset of encephalitis and significantly higher incidence of HHV-6 encephalitis in the broad-spectrum antiviral prophylaxis group, even in the MTX-based GVHD prophylaxis group of the CBT cohort.
Several hypotheses were proposed to explain why HHV-6 encephalitis was more prevalent in the broad-spectrum antiviral group. First, these prophylactic drugs may not penetrate the CNS owing to their low dosage. Second, the broad-spectrum antiviral group included more CBT patients than the other two groups (Table 1). Of note, approximately 30–40% of transplant patients in Japan received CBT [29], which is higher than in the US and Europe where CBT accounts for only 10% of HSCTs due to the expansion of haploidentical transplantation [30, 31]. This higher use of CB units may contribute to the higher incidence of HHV-6 encephalitis. Third, although no direct association has been observed between ciHHV-6 and HHV-6 encephalitis [19], there is a difference in terms of the genotype of ciHHV-6 in Japan and the US: HHV-6B accounts for 43% of ciHHV-6 cases in Japan [20] and 71% of ciHHV-6 cases in the US [19]. Fourth, reports of patients with HHV-6 myelitis, one of the HHV-6 end-organ diseases [8], have come mainly from Japan [6, 32, 33], suggesting that HHV-6 end-organ disease may depend on race. Taken together, the paradoxical finding that patients treated with broad-spectrum antiviral drugs exhibited a higher incidence of HHV-6 encephalitis may be attributed to not only the higher CBT patients in the broad-spectrum antiviral drug group, but also the differences in HHV-6 genotype, race, and phenotype of end-organ disease. However, further studies are warranted to validate our findings and to elucidate the detailed mechanisms underlying these new insights.
This study had some limitations. First, this was a retrospective, multicenter registry study, with various protocols. Second, as described in the methods section, the dosage of antiviral prophylactic drugs was unknown. However, it is generally challenging to describe the dosage of antiviral drugs because it is adjusted in clinical practice based on renal function, the severity of cytopenia, and the concomitant medications administered to each patient, which were unavailable in this study. Third, HHV-6-related outcomes other than HHV-6 encephalitis (such as HHV-6 reactivation in the blood/plasma detected by PCR or HHV-6 myelitis) were unknown because the database did not include these events of interest. Finally, although we confirmed the presence of previously reported risk factors for the development of HHV-6 encephalitis [2, 4,5,6], potential selection bias has to be considered—that is, patients with other unknown risk factors of HHV-6 encephalitis may have been more likely to receive broad-spectrum antivirals or more likely to undergo the HHV-6 DNA quantification in the CSF. However, HHV-6 DNA quantification is not covered by Japanese health insurance.
In summary, although LTV prophylaxis reduced the use of anti-CMV drugs, it was not a risk factor for HHV-6 encephalitis. However, paradoxically, broad-spectrum antiviral prophylaxis was associated with HHV-6 encephalitis in the LTV era. Further studies are warranted to validate these paradoxical findings and to elucidate the mechanisms underlying these new insights.
Data availability
The data in this study are not publicly available due to ethical restrictions that exceed the scope of the recipient/donor’s consent for research use in the registry. Data may be obtained from the corresponding author upon reasonable request and with permission from the JSTCT/JDCHCT.
References
Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;57:671–81.
Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transpl. 2012;18:1638–48.
Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transpl. 2013;48:574–80.
Ogata M, Kikuchi H, Satou T, Kawano R, Ikewaki J, Kohno K, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193:68–79.
Ogata M, Oshima K, Ikebe T, Takano K, Kanamori H, Kondo T, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 2017;52:1563–70.
Miyashita N, Endo T, Onozawa M, Hashimoto D, Kondo T, Fujimoto K, et al. Risk factors of human herpesvirus 6 encephalitis/myelitis after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2017;19:e12682.
Hakki M, Aitken SL, Danziger-Isakov L, Michaels MG, Carpenter PA, Chemaly RF, et al. American Society for transplantation and cellular therapy series: #3—prevention of Cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:707–19.
Ward KN, Hill JA, Hubacek P, de la Camara R, Crocchiolo R, Einsele H, et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019;104:2155–63.
Ogata M, Takano K, Moriuchi Y, Kondo T, Ueki T, Nakano N, et al. Effects of prophylactic foscarnet on human Herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transpl. 2018;24:1264–73.
Kim ES. Letermovir: first global approval. Drugs. 2018;78:147–52.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl J Med. 2017;377:2433–44.
Mori Y, Jinnouchi F, Takenaka K, Aoki T, Kuriyama T, Kadowaki M, et al. Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transpl. 2021;56:853–62.
Vyas A, Raval AD, Kamat S, LaPlante K, Tang Y, Chemaly RF. Real-world outcomes associated with letermovir use for cytomegalovirus primary prophylaxis in allogeneic hematopoietic cell transplant recipients: a systematic review and meta-analysis of observational studies. Open Forum Infect Dis. 2023;10:ofac687.
Kampouri E, Krantz EM, Zamora D, Kimball LE, Kiem E, Lovas EA, et al. 2109 HHV-6 and EBV reactivation after allogeneic hematopoietic cell transplantation in the era of letermovir for CMV prophylaxis: a retrospective cohort study. Open Forum Infect. Dis. 2022;9:ofac492.1730.
Srinivasan K, Spallone A, Khawaja F, Sassine J, Aramburo OM, Febres-Aldana AJ, et al. 2122. The Impact of HHV-6 DNAemia on hematopoietic cell transplant (HCT) recipients at high risk for CMV reactivation in the era of Letermovir. Open Forum Infect. Dis. 2022;9:ofac492.1743.
Kampouri E, Zamora D, Kiem ES, Liu W, Ibrahimi S, Blazevic RL, et al. Human herpesvirus-6 reactivation and disease after allogeneic haematopoietic cell transplantation in the era of letermovir for cytomegalovirus prophylaxis. Clin Microbiol Infect. 2023;29:1450.e1–1450.e7.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74.
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10.
Hill JA, Magaret AS, Hall-Sedlak R, Mikhaylova A, Huang ML, Sandmaier BM, et al. Outcomes of hematopoietic cell transplantation using donors or recipients with inherited chromosomally integrated HHV-6. Blood. 2017;130:1062–9.
Miura H, Kawamura Y, Hattori F, Kozawa K, Ihira M, Ohye T, et al. Chromosomally integrated human herpesvirus 6 in the Japanese population. J Med Virol. 2018;90:1636–42.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2009;15:367–9.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e381.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
El Jurdi N, Rogosheske J, DeFor T, Bejanyan N, Arora M, Bachanova V, et al. Prophylactic Foscarnet for Human Herpesvirus 6: effect on hematopoietic engraftment after reduced-intensity conditioning umbilical cord blood transplantation. Transpl Cell Ther. 2021;27:84.e81–84.e85.
Ogata M, Satou T, Kawano R, Goto K, Ikewaki J, Kohno K, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant. 2008;41:279–85. 2008/02/01
Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transpl. 2011;46:863–9.
Ogata M, Satou T, Inoue Y, Takano K, Ikebe T, Ando T, et al. Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transpl. 2013;48:257–64.
Yamamoto H. Single cord blood transplantation in Japan; expanding the possibilities of CBT. Int J Hematol. 2019;110:39–49.
Nagler A, Mohty M. In 2022, which is preferred: haploidentical or cord transplant? Hematol Am Soc Hematol Educ Program. 2022;2022:64–73.
Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transpl. 2016;51:778–85.
Ueki T, Hoshi K, Hiroshima Y, Sumi M, Ichikawa N, Ogata M, et al. Analysis of five cases of human herpesvirus-6 myelitis among 121 cord blood transplantations. Int J Hematol. 2018;107:363–72.
Nishimoto M, Nakamae H, Hayashi Y, Koh H, Nakane T, Yoshida M, et al. Prolonged sinus tachycardia caused by human herpesvirus 6 (HHV6) encephalomyelitis after allogeneic bone marrow transplantation. Intern Med. 2012;51:1265–7.
Acknowledgements
The authors are grateful for the work of all the physicians and data managers at the centers that contributed valuable data on transplantation to the JSTCT. We would also like to thank all of the members of the Transplant Registry Unified Management committees at JSTCT for their dedicated data management. We also thank Editage (https://www.editage.jp/) for English language editing. In addition, I, the first author, would like to thank Dr. Yoshimitsu Shimomura (Department of Hematology, Kobe City Hospital Organization Kobe City Medical Center General Hospital), Dr. Ayumi Fujimoto (Department of Hematology, Shimane University Hospital), and Dr. Kimimori Kamijo (Department of Hematology, Rinku General Medical Center) for giving me the technical advice to start this research. The authors did not receive financial support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Toshiki T designed the study, analyzed the data, and wrote the draft of the paper. K-I M, Shigeo F, SK, Takashi T, JK, MO, and KY designed the study or advised on methods and wrote the manuscript. YK, FI, and TF collected data, revised the manuscript, and were responsible for data management at JSTCT. YA managed the unified registry database and revised the manuscript. HN designed the study, advised on the methods, revised the manuscript, and was responsible for the project of JSTCT Donor/Source Working Group. All the other authors contributed to data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT (chat.openai.com), an AI language model developed by OpenAI, for English language proofreading and editing in order to improve the quality of the manuscript and to modify of statistical analysis code. After using ChatGPT, the authors reviewed and edited the content as needed to ensure accuracy and clarity and take full responsibility for the content of the publication.
Ethical approval
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was designed by the Donor/Source Working Group and the Transplant Complications Working Group of the JSTCT and was approved by the Transplant Registry Unified Management Program Data Management Committee of the JSTCT and the Institutional Review Board of Okayama University Hospital (2401-004), where the study was conducted.
Consent to participate
All patients provided written informed consent for data reporting.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terao, T., Matsuoka, Ki., Fuji, S. et al. Association between human herpesvirus-6 encephalitis and antiviral prophylaxis after allogeneic hematopoietic stem cell transplantation in the letermovir era. Bone Marrow Transplant 59, 1224–1231 (2024). https://doi.org/10.1038/s41409-024-02313-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02313-3
- Springer Nature Limited