Abstract
The efficacy of posttransplant cyclophosphamide (PTCy) and antithymocyte globulin (ATG) in controlling GVHD has been previously reported. We aim to study the safety and efficacy of the use of dual T-cell depletion with ATG and PTCy for peripheral blood reduced intensity conditioning regimen allo-HSCT in 270 patients with hematological malignancies. Median follow-up was 12.7 months. Nineteen percent of patients received grafts from a matched related donor, 46% from 10/10 matched unrelated donors (MUD), 16% from 9/10 MUD and 19% from haploidentical donors. Graft failure rate was 9%. CMV and EBV reactivation rates were 58 and 64%. The cumulative incidence of grade II–IV and III–IV acute GVHD at day + 100 was 20.1% and 4.6%, respectively. The CI of moderate/severe chronic GVHD at 1 year was 12.4%. There were no differences in the incidence of GVHD according to donor type. One-year OS, RFS, NRM, CIR, and GVHD-free/RFS respectively were 65.2%, 56.9%, 22.7%, 20.3%, and 47.6%. Higher disease-risk index and worse Karnofsky performance status were significant factors for poor outcomes. In conclusion, the use of T-cell dual depletion with ATG and PTCy results in very low rates of acute and chronic GVHD and acceptable relapse rates and NRM.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (allo-HSCT) is a potentially curative treatment for patients diagnosed with high-risk hematological disorders [1, 2]. However, it remains a challenging treatment with high morbidity and mortality [3].
Posttransplant cyclophosphamide (PTCy) decreases the risk of GVHD and graft rejection by inducing apoptosis of rapidly proliferating early alloreactive T cells and sparing regulatory T cells [4,5,6]. The efficacy of PTCy in combination with other immunosuppressive drugs preventing GVHD has been reported by several studies [7,8,9,10]. PTCy effectiveness using different graft sources was explored in a retrospective registry-based analysis and PTCy in combination with bone marrow sources resulted in lower rates of GVHD but higher cumulative incidence of relapse [11].
Anti-thymocyte globulin (ATG) is a commonly used agent for GVHD prophylaxis [12]. ATG depletes donor and recipient T cells reducing the risk of graft rejection and GVHD [12,13,14,15,16,17,18]. The efficacy of ATG in controlling cGVHD has been demonstrated on six randomized clinical trials [12,13,14,15,16,17,18]. In five out of six studies [12,13,14,15,16,17], the use of ATG did not affect survival or relapse rates. However, there remains a concern that the use of ATG may reduce graft-versus-leukemia effect, and impair passive transfer of memory T cells that reconstitute early immunity [18,19,20].
In October 2015, RIC regimen combined with rabbit-ATG, PTCy and cyclosporine (CsA) for the prevention of GVHD was established as the institutional standard of care in the Princess Margaret Cancer Centre, using peripheral blood stem cell (PBSC) grafts. The hypothesis for this protocol was that combining ATG and PTCy in T-cell replete-PBSC transplants would minimize GVHD rates without affecting engraftment and relapse rates. Peripheral blood was the selected donor source in order to reduce relapse rate, reduce time to engraftment, and thereby reduce infection rates.
The aim of the study is to report the outcome for all patients who underwent allo-HSCT with this protocol from October 2015 to May 2018 in the Center, and to explore the impact of donor type on GVHD and survival rates. There have been published reports documenting the safety of this protocol in MUD and haplo-HSCT [21,22,23,24,25,26]. However, this study includes a larger and more heterogeneous cohort of patients with an updated follow-up.
Patients and methods
Patient selection
Between October 2015 and May 2018, 270 adults diagnosed with hematological malignancies underwent allo-HSCT in the Center. All patients received reduced conditioning regimen and ATG–PTCY–CsA for GVHD propylaxis and were included in the analysis. General eligibility criteria for allo-HSCT are summarized in the Supplementary material. All patients provided informed consent prior allo-HSCT.
The present study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee at the Princess Margaret Cancer Centre, Toronto, Canada.
Donor selection and source of graft
High resolution molecular typing for HLA classes I (A, B, C) and II (DR, DQ) was performed for recipients and donors. Donor-specific antibodies (DSA) were assessed once the donor was identified, and repeated prior to transplant in all recipients. A MRD was preferred as first choice. In the absence of a MRD, a 10/10 MUD was selected as the second choice. For recipients who did not have a suitable MRD or MUD, alternative stem cell sources such as 9/10 HLA mismatched unrelated donors, and HLA haploidentical family members were considered.
All recipients received T-cell replete-PBSC grafts. The median CD34 + cell dose requested for infusion was 4 × 10^6 CD34+/kg for MRD, 10/10MUD and 9/10MUD, and 6 × 10^6 CD34+/kg for haploidentical donors.
Conditioning regimen, posttransplant immunosuppression, and supportive care
The present conditioning regimen and GVHD prophylaxis are summarized in Fig. 1.
RIC regimen comprised fludarabine (Flu) (30 mg/m2/day × 4 days from days-5 to -2), busulfan (Bu) (3.2 mg/kg/day × 2 days from days-3 to -2), and 200 cGy of TBI administered on day-1. GVHD prophylaxis consisted of rabbit- ATG (Thymoglobulin; Genzyme-Sanofi, Lyon, France), administered in doses of 0.5 mg/kg on day -3, 2 mg/kg on day-2 and 2 mg/kg on day-1 (total dose of 4.5 mg/kg), PTCy (50 mg/kg/day on days +3 to +4), and CsA, started at a dose of 2.5 mg/kg q12h IV on day +5 adjusted to achieve a therapeutic level of 200-400 ng/L, d. CsA tapering was started around day +45 to +60 for all patients without GVHD. Pharmacokinetics levels of ATG and cyclophosphamide were not routinely measured.
Definitions and disease monitoring are reported in the Supplementary material
Statistical method
Data was collected through retrospective chart review and updated in January 2019. Categorical variables were presented as counts and percentages. Continuous variables were presented with medians with ranges. Overall survival (OS), relapse-free survival (RFS) and GVHD-free/RFS survival (GRFS) were calculated using the Kaplan–Meier product-limit method and the impact of variables was assessed using Log-rank test. Non-relapse mortality (NRM) was estimated using the cumulative incidence method considering relapse as a competing risk. Cumulative incidence of relapse (CIR) was estimated considering death without relapse as competing event. The impact of variables on competing event analysis was compared using Gray’s test. Cumulative incidence of GHVD was estimated considering death and relapse as competing events. A multivariable Cox proportional hazards regression model was used to determine the effect of factors that were found to be statistically significant on the univariate analysis, and other factors considered clinically important. The multivariable analysis for OS and RFS included the following independent variables as control variables: Karnofsky performance status (70–80% vs 90–100%); disease risk index (low moderate risk vs high very-high risk); and donor type (all four donor types were included).
All p-values were two-sided and for the statistical analyses p < 0.05 was considered to indicate a statistically significant result. Statistical analysis was performed using the version 9.4 of the SAS system for Windows (Copyright© 2002–2012 SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
Patient characteristics are detailed in Table 1. Median age was 58 years (range: 18–74.5 years), and 154 (57%) of patients were males. Ethnicity and race were not recorded. Acute myeloid leukemia (AML) was the most prevalent diagnosis representing 53% of the entire cohort, followed by myelodysplastic syndrome (18%) and myelofibrosis (10%). Among patients diagnosed with AML, 26 (18%) had complex/high risk cytogenetic diagnosis. One hundred and twenty-eight (88.5%) recipients were in first complete remission (CR), 12 (8.3%) in second CR, and one in third CR. Three (0.2%) recipients were not in remission prior allo-HSCT. The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) and Karnofsly performance status (KPS) were assessed routinely at the time of the pre-transplant assessment. Median HCT-CI score was 1 (range: 0–7). Seventy-one (26%) of patients had a HCT-CI score ≥3, and 44 (16%) of recipients had a KPS ranged between 70 and 80%. Median follow-up in all 270 patients was 12.7 months (range 0.6–39).
Disease risk index (DRI) was the prognosis tool used to stratify disease-risk prior transplantation. Fifty-five (20%) patients had high (52) and very high [3] DRI prior allo-HSCT. Among these 55 patients, the median age was 61 (range: 20–74) and the baseline diagnoses were as follows: AML in 25 (45.4%) cases (22 patients had AML with complex cytogenetics in CR prior allo-HSCT and three recipients were not in CR), myelodysplastic syndrome with complex karyotype in 25 (45.5%) cases, acute lymphoblastic leukemia in second CR in five (9%) patients. Seven (12.7%) patients had an HCT-CI score ≥ 3, and 12 (21.8%) a KPS between 70 and 80%. Nine (16.4%) patients received grafts from MRD, 25 (45.5%) from 10/10 MUD, 7 (12.7%) from 9/10 MUD and 14 (25.5%) from haploidentical sibling donors.
Transplant characteristics
Transplant details are shown in Table 2. Cytokine release syndrome (CRS) was observed in five cases and appeared between day 0 and +1. It presented with fever in all cases, only grade 1 and 2 were documented, and were managed supportively in all cases. Engraftment syndrome occurred in 13 (5%) recipients, and all of them were successfully treated with steroids. Day 60 chimerism was assessed in 233 (86%) recipients; and in 198 (85%) cases was higher than 95% donor cells.
Graft failure (GF) was documented in 23 (9%) patients. Four (2%) recipients had primary GF and 19 (7%) secondary GF. Patients who underwent 9/10 MUD (21%) and haploidentical donor (17%) allo-HSCT, had a higher incidence of GF than those recipients who received MRD (2%) and MUD (3%) grafts (p < 0.0001). DSA were reassessed and in all cases were negative. Nine recipients underwent second salvage allo-HSCT and one recipient received donor lymphocyte infusions. Seventeen (74%) patients with GF died, and in most of them infection was the immediate cause of death.
Median length of transplant hospitalization was 29 days (range 20–180). Two hundred and forty-seven (92%) recipients had mucositis; 15 (8%) of them were grade 3–4, and 11 required a short course of total parenteral nutrition. Neutropenic fever occurred in 221 (82%) patients and 108 (40%) of them showed positive blood cultures. Forty-four (16%) recipients developed veno-occlusive syndrome (VOD). Moderate VOD was documented in 18 (7%) recipients and severe VOD in one. All cases resolved with fluid restriction, diuretics, dopamine intravenous infusion, and defibrotide (in one case).
Infectious complications
Cytomegalovirus (CMV) reactivation was observed in 156 (58%) patients. CMV titers were monitored by quantitative PCR in plasma samples during the first six months of follow-up or longer if on immunosuppression. The cut-off value for test positivity was >200 copies of CMV DNA per milliliter of plasma. Any positive result above that level was considered a CMV reactivation and preemptive treatment was initiated. First line of treatment was oral valgancyclovir or intravenous ganciclovir until viremia clearance. Eleven (4%) recipients developed CMV disease. First line of treatment was intravenous ganciclovir and intravenous immunoglobulin (Ig) replacement in those recipients with CMV pneumonitis, and in those with low IgG levels. Eight cases resolved with intravenous ganciclovir therapy.
Epstein–Barr virus (EBV) reactivation was documented in 171 (63.6%) recipients. The cut-off value for test positivity was >600 copies of EBV DNA per milliliter of plasma. Any positive result over that level was considered EBV reactivation. Preemptive treatment with rituximab for high EBV-DNA-emia was not routinely done. Twenty-three (8.5%) patients developed probable (6 patients) or biopsy proven (17 patients) posttransplant lymphoproliferative disease (PTLD). PTLD was managed by tapering immunosuppression and weekly rituximab for a maximum of four doses. One recipient required second line of treatment with R-CHOP chemotherapy. Twenty-one (91%) recipients achieved clinical responses with regression of affected nodal tissue (if any) and clearance of EBV-viremia. Two patients died secondary to PTLD.
Fifty-seven (21%) recipients developed BK hemorrhagic cystitis. Among these 57 patients, only four recipients had grade 3 and 4 BK cystitis, presenting significant urinary retention caused by macroscopic hematuria with clots. These four recipients required prolonged hospitalization, intravesical cidofovir, and one of them IV cidofovir. No patient developed acute kidney injury. Three of these four recipients had clinical response to treatment while the fourth developed secondary graft failure concomitantly with multiple infections.
Other viral infections were diagnosed in 80 (30%) patients. Proven or probable invasive fungal infection was identified in 21 (8%) recipients during the posttransplant course. Nine of these 21 patients died with an ongoing fungal infection. Three of these nine patients had antecedent graft failure.
Graft versus host disease
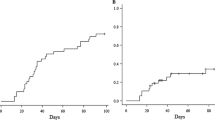
Detailed results are summarized in Table 3 and Supplementary Fig. 1. Overall, aGVHD was identified in 94 (35%) patients. However, grade III and IV aGVHD was diagnosed in 12 (4%) and 1 (1%) recipient, respectively. Nine (3%) cases had steroid refractory aGVHD. Fifty-three (20%) recipients were diagnosed with cGVHD. Moderate cGVHD was documented in 29 (11%) recipients and severe cGVHD in 7 (3%). Five patients with moderate/severe cGVHD had steroid refractory cGVHD. Four patients died secondary to severe GVHD: one due to acute gut GVHD, one secondary to steroid refractory acute gut and liver GVHD, one due to idiopathic pneumonia syndrome, and one secondary to chronic severe lung GVHD.
Figure 2 shows the cumulative incidence of GVHD according to donor type. The difference between the cumulative incidence of grade II–IV aGVHD (p = 0.440) and moderate–severe cGVHD according to donor type was not statistically significant (p = 0.738). Grade III–IV aGVHD was not analyzed according to donor type because of the low number of events.
Outcome
Main outcome information is summarized on Table 2 and on Table 4, and Figs. 2, 3 and 4. With a median follow-up of 12.7 months (range 0.6–39), 105 (39%) patients died and 62 (23%) relapsed. Main causes of death were infection (15%) and relapse (14%).
Results from the univariate and multivariate analysis are provided in the Table 4. Donor type had a significant impact on OS, RFS, NRM, CIR, and GRFS in the univariate analysis. Karnofsky performance status between 70 and 80% (HR 1.92 (95% 1.25–2.9); p = 0.003) and high/very high DRI prior allo-HSCT (HR 1.79 (95% CI 1.21–2.66); p = 0.003) were significant predictors for worse RFS in the multivariate analysis. KPS between 70 and 80% (HR 1.99 (95% CI 1.3–3.04); p = 0.008) and higher DRI (HR 1.99% (95% CI 1.3–3.04); p = 0.001) were also significant risk factors for worse OS. The use of 10/10 matched unrelated donors provided a protective effect in comparison with other donor sources for OS and RFS.
A sub-analysis among the 55 patients with higher DRI was done to explore this cohort in detail. Among these 55 patients, 33 (60%) died and 20 (36.4%) relapsed. Among these 20 recipients who relapsed, 80% died during the follow-up. Two years OS and RFS were respectively 31.2% (95% CI 17.8–45.6) and 29–8% (95% CI 17.1–43.5). NRM and CIR at 2 years were respectively 31% (95% CI 18.1–45) and 39.2% (95% CI 25.8–52.5).
Discussion
Allo-HSCT using un-manipulated-T-cell replete-PBSC grafts with ATG, PTCy, and cyclosporine, results in low rates of GVHD. A pilot report with data from patients undergoing unrelated and haploidentical allo-HSCT was reported [21,22,23, 26], and the efficacy of this protocol in AML and myelofibrosis patients has been explored [24, 25]. This study provides additional information from a more heterogeneous cohort and with an updated follow-up.
The combination of fludarabine, low-dose of busulfan, and TBI (200 cGy) is a well-tolerated regimen and induces sufficient immunosuppression to allow consistent engraftment. Transplant age was not a significant risk factor for survival. These results support that age may have less of an impact on defining eligibility criterion for allo-HSCT, and they underline the importance of pre-transplant performance status [27]. Relapse rates documented in the study were 23%. The feasibility of RIC allo-HSCT for high-risk hematological malignancies is well documented [28,29,30,31]. Those patients with high/very-high DRI had a significant higher risk of mortality in our study. DRI is a valuable prognostic tool [32] that can be used to identify a very high-risk population. Posttransplant disease control strategies could be considered for these patients, such as prophylactic donor lymphocyte infusions or maintenance chemotherapy.
Survival rates differed among donor type. Those recipients who received 10/10 MUD grafts had higher survival rates than those who received grafts from MRDs. This results contrast with findings from other studies [33], and may be partly explained by the smaller number of patients who received MRD grafts. However, with the evidence reported here, we hypothesize that MRD transplants would require less immunosuppression to control GVHD. The optimal dose of immunosuppression for each donor source is not well established. Further investigations are needed to determine the best dose of ATG, and to determine if it should be used in MRD transplant. Survival rates were comparable between patients who received 9/10 MUD and haploidentical donor grafts. Taking into account these results, and easier availability and lower costs, haploidentical donors may be preferred to 9/10 MUD for allo-HSCT, using the present conditioning regimen.
The combination of ATG, PTCy, and CsA reduces GVHD rates when PBSC grafts are used. PTCy may also potentiate the antineoplastic activity of the conditioning regimen decreasing relapse rates in patients with higher risk diseases [34]. PTCy decreases the risk of GVHD and graft rejection by inducing cell apoptosis of rapidly proliferating early alloreactive T-cells sparing regulatory T-cells and preserving nondividing hematopoietic stem cells [4,5,6]. The addition of ATG results in faster achievement of donor chimerism and increases control of GVHD [12,13,14,15,16,17,18]. A remarkable finding of the study is the absence of statistically significant differences in clinically relevant GVHD between donor types, suggesting that the new combination of ATG, PTCy, and CsA overcomes the HLA-barrier and its effect on GVHD.
Primary GF rates were comparable with published data; however secondary GF rates were higher [35, 36]. Observed rates of secondary GF, especially in mismatched allo-HSCT, could be secondary to the protracted T-cell depletion induced by this GVHD prophylaxis, leading to delayed donor T-cell engraftment. Unfortunately, T-cell reconstitution data are not available.
Infection was the most frequent cause of death. Despite the use of T-cell replete-grafts, observed rates CMV were high. The use of novel CMV prophylactic agents such as letermovir could be explored [37]. We attribute the high proportion of EBV-R and PTLD observed in the study to the use of ATG for T-cell depletion [38,39,40]. The selection of EBV matched donors and the use of pre-emptive rituximab could be considered when this GVHD prophylaxis is used [41, 42]. To reduce infectious toxicity and with the hypothesis of preserving the GVHD-preventing properties of this regimen, we have decided to reduce the dose of ATG to 2 mg/kg from current dose of 4.5 mg/kg for our patients. This modification may stimulate the recovery of donor T-cells and thereby reduce secondary GF rates.
The main limitations of this study are the retrospective design and the heterogeneity of the sample size. Future research will focus on differences in the effectiveness of the new treatment across different hematological disorders. Prospective multicenter clinical trials could be considered to validate the results in different transplant centers.
In conclusion, the evidence reported in this paper indicates that the combination of ATG, PTCy, and CsA is effective in reducing both acute and chronic GVHD in all donor types. Further optimization of the present protocol is needed to reduce NRM and disease relapse, for example using posttransplant disease control strategies for the very-high risk group of patients.
References
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–24.
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2002;8:377–86.
Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30.
Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
Fuchs EJ. Selective allodepletion: have we finally found the holy grail? Biol Blood Marrow Transpl. 2013;19:1413–4.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6.
Bashey A, Zhang MJ, McCurdy SRSt, Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for t-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transpl. 2006;12:560–5.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Baron F, Mohty M, Blaise D, Socie G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:224–34.
Lee KH, Lee JH, Lee JH, Kim DY, Park HS, Choi EJ, et al. Reduced-Intensity conditioning with busulfan, fludarabine, and antithymocyte globulin for hematopoietic cell transplantation from unrelated or haploidentical family donors in patients with acute myeloid leukemia in remission. Biol Blood Marrow Transpl. 2017;23:1555–66.
Deotare U, Atenafu EG, Loach D, Michelis FV, Kim DD, Thyagu S, et al. Reduction of severe acute graft-versus-host disease using a combination of pre transplant anti-thymocyte globulin and post-transplant cyclophosphamide in matched unrelated donor transplantation. Bone Marrow Transpl. 2018;53:361–5.
Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral blood stem cell transplants. Eur J Haematol. 2019;102:486–93.
Salas MQ, Kumar R, Viswabandya A. Impressive graft-versus-host disease-free, relapse-free survival in matched unrelated donor allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning and a combination of antithymocyte globulin and post-transplantation cyclophosphamide. Biol Blood Marrow Transpl. 2019;25:PE352–E353.
Salas MQ, Lam W, Datt Law A, Kim DDH, Michelis FV, Loach D, et al. Reduced intensity conditioning allogeneic transplant with dual T-cell depletion in myelofibrosis. Eur J Haematol. 2019;103:597–606.
Salas MQ, Prem S, Atenafu EG, Law AD, Lam W, Al-Shaibani Z, et al. Reduced intensity allogeneic stem cell transplant with anti-thymocyte globulin and post-transplant cyclophosphamide in acute myeloid leukemia. Eur J Haematol. 2019;103:510–8.
Law AD, Salas MQ, Lam W, Michelis FV, Thyagu S, Kim DDH, et al. Reduced-intensity conditioning and dual T lymphocyte suppression with antithymocyte globulin and post-transplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical hematopoietic stem cell transplants for hematological malignancies. Biol Blood Marrow Transpl. 2018;24:2259–64.
McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
Hamadani M, Mohty M, Kharfan-Dabaja MA. Reduced-intensity conditioning allogeneic hematopoietic cell transplantation in adults with acute myeloid leukemia. Cancer Control. 2011;18:237–45.
Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–84.
Santoro N, Labopin M, Ciceri F, Van Lint MT, Nasso D, Blaise D, et al. Impact of conditioning intensity on outcomes of haploidentical stem cell transplantation for patients with acute myeloid leukemia over 45 years of age. Cancer. 2019;125:1499–1506.
Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13.
Shouval R, Fein JA, Labopin M, Kroger N, Duarte RF, Bader P, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019.
Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A, Schmid C, et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:1810–22.
Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transpl. 2013;48:537–43.
Ozdemir ZN, Civriz Bozdag S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57:163–7.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–44.
Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–62.
Kanakry JA, Kasamon YL, Bolanos-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, et al. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis. Biol Blood Marrow Transpl. 2013;19:1514–7.
Kalra A, Roessner C, Jupp J, Williamson T, Tellier R, Chaudhry A, et al. Epstein-barr virus DNAemia monitoring for the management of post-transplant lymphoproliferative disorder. Cytotherapy. 2018;20:706–14.
Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transpl. 2011;17:591–7.
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802.
Acknowledgements
We thank our patients and the nursing and support staff in the Hans Messner Allogeneic Blood and Marrow Transplant Program.
Author information
Authors and Affiliations
Contributions
QS and SP collected the data, analyzed results, made the figures and wrote the paper. Both contributed equally to the writing and development of this paper. EA did the statistical analysis of the data AL, WL, ZA, DL, DK, FM, JL, RK, JM and AV provided valuable input into the study design, analysis, and interpretation and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Salas, M.Q., Prem, S., Atenafu, E.G. et al. Dual T-cell depletion with ATG and PTCy for peripheral blood reduced intensity conditioning allo-HSCT results in very low rates of GVHD. Bone Marrow Transplant 55, 1773–1783 (2020). https://doi.org/10.1038/s41409-020-0813-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0813-9
- Springer Nature Limited
This article is cited by
-
Reduced-toxicity conditioning regimen with low dose post-transplantation cyclophosphamide and low-dose anti-thymocyte globulin as graft-versus-host disease prophylaxis for haploidentical stem cell transplantation in older patients
Annals of Hematology (2024)
-
Post-transplant cyclophosphamide compared to sirolimus/tacrolimus in reduced intensity conditioning transplants for patients with lymphoid malignancies
Bone Marrow Transplantation (2024)
-
Acute kidney injury within 100 days post allogeneic hematopoietic cell transplantation is associated with increased risk of post-transplant complications and poor transplant outcomes
Bone Marrow Transplantation (2022)
-
Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial
Bone Marrow Transplantation (2022)
-
Allogeneic hematopoietic cell transplantation with cord blood versus mismatched unrelated donor with post-transplant cyclophosphamide in acute myeloid leukemia
Journal of Hematology & Oncology (2021)