Abstract
Background and importance
Spontaneous spinal subdural hematomas are rare. Their occurrence in a child with congenital von Willebrand disease and the complication of their surgery by a large secondary syringomyelia have never been previously reported.
Case presentation
A 13-year-old girl with congenital von Willebrand disease presented to our emergency department in January 2011 for sudden onset of severe back pain centered in her thoracic spine rapidly aggravated by signs of acute myelopathy without any precipitating factor. MRI scan revealed a thoracic subdural collection anterior to the spinal cord at the T7–T9 level, hyperintense on T1- and T2-weighted sequences consistent with an acute spinal subdural hemorrhage. Evacuation of the subdural hematoma was realized immediately after hemostasis parameter correction, and post-operative course was uneventful with full functional recovery. One year later, the patient presented once again but with progressive and more severe myelopathy caused by a large syringomyelia extending from the T5 level to the conus medullaris. A syringopleural shunting was performed and the patient was unrolled under an intensive care and rehabilitation program. Her condition remarkably improved and she became able to walk independently within 2 weeks post-operatively.
Conclusions
von Willebrand disease should be included as a possible factor of spontaneous spinal subdural hemorrhage. Surgery is advised in emergency and can be associated with remarkable recovery especially in children. Delayed syringomyelia can complicate the post-operative course and can be successfully addressed by syringopleural shunting. Long-term clinical and radiological follow-up is advocated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with congenital or acquired clotting disorders are at increased risk for experiencing spontaneous hemorrhage into unusual sites. Subdural hematomas and other central nervous system (CNS) hemorrhages are uncommon but can represent a major cause of death and disability when they occur.
The occurrence of a spontaneous acute spinal subdural hematoma in a child with congenital von Willebrand disease and the development of a large syringomyelia as a late post-operative complication, 1 year after initial surgery, have never been previously reported. The case is presented and the literature is reviewed.
Case report
A 13-year-old girl presented to the emergency department of our institution in January 2011 for sudden onset of severe back pain centered in her thoracic spine without any precipitating factor such as sports or trauma, rapidly aggravated few hours later by progressive weakness of both her lower limbs.
Her medical history was consistent for a congenital type I von Willebrand disease diagnosed in the perinatal period for recurrent epistaxis episodes. Two members of her family were also harboring the same disease diagnosed after several and various hemorrhagic episodes. She was under desmopressin treatment since the age of three.
On admission, her bladder function was also altered, with acute urinary retention. Examination of motor function in the lower limbs demonstrated anti-gravity strength proximally and 3/5 strength in the distal muscle groups. Tone in the lower extremities was decreased bilaterally. Sensory examination demonstrated a pinprick level at T7 and proprioception was significantly impaired in both lower extremities.
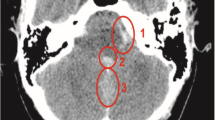
An MRI scan of the thoracolumbar spine was realized and demonstrated an extramedullary intradural fluid collection spanning anteriorly to the spinal cord from the T7 to T9 levels, hyperintense on T1- and T2-weighted sequences consistent with an acute spinal subdural hemorrhage [Fig. 1].
Based on these findings and the patient’s clinical status, urgent surgical evacuation was decided. Laboratory screening tests showed prolonged activated partial thromboplastin time, and concentrated blood-clotting factors containing von Willebrand factor and factor VIII were administered.
Immediately after the hemostasis parameter correction, the patient was taken to the operative theater where a T7–T9 laminectomy was realized. The hematoma was completely evacuated and the visible arachnoid layer seemed to be intact.
The post-operative course was uneventful and the patient recovered quickly. She was discharged home 3 days after surgery, and serial follow-up examinations were planned but unfortunately she was lost to view.
One year after initial surgery, she presented once again with a progressive but more severe myelopathy involving her lower limbs. Examination of motor function demonstrated 2/5 muscle strength in all groups. Second MRI examination identified a large syrinx extending from the T5 level to the conus medullaris [Fig. 2a]. Post-contrast MRI demonstrated no focal enhancement in the spinal cord or syrinx and a second surgery targeting the syrinx was decided.
We opted for a syringopleural shunting via the same approach. The dura was opened with care to preserve the underlying arachnoid membrane. The arachnoid was grossly abnormal and appeared white, opaque, and unusually vascular. The dense “arachnoid” was opened and lysis of adhesions was performed. The spinal cord was punctured in the midline between the two posterior recesses at the level of T8. The T-shaped catheter was then introduced into the syrinx cavity and subsequently attached to the dura and muscles as well. The other tip of the catheter was inserted into the pleural cavity via the T9–T10 intercostal space.
The patient was enrolled post-operatively under an intensive care and rehabilitation program. Her gait and balance disturbances improved substantially. Serial post-operative MRI scans showed progressive syrinx cavity regression [Fig. 2b] and stabilization of her delayed post-operative kyphotic deformity. She is able to walk independently at the most recent follow-up examination 2.5 years after the second surgery.
Discussion
Spontaneous acute spinal subdural hematoma was first described by Shiller and coworkers in 1948 [28].
For this article, we reviewed the PubMed and MEDLINE databases. Inclusion criteria were as follows: “Acute spinal subdural hematoma”/“Spontaneous spinal subdural hematoma”/“Nontraumatic spinal subdural hematoma” with “diagnostic imaging”/“factors”/“surgery”/“corticosteroids”. Spinal subdural hematomas associated with cranial subdural hematomas and those appearing spontaneously following lumbar puncture, anesthesia, or surgery were excluded. The total review population of spontaneous acute spinal subdural hematomas was 68 cases.
Age at onset varies from 1.4 to 81 years [17, 21, 28] with higher incidence in the fifth to the sixth decades of life (68 % of all cases). The thoracolumbar and lumbar spine segments were the most frequently involved with a slight male predominance (sex ratio M/F = 1.09).
Presenting symptoms include mainly low back pain, signs of acute myelopathy, or cauda equina syndrome, and from the imaging perspective, MR imaging best shows the extent of the hemorrhage and permits delineation from the epidural space.
In case of acute spinal subdural hematoma, like ours, an iso-hyperintensity is found on T1-weighted images and heterogeneous hyperintensity is found on T2-weighted images [2]. If no vascular malformation is found, selective spinal angiography must be considered [14].
The causative aspects of spontaneous acute spinal subdural hemorrhage are still speculative and no cause was found in 42 % of cases (n = 29). Anticoagulant therapies were incriminated in 33 % of cases (n = 23), and other responsible factors include hemophilia [28], cryoglobulinemia [34], thrombocythemia [16], polycythemia vera [9, 13], primary central nervous system vasculitis [10], systemic vasculitis (Wegener’s Granulomatosis) [11], fibromuscular dysplasia [15], increased pressure of intraspinal vessels in case of bilateral incarcerated inguinal hernia [24], poisoning with coumarinic rodenticide [22], and association to Churg-Strauss syndrome [18].
An intradural tumor hemorrhage [31, 35] and the rupture of a radicular artery aneurysm [19] and of an angiographically occult vascular malformation [14] were also described.
Only two cases of pediatric spinal subdural hematomas have been described [Table 1] and are to worth note: although congenital von Willebrand disease is the most common hereditary coagulation abnormality, no previous cases related to this condition have been reported to date.
Moreover, the pathogenesis of the spinal subdural hemorrhage is still unclear due to the lack of bridging veins in the spinal subdural space and the relatively too small size of the vessels that run on the undersurface of the dura.
Russell and Benoit [25] point out those vessels of substantive caliber (such as the major radiculomedullary arteries) that travel for the most part in the subarachnoid space. In fact, some authors have suggested that the spinal subdural hemorrhage may be caused by the extension of a spinal subarachnoid hemorrhage into the subdural space [1, 25] and that minor trauma leading to an increased intrathoracic and then intraluminal pressure of these vessels may cause rupture [6]. Such a theory is supported by three previously reported cases of associated subdural and subarachnoid hemorrhage we found in our review [5, 12, 14], but these findings are tempered by other reports, like ours, in which the subarachnoid space seems to be normal.
Treatment of an acute spinal subdural hematoma typically consists of surgical decompression and management of basal blood disorders. However, some recent reports emphasize the possible recovery under high-dose corticosteroids [32, 36] or simple conservative treatment [8, 12, 13, 23], especially in case of mild neurologic deficit and early recovery.
In 1984, Swann et al. were the first to treat a dorsally located spinal subdural hematoma by suction via a lumbar catheter [33], and in 1990, Schwerdtfeger et al. [27] added irrigation to suction with a good surgical outcome.
In the present case, a 13-year-old girl with congenital von Willebrand disease presented with signs of acute myelopathy due to a ventrally located spinal subdural hematoma. We preferred an urgent decompressive surgery via a posterior conventional laminectomy immediately after the infusion of prepared doses of concentrated blood-clotting factors containing von Willebrand factor and factor VIII leading to activated partial thromboplastin time correction. The recovery was full within few hours after surgery.
Post-operative complications are rare. Kim et al. [14] reported a neurologic aggravation 10 days following surgery due to a small remaining hematoma and severe adhesion between the arachnoid membrane and the cauda equina. A case of massive rebleeding necessitating urgent decompression 3 days after initial surgery, in a 73-year-old woman with polycythemia vera, was also described [9].
Syringomyelia as a late post-operative complication of an acute spinal subdural hematoma has never been previously reported. In fact, Siddiqi et al. [29] presented in 2005 the sole case of the literature in which a chronic spinal subdural hematoma was associated to a thoracic syrinx. But in that report, the 76-year-old woman presented with a 14-year history of progressive myelopathy and the two lesions were diagnosed simultaneously.
As the pathogenesis of syringomyelia, its relation to arachnoiditis, and the role of the spinal subdural hemorrhage are still unclear, several hypotheses have been postulated: the spinal subdural hematoma could either be an etiological factor in the formation of spinal arachnoiditis and then syringomyelia by means of its breakdown products which is probably the cause in the present case as the subarachnoid space seemed to be normal initially, or may be secondary to a subarachnoid hemorrhage diffusing secondarily into the subdural space, a condition Barnett, in 1973, already classified as a possible direct cause of spinal arachnoiditis [4].
Two possible mechanisms have been proposed for syrinx formation in case of spinal arachnoiditis: an ischemic model and a CSF flow model: inflammation of the meninges can lead to vessel scarring and subsequent cord ischemia, liquefaction, and cavitation [7]. Alternatively, arachnoiditis can cause cord tether into the dural tube and impair CSF flow [20].
Pressure dissociations between the upper and lower compartments of the subarachnoid space can form as a result, and pressure waves transmitted within the epidural veins during Valsalva maneuvers, along with the pressure dissociation, can force CSF into the central canal and expand the syrinx [7, 20].
In the present case, the syrinx occurrence was unexpected and its extension above the level of the hematoma was more surprising. Interestingly, although the neurological deterioration was more severe in the second admission, the post-operative outcome was excellent, which underscores the efficacy of the syringopleural shunting and outlines the possible recovery after second surgery especially in children.
If we observed such a remarkable recovery, this was not to prevent the kyphotic deformity that developed later at the level of the laminectomy. Such a delayed complication can be associated with a functional decline and should be taken seriously into consideration. Fortunately, we observed no additional deterioration both clinically and radiologically till the most recent follow-up evaluation, and whether it was better to use a laminoplasty rather than a laminectomy or not is still a matter of debate. In fact, although Ahmed et al. [3] and Simon et al. [30] emphasize that the surgical risk for developing a spinal deformity becomes statistically significant only when more than four laminae are removed, such a complication was also recently documented in a 17-year-old boy following a double spinal level laminoplasty [26].
Finally, lessons must be drawn from the present case and future challenges should be taken into consideration:
-
Spontaneous acute spinal subdural hematoma can complicate the course of congenital von Willebrand disease in young patients.
-
Surgery is advised in emergency after missing factor administration and can be associated with remarkable recovery.
-
Syringomyelia may occur as a late post-operative complication outlining the need for a long-term clinical and radiological follow-up.
-
The fact that this patient improved remarkably after the second surgery suggests that lysis of adhesions associated to syringopleural shunting is a suitable option to address this lesion and can carry an excellent prognosis especially in children.
-
As the causative aspects of the syrinx and its extension are still unclear, a higher number of cases and a better understanding of the pathogenesis of these forms of syringomyelia will be crucial.
Abbreviations
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
References
Abla AA, Oh MY (2000) Spinal chronic subdural hematoma. Neurosurg Clin N Am 3:465–471
Adamson DC, Bulsara K, Bronec PR (2004) Spontaneous cervical epidural hematoma: case report and literature review. Surg Neurol 62:156–159
Ahmed R, Menezes AH, Awe OO, Mahaney KB, Torner JC, Weinstein SL (2014) Long-term incidence and risk factors for development of spinal deformity following resection of pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr 13(6):613–621
Barnett H (1973) Syringomyelia associated with spinal arachnoiditis. In: Barnett H, Foster J, Hudgson P (eds) Syringomyelia. Saunders, London, pp. 220–224
Bernsen RA, Hoogenraad TU (1992) A spinal haematoma occurring in the subarachnoid as well as in the subdural space in a patient treated with anticoagulants. Clin Neurol Neurosurg 94:35–37
Calhoun JM, Boop F (1991) Spontaneous spinal subdural hematoma: case report and review of the literature. Neurosurgery 29(1):133–134
Caplan LR, Norohna AB, Amico LL (1990) Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry 53:106–113
Chung TT, Hsieh CT, Liu MY, Ju DT (2011) Spontaneous spinal subdural hematoma: a rare case report and review of the literature. J Med Sci 31(4):181–183
Cincu R, de Asis LF, Rivero D, Eiras J, Ara JR (2009) Spontaneous subdural hematoma of the thoracolumbar region with massive recurrent bleed. Indian J Orthop 43(4):412–415
Fu M, Omay SB, Morgan J, Kelley B, Abbed K, Bulsara KR (2012) Primary central nervous system vasculitis presenting as spinal subdural hematoma. World Neurosurg 78(1–2):192
Guilfoyle MR, Khan S, Helmy A, Jalloh I, Trivedi S, Trivedi R, et al (2010) Spinal intradural haemorrhage in a patient with Wegener’s Granulomatosis. Clin Neurol Neurosurg 112(4):341–343
Kakitsubata Y, Theodorou SJ, Theodorou DJ, Miyata Y, Ito Y, Yuki Y, et al (2010) Spontaneous spinal subarachnoid hemorrhage associated with subdural hematoma at different spinal levels. Emerg Radiol 17(1):69–72
Kalina P, Drehobl KE, Black K, Woldenberg R, Sapan M (1995) Spinal cord compression by spontaneous spinal subdural haematoma in polycythemia vera. Postgrad Med J 71(836):378–379
Kim JH, Lee SH, Kim ES, Eoh W (2011) Angiographically occult vascular malformation of the cauda equina presenting massive spinal subdural and subarachnoid hematoma. J Korean Neurosurg Soc 49(6):373–6
Kim SD, Park JO, Kim SH, Lee YH, Lim DJ, Park JY (2008) Spontaneous thoracic spinal subdural hematoma associated with fibromuscular dysplasia. J Neurosurg Spine 8(5):478–481
Konitsiotis S, Glantzouni A, Argyropoulou MI, Tsapoga T, Elisaf M, Efremidis SC (2003) Acute spontaneous spinal subdural haematomas in a patient with essential thrombocythaemia. J Neurol 250(9):1109–1111
Küker W, Thiex R, Friese S, Freudenstein D, Reinges MH, Ernemann U, et al (2000) Spinal subdural and epidural haematomas: diagnostic and therapeutic aspects in acute and subacute cases. Acta Neurochir 142(7):777–785
Kukita CC, Gobatto AL, Lobo AZ, Taniguchi LU (2012) Spinal hematoma complicating a Churg-Strauss syndrome patient: a previously unreported association. Clinics (Sao Paulo) 67(7):855–7
Langmayr JJ, Ortler M, Dessl A, Twerdy K, Aichner F, Felber S (1995) Management of spontaneous extramedullary spinal haematomas: results in eight patients after MRI diagnosis and surgical decompression. J Neurol Neurosurg Psychiatry 59(4):442–447
Levy A, Sonntag V (1998) Management of posttraumatic syringomyelia using an expansile duraplasty: a case report. Spine 23:128–132
Levy JM (1990) Spontaneous lumbar subdural hematoma. AJNR Am J Neuroradiol 11(4):780–781
Nighoghossian N, Ruel JH, Ffrench P, Froment JC, Trouillas P (1990) Hematome sousdural cervico-dorsal par intoxication aux raticides coumariniques. Rev Neurol 146:221–223
Oh SH, Han IB, Koo YH, Kim OJ (2009) Acute spinal subdural hematoma presenting with spontaneously resolving hemiplegia. J Korean Neurosurg Soc 45(6):390–393
Ozdemir O, Calisaneller T, Yildirim E, Caner H, Altinors N (2008) Acute spontaneous spinal subdural hematoma in a patient with bilateral incarcerated inguinal hernia. Joint Bone Spine 75(3):345–347
Russell NA, Benoit BG (1983) Spinal subdural hematoma: a review. Surg Neurol 20:133–137
Safain MG, Engelberg RB, Riesenburger R, Kryzanski J, Jea A, Hwang SW (2014) Pediatric iatrogenic thoracic kyphosis and tension myelopathy treated with a thoracic pedicle subtraction osteotomy: a case report and review of the literature. Childs Nerv Syst 30(7):1293–1299
Schwerdtfeger K, Caspar W, Alloussi S, Strowitzki M, Loew F (1990) Acute spinal intradural extramedullary hematoma: a nonsurgical approach for spinal cord decompression. Neurosurgery 27(2):312–314
Shiller F, Neligan G, Budtz-Olsen O (1948) Surgery in hemophilia: a case of spinal subdural hematoma producing paraplegia. Lancet 2:842–845
Siddiqi F, Hammond R, Lee D, Duggal N (2005) Spontaneous chronic spinal subdural hematoma associated with spinal arachnoiditis and syringomyelia. J Clin Neurosci 12(8):949–953
Simon SL, Auerbach JD, Garg S, Sutton LN, Telfeian AE, Dormans JP (2008) Efficacy of spinal instrumentation and fusion in the prevention of postlaminectomy spinal deformity in children with intramedullary spinal cord tumors. J Pediatr Orthop 28(2):244–249
Smith RA (1985) Spinal subdural hematoma, neurilemmoma and acute transverse myelopathy. Surg Neurol 23:367–370
Song TJ, Lee JB, Choi YC, Lee KY, Kim WJ (2011) Treatment of spontaneous cervical spinal subdural hematoma with methylprednisolone pulse therapy. Yonsei Med J 52(4):692–4
Swann KW, Ropper AH, New PF, Poletti CE (1984) Spontaneous spinal subarachnoid hemorrhage and subdural hematoma. Report of two cases. J Neurosurg 61(5):975–980
Teruel JL, Herrero JA, Desuki A, Felipe C, Ortuño J (1988) Hematoma subdural medular como complicacion neurologica de una crioglobulinemia. Arch Neurobiol 51:204–206
Vázquez-Barquero A, Pascual J, Quintana F, Figols J, Izquierdo JM (1994) Cervical schwannoma presenting as a spinal subdural haematoma. Br J Neurosurg 8(6):739–741
Yang NR, Kim SJ, Cho YJ, Cho do S (2011) Spontaneous resolution of nontraumatic acute spinal subdural hematoma. J Korean Neurosurg Soc 50(3):268–270
Acknowledgments
Disclosure
“The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.”
Consent
Although written informed consent is not needed in this paper as illustrations do not permit the recognition of the patient, we have obtained such consent for the publication of this clinical picture and the accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of the journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Nsir, A., Boubaker, A. & Jemel, H. Syringomyelia following surgery for a spontaneous spinal subdural hematoma in a 13-year-old girl with congenital von Willebrand disease: case report and literature review. Childs Nerv Syst 32, 727–731 (2016). https://doi.org/10.1007/s00381-015-2875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2875-3