Abstract
Background and importance
Syringomyelia, or the formation of fluid-filled cysts within the spinal cord, associated with delayed spinal arachnoiditis is an uncommon complication of aneurysmal subarachnoid haemorrhage. To date, about 18 cases have been reported in medical literature, with just two reported in patients under the age of 35 years.
Clinical presentation
A 27-year-old female patient complained of sudden, severe headaches in the occipital region, nuchal rigidity, and drowsiness when she presented at our institution. A head computed tomography scan revealed intraventricular bleeding in the lateral and fourth ventricles with more extensive haemorrhaging in the frontal horns. A left posterior inferior cerebellar artery (PICA) aneurysm was confirmed via digital subtraction angiogram, and endovascular embolization was done. Two years later, the patient reported intense pain in the lower back along with symptoms suggestive of spinal cord compression. Spinal magnetic resonance imaging (MRI) showed spinal adhesions from C1 to L4, syringomyelia with some vasogenic oedema extending from T3 to T9 level, and a cyst in the lumbar region. Consequently, a right hemilaminectomy was performed along with microsurgical release of arachnoid adhesions and placement of a subdural drain. Radiological and symptomatic improvements were observed. Since then, the patient’s clinical condition has remained stable during the past three years of follow-up visits.
Conclusions
Literature on optimal treatment modalities and patient prognosis is scarce and debated. The time for symptom improvement depends on the level and extent of spinal cord involvement. Rehabilitation may be required for most patients, as complete symptomatic recovery may not be attainable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background and importance
Aneurysmal subarachnoid haemorrhage (aSAH) accounts for ~85% of all subarachnoid haemorrhages and is linked to a high early mortality rate [1, 2]. It has a global incidence of 7.9 per 100,000 person-years, with females at a higher relative risk [3]. Other significant risk factors include age (>60 years), high blood pressure, smoking, alcohol use, oestrogen deficient status, and family history [2, 4]. A rare complication of aSAH is the development of delayed spinal arachnoiditis, which is characterized as persistent inflammation in the arachnoid mater that leads to scarring and nerve root tethering [5]. Spinal arachnoiditis appears to have a post-infectious pathoetiology, particularly in patients with compromised immune systems [6]. In isolated cases, spinal arachnoiditis has been reported to be associated with syringomyelia, the formation of fluid-filled cysts called syrinxes within the spinal cord.
Because of the rarity of the condition, knowledge of its clinical presentation, pathogenesis, management, and long-term outcomes is limited. In reviewing the literature, we were able to identify 18 cases with syringomyelia post-aSAH that were reported between 1940 and 2023 (Table 1) [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. All patients were above 45 years old, with two exceptions aged 22 and 35 years [7, 20]. Among the reported cases, seven patients were male and the most frequent cause of SAH was an aneurysm of the posterior inferior cerebellar artery (PICA). The latency period between surgical management of SAH and diagnosis of syringomyelia ranged from two weeks to 11 years. Patient follow-up period ranged from four months to six years. Herein, we present an illustrative case report of a young adult patient without known risk factors for aSAH who experienced delayed spinal arachnoiditis in the cervical region concomitant with syringomyelia in the upper thoracic spine. Additionally, we present the patient’s long-term follow-up, including her perspective on quality-of-life issues.
Clinical presentation
A 27-year-old female patient, with no prior history of serious illness, presented to the ambulance during the night with sudden-onset severe headaches in the occipital region while sleeping, nuchal rigidity when moving the head, drowsiness, and two episodes of vomiting. The patient, working as a nurse, reported no prior medication use, alcohol consumption, smoking habit, or use of narcotics. At the time of presentation to the ambulance, her vital signs were stable (BP—120/80 mmHg; pulse—90 beats per minute; respiratory rate—16 breaths per minute; and Glasgow Coma Scale GCS—15 points). The patient received an intravenous 2 ml bolus of 500 mg/ml Metamizole for pain management, intravenous 2 ml bolus of 10 mg/2 ml Metoclopramide as an antiemetic, and saline for fluid replenishment.
A CT scan of the head without contrast was done and showed intraventricular bleeding in the lateral and fourth ventricles, with more extensive bleeding in the frontal horns. Additionally, bleeding was found around the foramen magnum that extended into the spinal canal (Fig. 1). The on-call radiologist recommended a magnetic resonance imaging (MRI) scan to eliminate differential diagnoses including malignant lesions and arteriovenous malformations. Clinically, the patient rapidly deteriorated and experienced a decline in consciousness within one hour, registering a GCS score of nine points. Consequently, the patient was transferred to the intensive care unit and underwent a ventriculostomy. The following morning, the patient was electively intubated, and a digital subtraction angiography (DSA) was performed, revealing an aneurysm of the left PICA in the P1-P2 segment (Fig. 2). The patient was prescribed Tab. Nimodipine 60 mg six times a day.
The left PICA aneurysm was successfully treated through endovascular embolization. An intraoperative CT scan was done to rule out any procedural complications. The CT revealed an enlarged ventricular system, diffuse subarachnoid haemorrhage (Fisher IV), and intraventricular haemorrhage. The patient was extubated during the day with complaints of headache. Three days later the patient complained of increasing intensity of headache, nuchal rigidity, and became bradycardic (pulse 56 bpm). Another CT head scan was performed, revealing infratentorial and supratentorial oedema, subarachnoid bleeding, and signs of haemorrhage in the cerebral aqueduct and fourth ventricle. Consequently, the drainage rate of cerebrospinal fluid (CSF) was increased to decrease the oedema. A check CT taken a week later showed residual blood in the ventricles and SAH spaces without ischaemic complications or hydrocephalus. Consequently, the ventriculostomy was evacuated after being in place for about ten days.
The patient was transferred to the semi-intensive neurosurgery ward with a GCS score of 15, no focal deficits, and mild nuchal rigidity. Three days later, the patient reported feeling drowsy, sleepy, and experiencing increased nuchal rigidity, resulting in a GCS score of fourteen points. No changes were seen in the CT head scan. A lumbar puncture was performed at the L3-L4 level, and the purulent CSF so obtained was found to be positive for Klebsiella pneumoniae. The bacteria were sensitive to meropenem. Based on the recommendation of the infectologist, intravenous meropenem 2 g was started thrice daily for six weeks due to suspected secondary meningitis and ventriculitis. The patient’s condition remained stable for the following week with signs of infection reduction as shown by repeated lumbar punctures.
A physiotherapist examined the patient and reported symmetrical muscle strength, with no signs of paresis or sensory deficits. The patient was fully independent and able to walk outside of the ward. However, the physiotherapist observed slight coordination deficits and emotional disturbances, along with right-sided paresis of the abducens nerve as confirmed by the ophthalmologist’s consultation. Two weeks later, the patient was discharged from the hospital with a referral to undergo DSA in three months and to enrol in a rehabilitation centre. At the time of discharge, the patient complained of mild pain in the lumbar region, though, we did not observe any paresis or meningeal signs. Four months later, the patient underwent the follow-up DSA, revealing full occlusion of the PICA aneurysm (Montreal A). No cognitive changes were observed (GCS 15 points), and no signs of abducens nerve palsy were noted. The patient was instructed to return for another DSA after one year. The subsequent follow-up DSA confirmed stable full occlusion of the aneurysm.
Re-hospitalization due to delayed spinal arachnoiditis and syringomyelia
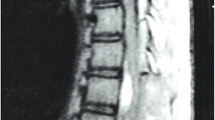
However, several months later, the patient reported severe lower back pain and symptoms of spinal cord compression, including weakened muscle strength (particularly on the right side), slight clumsiness in the right leg for six months, and loss of deep sensations, including proprioception, in the legs. The patient also experienced difficulties in beginning and maintaining urinary stream and loss of temperature sensation in the lower body. The patient underwent an MRI scan that revealed a SAH bleed and bacterial meningitis complications (spinal adhesions) from C1-L4. Syringomyelia with some vasogenic oedema and spinal deformity were observed extending from T3 to T9 level with maximal deformity in the T3-T4 region. A cyst was also visualized in the lumbar region (Fig. 3). The Babinski sign was found to be positive.
Preoperative T2 sequence MRI images in the (A) sagittal and (B) axial axis of thoracic spine showing dural adhesions (red circle) and septations, more pronounced at T2-T3 level, with arachnoid cysts (red and green arrows) above and below this level; (C) Spinal cord deformity and dislocation, with slightly widened central canal suggesting syringomyelia (yellow arrow) and some vasogenic oedema in spinal cord central parts.
Accordingly, a right hemilaminectomy was performed at the T3-T4 level with microsurgical release of arachnoid adhesions. The patient requested to postpone the lumbar cyst drainage procedure. A subdural drain was then inserted to prevent adhesions from blocking CSF flow through the spinal canal. MRI showed improvement in CSF circulation and marked reduction of spinal cord oedema (Fig. 4). Post-operative consultation with the physiotherapist showed unstable gait and decreased muscle strength on the right side. Although voiding dysfunction persisted, the patient was able to walk unassisted. The patient was discharged from the hospital after nine days with mild neurological deficit and a referral to a rehabilitation centre. Three months later, a follow-up DSA was conducted, which showed no changes in the findings—full occlusion of the aneurysm (Montreal A). Since then, patient’s clinical and radiological status has remained unchanged for the past three years during follow-up visits.
Postoperative T2 sequence MRI images in the (A) sagittal axis and (B) T2 merge axial images after right sided laminotomy, arachnoidolysis, and placement of a cysto-subdural shunt (red circle) at the T2-T3 level posterior to the spinal cord. Improvement of CSF circulation and marked reduction of spinal cord oedema can be visualized (pink arrow).
Patient perspective
About a year and a half after experiencing SAH with an aneurysm rupture and bacterial meningitis, I began feeling a sense of weightiness in my right leg. I became clumsier, my gait became unsteady, and I experienced a change in temperature sensation. It progressively became more challenging to engage in physical activities such as running and sports. I also observed issues with the pelvic organ functions, such as difficulty emptying my bladder and decreased sensation in the perineal area. I did various MRIs and other functional exams to establish the diagnosis. After consulting with a few neurologists, the final diagnosis of spinal cord compression was determined, accompanied by spinal cord oedema and spinal arachnoiditis with arachnoid cysts in the spinal canal.
Currently, after surgery and multiple physiotherapy and rehabilitation sessions, I feel an increase in the load tolerance and muscle strength in my right leg. However, significant balance disorders and muscle spasms persist. I continue to experience a loss of sensation, particularly in my foot, leading to frequent falls. Additionally, I feel uncomfortable when sitting for prolonged periods of time. Also, functional disorders of the pelvic organs persist - including neurogenic bladder, bowel dysfunction, and sexual dysfunction—which disrupt daily life and emotional well-being. I do engage in physical activity such as walking, running, and stretching exercises, as well as deep breathing. According to the Adhesive Arachnoiditis classification developed by the Tennant Foundation, I am categorized as Stage II (moderate) [22]. I maintain a balanced diet and supplement it with nutrients such as curcumin, vitamins C and B12, choline and inositol, and magnesium.
Discussion
In our patient, it is plausible to suggest that both aSAH (non-infectious aetiology) and Klebsiella pneumoniae infection (infectious aetiology) may have contributed to the development of spinal arachnoiditis and syringomyelia. Although the pathophysiology underlying the development of spinal arachnoiditis following aSAH remains to be determined, it is hypothesized that the haemorrhagic inflammatory response to aneurysmal rupture plays a pivotal role. This process, initiated at the rupture site, continues through the fibroproliferative cascade, leading to the development of arachnoiditis at any point along the neural axis [15].
With regard to the infectious aetiology of post-meningitis spinal arachnoiditis and syringomyelia, several bacterial, fungal, and viral agents have been described in the literature. The most frequently reported pathogen appears to be Mycobacterium tuberculosis [5, 23,24,25], followed by isolated cases of pathogens such as Listeria and Candida [26, 27]. In the context of Klebsiella infections, while meningitis has been routinely described in the literature as a post-neurosurgical nosocomial infection, other central nervous system infections have been observed to be exceedingly rare. Thus far, we have been unable to find a documented case of Klebsiella meningitis progressing to spinal arachnoiditis and syringomyelia.
It is possible that spinal arachnoiditis may develop because of persistent aseptic inflammation in individuals who have previously developed meningitis [28]. The associated leptomeningeal scarring could trigger a delayed inflammatory fibroproliferative reaction resulting in fibrino-collagenous exudate that adheres the nerve roots to either themselves or the thecal sac (adhesive arachnoiditis) [28, 29]. These scarring changes can further induce thrombotic changes in the meningeal and spinal cord vessels, obstructing vascular flow to the spinal cord and resulting in focal ischaemia and necrosis [23]. Alternatively, the scar can block the communication between the subdural and subarachnoid spaces, thereby shortening the length of the subarachnoid space and impairing the CSF flow dynamics. This attenuates the ability of the spinal theca to absorb the subarachnoid CSF pressure waves that act on the spinal cord above the scar [30], driving the CSF into these spaces and eventually into the central canal, leading to syrinx formation [5, 31, 32].
In adhesive arachnoiditis, it has been postulated that the scarring also exerts an outward pressure on the spinal cord, causing enlargement of the central canal and creation of a negative suction pressure, which in turn leads to accumulation of fluid within the central canal [32]. The sustained drainage of the syrinx can result from several different sources, including the disrupted tissue planes (due to shearing stress from scarred meninges) and venous congestion of supplying vessels [33]. Obstruction and subsequent expansion of the perivascular Virchow-Robin spaces (due to compression of capillary circulation by dilated parenchyma) may result in the formation of small pools of extracellular fluid that drain into the syrinx [34, 35]. Additionally, unabsorbed subarachnoid pressure waves exert compressive force on the spinal cord, pushing more CSF into the syrinx [30]. Lastly, the compression of the capillaries under force reduces the absorptive surface area, limiting capillary capacity to remove CSF from the syrinx [36].
The primary treatment reported in literature for syringomyelia associated with arachnoiditis is the laminectomy procedure accompanied by adhesion microlysis and/or insertion of a shunt. It has been demonstrated that surgical removal of the obstruction has the potential to reverse the clinical symptoms and to provide long-term resolution [30]. However, the extent to which these symptoms are alleviated is directly proportional to the number of spinal segments affected, with involvement of four or more spinal segments necessitating shunting [30]. Shunting CSF to the subarachnoid, pleural, or peritoneal space enables unobstructed and continuous flow, eliminating the necessity for multiple cystic drainages while also producing sustained enhancements in neurological complications [37, 38]. Nonetheless, the efficacy of shunting has been a topic of debate in literature due to potential complications such as blockage, migration, or infection [38, 39]. Naturally, it is challenging to conduct comparative trials or observational studies to evaluate these shunting techniques due to the small sample size, lack of long-term follow-up, and failure to reach statistical significance.
Patient prognosis also is a topic of ongoing debate in the literature. While a review of the literature (Table 1) indicates symptomatic and radiological improvement in all observed patients, full recovery is exceptionally rare. Time to symptom improvement also varies depending on the severity of symptoms and the level and extent of involvement of the spinal cord. A few patients also experienced recurrence, although the cause remains to be investigated. Repeated surgical manipulation seems to provide relief [13]. Patients often require physical rehabilitation to improve day-to-day function and quality of life.
Conclusions
Delayed spinal arachnoiditis associated with syringomyelia is a rare complication of aneurysmal SAH. Risk factors include female gender, aneurysm in the posterior circulation, and advanced age. Primary treatment includes laminectomy accompanied by adhesion microlysis and shunt placement. Rehabilitation may be necessary in most patients, as full symptomatic recovery may not be achieved.
Timeline
A timeline of the key events has been summarized in Fig. 5.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18. https://doi.org/10.1016/S0140-6736(07)60153-6.
Ziu E, Khan Suheb MZ, Mesfin FB. Subarachnoid Hemorrhage. In: StatPearls. Treasure Island (FL). StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK441958/.
Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–97. https://doi.org/10.1001/jamaneurol.2019.0006.
Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A. Trigger factors for rupture of intracranial aneurysms in relation to patient and aneurysm characteristics. J Neurol. 2012;259:1298–302. https://doi.org/10.1007/s00415-011-6341-1.
Nadeem SF, Baig AN, Tariq QUA, Shamim MS. Spinal arachnoiditis and syringomyelia: review of literature with emphasis on postinfectious inflammation and treatment. Surg Neurol Int. 2022;13:299. https://doi.org/10.25259/SNI_383_2022.
Di Ieva A, Barolat G, Tschabitscher M, Rognone E, Aimar E, Gaetani P, et al. Lumbar arachnoiditis and thecaloscopy: brief review and proposed treatment algorithm. Cent Eur Neurosurg. 2010;71:207–12. https://doi.org/10.1055/s-0029-1243201.
Lorenzana-Honrado L, Cabezudo-Artero JM, Gozez-Perals L. Arachnoid cyst. J Neurosurg. 1996;85:734–5. https://doi.org/10.3171/jns.1996.85.4.0734a.
Taguchi Y, Suzuki R, Okada M, Sekino H. Spinal arachnoid cyst developing after surgical treatment of a ruptured vertebral artery aneurysm: a possible complication of topical use of fibrin glue. Case report. J Neurosurg. 1996;84:526–9. https://doi.org/10.3171/jns.1996.84.3.0526.
Tumialán LM, Cawley CM, Barrow DL. Arachnoid cyst with associated arachnoiditis developing after subarachnoid hemorrhage. Case report. J Neurosurg. 2005;103:1088–91. https://doi.org/10.3171/jns.2005.103.6.1088.
Marshman LA, David KM, King A, Chawda SJ. Delayed fibrotic obliteration of the spinal subarachnoid space after cerebral aneurysmal subarachnoid hemorrhage: case report. Neurosurgery. 2007;61:E659–60. https://doi.org/10.1227/01.NEU.0000290920.55470.EC.
Eneling J, Boström S, Rossitti S. Subarachnoid hemorrhage-associated arachnoiditis and syringomyelia. Clin Neuroradiol. 2012;22:169–73. https://doi.org/10.1007/s00062-011-0082-5.
Abel TJ, Howard MA 3rd, Menezes A. Syringomyelia and spinal arachnoiditis resulting from aneurysmal subarachnoid hemorrhage: Report of two cases and review of the literature. J Craniovertebr Junction Spine. 2014;5:47–51. https://doi.org/10.4103/0974-8237.135227.
Abhinav K, Bradley M, Aquilina K, Patel NK. Spinal arachnoiditis and cyst formation with subarachnoid haemorrhage. Br J Neurosurg. 2012;26:574–5. https://doi.org/10.3109/02688697.2011.651512.
Ishizaka S, Hayashi K, Otsuka M, Fukuda S, Tsunoda K, Ushijima R, et al. Syringomyelia and arachnoid cysts associated with spinal arachnoiditis following subarachnoid hemorrhage. Neurol Med Chir. 2012;52:686–90. https://doi.org/10.2176/nmc.52.686.
Nakanishi K, Uchiyama T, Nakano N, Fukawa N, Yamada K, Yabuuchi T, et al. Spinal syringomyelia following subarachnoid hemorrhage. J Clin Neurosci. 2012;19:594–7. https://doi.org/10.1016/j.jocn.2011.07.035.
Rahmathulla G, Kamian K. Compressive cervicothoracic adhesive arachnoiditis following aneurysmal subarachnoid hemorrhage: a case report and literature review. J Neurol Surg Rep. 2014;75:e56–61. https://doi.org/10.1055/s-0033-1363506.
McAlpine H, Adamides AA. Acute cervical cord syrinx after aneurysmal subarachnoid haemorrhage. J Clin Neurosci. 2016;32:143–5. https://doi.org/10.1016/j.jocn.2016.03.016.
Davidoff CL, Liu S, Wong JHY, Koustais S, Rogers JM, Stoodley MA. Treatment of syringomyelia in patients with arachnoiditis at the craniocervical junction. World Neurosurg. 2017;107:565–73. https://doi.org/10.1016/j.wneu.2017.08.064.
Machida A, Fujii M, Ishihara T, Amano E, Otsu S, Fujii S, et al. Syringomyelia due to lumbar spinal fluid drainage in the acute phase of subarachnoid hemorrhage: a case report. J Stroke Cerebrovasc Dis. 2018;27:e11–e14. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.038.
Huang S-S, Ke Y-L, Hsieh W-C, Hsu H-C, Chen C-M. Secondary syringomyelia after subarachnoid hemorrhage: a case report. Rehabil Pract Sci. 2020;48:8 https://doi.org/10.6315/TJPMR.202012_48(2).0008
Nagashima Y, Nishimura Y, Ito H, Nishii T, Oyama T, Saito R. Diagnosis and treatment strategies for arachnoiditis ossificans following subarachnoid hemorrhage: a case report. NMC Case Rep J. 2022;9:295–9. https://doi.org/10.2176/jns-nmc.2022-0036.
Tennant Foundation. Stages and categorization of AA. Adhesive Arachnoiditis (AA): Bulletin No. 23. 2023. https://www.acmcrn.org/_files/ugd/5a5fb2_67b13790ecb24413a45972280b11fbbc.pdf?index=true.
Fehlings MG, Bernstein M. Syringomyelia as a complication of tuberculous meningitis. Can J Neurol Sci. 1992;19:84–7.
Hui AC, Chan YL, Kay R. Syrinx and tuberculoma formation in tuberculous arachnoiditis. Can J Neurol Sci. 2001;28:148–9. https://doi.org/10.1017/s0317167100052847.
Kannapadi NV, Alomari SO, Caturegli G, Bydon A, Cho SM. Management of syringomyelia associated with tuberculous meningitis: a case report and systematic review of the literature. J Clin Neurosci. 2021;87:20–5. https://doi.org/10.1016/j.jocn.2021.01.052.
Nardone R, Alessandrini F, Tezzon F. Syringomyelia following Listeria meningoencephalitis: report of a case. Neurol Sci. 2003;24:40–3. https://doi.org/10.1007/s100720300021.
Phanthumchinda K, Kaoropthum S. Syringomyelia associated with post-meningitic spinal arachnoiditis due to Candida tropicalis. Postgrad Med J. 1991;67:767–9. https://doi.org/10.1136/pgmj.67.790.767.
Karschnia P, Kaulen L, Thon N, Baehring JM. Clinical reasoning: a 64-year-old man with history of meningitis presenting with proximal weakness of the arms. Neurology. 2022;98:208–13. https://doi.org/10.1212/WNL.0000000000013085.
Safi S, Thabat A, Arshad M, Hanoun M. Arachnoiditis—a challenge in diagnosis and success in outcome—case report. Interdiscip Neurosurg. 2021;25:101219 https://doi.org/10.1016/j.inat.2021.101219
Heiss JD, Snyder K, Peterson MM, Patronas NJ, Butman JA, Smith RK, et al. Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine. 2012;17:367–80. https://doi.org/10.3171/2012.8.SPINE111059.
Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990;53:106–13. https://doi.org/10.1136/jnnp.53.2.106.
Tsitouras V, Sgouros S. Syringomyelia and tethered cord in children. Childs Nerv Syst. 2013;29:1625–34. https://doi.org/10.1007/s00381-013-2180-y.
Williams B. On the pathogenesis of syringomyelia: a review. J R Soc Med. 1980;73:798–806. https://doi.org/10.1177/014107688007301109.
Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet. 1972;2:799–801. https://doi.org/10.1016/s0140-6736(72)92152-6.
Kakar A, Madan VS, Prakash V. Syringomyelia—a complication of meningitis—case report. Spinal Cord. 1997;35:629–31. https://doi.org/10.1038/sj.sc.3100420.
Giner J, Pérez López C, Hernández B, Gómez de la Riva Á, Isla A, Roda JM. Update on the pathophysiology and management of syringomyelia unrelated to Chiari malformation. Neurologia. 2019;34:318–25. https://doi.org/10.1016/j.nrl.2016.09.010.
Zuev AA, Lebedev VB, Pedyash NV, Epifanov DS, Levin RS. Lechenie siringomielii, assotsiirovannoĭ s adgezivnym arakhnoiditom [Treatment of syringomyelia associated with adhesive arachnoiditis]. Zh Vopr Neirokhir Im N N Burdenko. 2017;81:39–47. https://doi.org/10.17116/neiro201781339-47.
Cacciola F, Capozza M, Perrini P, Benedetto N, Di Lorenzo N. Syringopleural shunt as a rescue procedure in patients with syringomyelia refractory to restoration of cerebrospinal fluid flow. Neurosurgery. 2009;65:471–6. https://doi.org/10.1227/01.NEU.0000350871.47574.DE.
Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233–40. https://doi.org/10.3171/jns.1997.86.2.0233.
Author information
Authors and Affiliations
Contributions
NJ and KA conceptualized the present report while KA, NJ, and IP were involved in data collection. KA and LJ were responsible for data interpretation and methodology. NJ and LJ were responsible for visualizations. IP, KA, and NJ wrote the initial draft while all authors were responsible for revisions. KA and AM supervised and administered the project and were responsible for funding. All authors have read the final manuscript and agree to its publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethics approval was not requested since the patient was treated under the local and institutional guidelines.
Consent for publication
The patient has given written and verbal consent for the current case report. Additionally, the patient had graciously agreed to write and publish their personal experiences and quality of life issues in the report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jain, N., Jaunozolina, L., Putraima, I. et al. Delayed spinal arachnoiditis with syringomyelia following aneurysmal subarachnoid haemorrhage: a case report with patient experience. Spinal Cord Ser Cases 10, 41 (2024). https://doi.org/10.1038/s41394-024-00654-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-024-00654-1
- Springer Nature Limited