Abstract
Fluctuations in progesterone (P4) and estradiol (E2) across the menstrual cycle can exert direct effects on biological systems implicated in neuropsychiatric disorders and represent a key biological source of variability in affective, cognitive, and behavioral disorders. Although these cyclical symptoms may be most readily identified when they occur exclusively in relation to the menstrual cycle, as in DSM-5 premenstrual dysphoric disorder, symptom changes of similar magnitude occur in a larger proportion of people with ongoing psychiatric disorders. Studies investigating cyclical regulation of brain and behavior often produce inconsistent results, which may be attributed to a lack of focus on specific hormonal events and individual differences in related sensitivities. We propose a transdiagnostic Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC) framework, postulating that atypical neural responses to several key hormonal events provoke specific temporal patterns of affective and behavioral change across the menstrual cycle. We review prospective and experimental evidence providing initial support for these dimensions, which include (1) luteal-onset negative affect caused by a sensitivity to E2 or P4 surges (mediated by neuroactive metabolites such as allopregnanolone), typified by irritability and hyperarousal; (2) perimenstrual-onset negative affect caused by a sensitivity to low or falling E2, typified by low mood and cognitive dysfunction; and (3) preovulatory-onset positive affect dysregulation caused by a sensitivity to E2 surges, typified by harmful substance use and other risky reward-seeking. This multidimensional, transdiagnostic framework for hormone sensitivity can inform more precise research on ovarian steroid regulation of psychopathology, including further mechanistic research, diagnostic refinement, and precision psychiatry treatment development. Additionally, given the high rates of hormone sensitivity across affective disorders, the DASH-MC may guide broader insights into the complex neurobiological vulnerabilities driving female-biased affective risk, as well as potential triggers and mechanisms of affective state change in psychiatric disorders.
Similar content being viewed by others
Introduction
The menstrual cycle as a source of fluctuating vulnerability for psychopathology
Changes in ovarian hormones across the menstrual cycle represent a key biological source of symptom variability in psychopathology among many individuals assigned female at birth (AFAB)Footnote 1. In conjunction with effects on the reproductive system, cyclical changes in both progesterone (P4) and estradiol (E2) exert direct effects on the central nervous system, including the majority of neurobiological systems implicated in psychiatric disorders [2]. However, the myriad ways in which these hormones regulate brain function, along with marked individual differences in sensitivity to these hormone changes, have led to scattered, sometimes contradictory findings without a coherent theoretical framework. We propose a unifying framework—the Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC)—based on observations of several cyclical symptom patterns that appear to have unique biological triggers or mechanisms.
The word “dimensional” indicates the hypothesis that more than one kind of ovarian steroid hormone sensitivity exists, and further that such dimensions of hormone sensitivity are conceptualized as continuous rather than categorical variables. We use “affective” to indicate that the primary outcomes of interest relate to emotional functioning (e.g., sadness, anger, joy, fear, anxiety), although we expect that direct cognitive or motor hormone sensitivities also exist. “Sensitivity” refers to the fact that only some people experience significant affective reactions to normative physiological hormone change, which implies an atypical reactivity. Here, “hormones” is used to refer to ovarian E2 and P4. We label this framework transdiagnostic to reflect that these patterns of hormone sensitivity appear to induce or exacerbate common neurobiological and behavioral processes relevant to many different psychiatric diagnoses, with relevance to a wide range of clinical populations. We aim to provide a useful conceptual framework for research in hormone sensitivity that explains the complex presentations of our patients, identifies possible neurobiological triggers and processes underlying their symptoms, and supports the advancement of precision medicine. While we focus here on applications of the DASH framework to symptom change across the menstrual cycle, we believe extending this framework would likely also hold promise for explaining pathophysiological heterogeneity of mental health risks associated with puberty, pregnancy, and the menopause.
The menstrual cycle
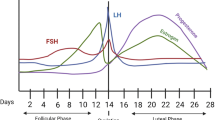
The monthly menstrual cycle is governed by the pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus, stimulating the anterior pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). During the follicular phase, ovarian follicles develop, creating a gradual rise in estrogen (E2) levels and a peak just before ovulation, when the dominant follicle releases an egg. After ovulation, the luteal phase begins when the remaining tissue from the dominant follicle transforms into the corpus luteum, which secretes both E2 and progesterone (P4). In the absence of fertilization, the corpus luteum regresses, causing E2 and P4 levels to decline rapidly, triggering menstruation. Hormone levels remain low and stable through the early follicular phase.
The need for a transdiagnostic approach to hormone sensitivity
These typical menstrual hormone shifts exert clinically significant effects on affect, cognition, or behavior for only a minority of ovulating people [3]. These symptoms arise not due to differences in hormone levels or trajectories but rather due to an altered brain sensitivity to typical cyclical hormone changes (hormone sensitivity) [4]. The DSM-5 diagnosis of premenstrual dysphoric disorder (PMDD), observed in approximately 5.5% of the cycling populationFootnote 2, is given when distressing or impairing emotional symptoms are present in the week before menses onset, begin to improve during menses, and become minimal or absent in the week following menses [6, 7]. For the diagnosis of PMDD, prospective daily symptom ratings must confirm the pattern in at least five total symptoms (from a list of 11 emotional, behavioral, and somatic symptoms), including at least one emotional symptom, across at least two cycles.
PMDD prevalence estimates represent only a subset of those with cyclical symptoms, because the DSM-5 diagnostic criteria exclude individuals whose symptoms exhibit substantial premenstrual increases but fail to decline to subclinical levels through the follicular phase [8]. This excluded group, while not included in an established diagnosis or specifier, has been defined by a group of international experts as premenstrual exacerbation (PME) [9], typically in reference to a presumed underlying disorder (e.g., major depressive disorder). A recent review of prospective studies suggests that a substantial percentage of those with chronic psychiatric disorders experience clinically significant cyclical worsening of symptoms [10]. Prospectively confirmed PME appears highly prevalent in major depressive disorder, with rates as high as 60% according to a large community-based study [11]. This is particularly notable given that the methods used for delineating “significant” PME in that study (i.e., premenstrual symptom increase of >=1 person-standard-deviation) penalize individuals with higher mean symptom levels, making it more difficult for those with greater baseline symptoms to cross the threshold (i.e., since person-mean and person-standard-deviation are always strongly correlated). Sample sizes in most studies of other disorders are too small to derive population-level estimates of hormone sensitivity. Still, substantial rates of prospectively-confirmed PME have been documented in bipolar disorder [12], borderline personality disorder (BPD) [13,14,15], bulimia nervosa [16, 17], attention-deficit/hyperactivity disorder (ADHD) [18], schizophrenia and psychosis [19, 20], substance misuse [21, 22], and post-traumatic stress disorder [23].
This broad pattern may reflect a shared underlying vulnerability to both psychopathology and hormone sensitivity, including beyond clinical samples. Even in healthy participants who did not meet criteria for any psychiatric diagnoses, mood changes in response to induced hormone fluctuations were present only for individuals who reported more extreme levels of neuroticism (chronic tendency toward experiencing negative affect) [24]. This heightened prevalence of hormone sensitivity across a range of psychopathology suggests that a transdiagnostic understanding of hormone sensitivity in these populations may inform not only our understanding of ovarian steroid effects on psychopathology but also provide insight into the broader neural vulnerability to psychiatric disorders [25,26,27].
Beyond premenstrual symptoms: the need for a multidimensional approach
Though most research focuses on premenstrual symptoms, the timing of cycle effects on symptom expression are more complex and variable. Patterns described as premenstrual or perimenstrual can include differences in timing of symptom onset (e.g., early vs late luteal phase) and clearance (e.g., rapid offset at the start of menses vs persistence into the follicular phase). Peri-ovulatory effects on symptoms, long described by some patients and suggested by animal studies, have also emerged in this growing evidence base. Identifying specific biobehavioral triggers and processes underlying these unique patterns of symptom presentation [28] may explain seemingly inconsistent findings in observational research and clinical trials.
To this end, we propose a novel Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC) framework, encompassing PMDD, PME, and periovulatory phenotypes, in which distinct cyclical hormone changes can trigger or exacerbate transdiagnostic psychiatric symptoms at different menstrual cycle phases. We present three initial hypothesized sensitivities (see Fig. 1): (1) luteal-onset symptoms, driven primarily by fluctuations in P4 metabolites, (2) perimenstrual-onset symptoms, driven by E2 withdrawal and/or low levels of E2, and (3) periovulatory symptoms, driven by sudden increases in E2. While based on a growing number of convergent findings across research on PMDD and PME, these candidate forms of hormone sensitivity may evolve with further research to identify definitive, underlying molecular pathways. Hormone-sensitive individuals may have one or a combination of sensitivities, including possible additional types yet to be identified.
Below we review evidence for these symptom patterns and potential contributing neurobiological processes. Currently available retrospective assessments of PMDD criteria (i.e., single-time-point surveys and interviews) are highly prone to false positives and do not outperform chance in predicting cyclical symptom patterns in daily ratings [3, 29]. Accuracy of retrospective report for detecting hormone sensitivity in other types of sensitivities, such as PME, has not yet been well evaluated, but may be similarly biased. Given that within-person, prospective methods are essential for validly assessing naturally occurring cycle effects on symptoms [3, 29, 30], only studies using these or experimental methods will be reviewed as primary evidence for these hormone sensitivities, unless explicitly noted.
Dimensions of hormone sensitivity
Dimension 1: Luteal-onset sensitivity to neuroactive steroid/allopregnanolone surges
Phenomenology of Midluteal-onset symptoms
The best-studied dimension of hormone sensitivity involves altered reactions to typical early to midluteal phase surges in P4 and E2, particularly the related surges in P4 metabolites such as allopregnanolone (ALLO). Across the menstrual cycle, systematic changes in ALLO levels follow those of P4 [31]. More specifically, ALLO levels are generally low during the follicular phase, begin to increase after ovulation, and reach their primary peak in the midluteal phase. A sensitivity to ALLO, which appears to characterize the majority of patients with DSM-5 PMDD, provokes a broad range of negative affective symptoms, with particularly strong increases in symptoms characterized by hypersensitivity, including irritability, stress sensitivity, somatic anxiety, mood lability (hypersensitive/reactive mood), rejection sensitivity (social hypersensitivity), reactive aggression (behavioral response to irritability), and other related symptoms in the midluteal phase [14, 15, 32,33,34,35,36]. Typically, these symptoms emerge after ovulation, increase across the luteal phase, peak in the late luteal phase, improve with menses onset, and show a nadir in the midfollicular phase. However, it should be noted that even in samples carefully recruited for luteal phase confinement of PMDD symptoms, there are prominent individual differences in the lag time between the postovulatory ALLO increase and symptom emergence, with some patients experiencing immediate symptom onset in the early luteal phase, and others experiencing symptoms only in the premenstrual week. Accordingly, we refer to these symptoms as “luteal-onset”, and they are typically luteally-confined.
Although PMDD patients experience a variety of emotional symptoms, including low mood and anhedonia, the hypersensitive phenotype predominates, with luteal-onset symptoms of irritability, anger, and interpersonal conflict among the most commonly observed [34, 35] and most impairing [36]. In a study using group-based trajectory models to identify subtypes of prospectively-diagnosed PMDD (N = 74), the most severe symptom trajectory (observed in 64% of the sample) involved irritability and mood lability rising post-ovulation and continuing throughout the luteal phase. Late luteal increases specifically in high arousal negative affect (e.g, upset, irritated, nervous) vs. lower arousal (e.g., down, bored, listless) also occurred in response to momentary stress in a sample diagnosed with PMDD (i.e., luteally-confined symptoms) [32], further supporting this irritable and hypersensitive luteal phenotype.
Similar patterns of luteal hypersensitivity have been observed in a number of small studies examining the degree of cyclical worsening in other psychiatric conditions or symptoms. In BPD (N = 15), irritability and anger demonstrated exacerbation across the luteal phase, with lowest levels before and at ovulation; this initial rise in irritability was shortly followed by elevated interpersonal reactivity (e.g., rejection sensitivity, perceived invalidation) [14, 15]. In a transdiagnostic sample of psychiatric outpatients recruited for past-month suicidality, irritability showed a different pattern of cyclical exacerbation than depressive symptoms. Both rose in the midluteal phase and peaked perimenstrually; however, irritability remitted much more rapidly with menses onset, suggesting a stronger luteal confinement of this symptom [37]. For some, this high arousal negative affect may present as anxiety—among ovulating people with panic disorder (N = 24), half demonstrated substantial luteally-confined exacerbations [38]. While less well studied, qualitative work interviewing people with lived experience of PMDD, as well as expert clinicians, identified misophonia (hypersensitivity to auditory stimuli) as a common and significant symptom alongside irritability [39]. These reports are consistent with findings of luteal accentuation of the acoustic startle response in PMDD, but not healthy controls [33], suggesting hypersensitivity to stimuli may contribute to this phenotype.
This increased luteal distress may lead to dysregulated behavioral responses. In both healthy and transdiagnostic outpatient samples, premenstrual increases in alcohol use were linked specifically to coping motives, potentially representing alcohol’s ability to acutely reduce high-arousal negative affect [21, 22]. Similarly, reactive aggression (i.e., aggression in response to frustration/provocation) was highest in the late luteal phase in BPD [15].
Biological processes relating to luteally-confined symptoms
Altered sensitivity to luteal E2 or P4 surges
Rigorous experimental evidence suggests this luteal phase pattern of symptom is triggered by a neural sensitivity to normative post-ovulatory steroid surges [4, 40, 41]. Several RCTs indicate that luteally-confined symptoms can be treated by suppressing ovulation and subsequent steroid flux, including some improvement with extended-cycle oral contraceptives [42] and substantial improvement with GnRH analogs (GnRHa) [43, 44] and selective P4 receptor modulators (ulipristal acetate) [45]. Among PMDD patients whose symptoms remit during the low, stable hormone state that results from GnRHa treatment, symptoms can be rapidly re-triggered by administering (or “adding back”) luteal phase levels of E2, P4, or both, to create an artificial steroid surge that mimics the periovulatory or early luteal phases [4, 41]—and the same hormone changes do not provoke symptoms in controls. Of note, even among PMDD patients carefully selected for luteally-confined symptoms, there was substantial variability as to whether symptoms were triggered during P4 add-back, E2 add-back, or both, highlighting the potential for further subtypes of luteal-onset hormone sensitivity [4].
Experiments further demonstrate that these luteally-confined symptoms are lagged, time-limited responses to post-ovulatory steroid surges specifically, rather than triggered either by elevated absolute steroid levels, and rather than the late luteal drop in steroid levels (or withdrawal) or subsequent low absolute levels [4, 40, 41]. In one study, for PMDD patients whose symptoms remitted during GnRHa (low, stable steroid levels) and returned following addback of luteal levels of E2 and P4 (i.e., steroid surges), this increase in symptoms lasted only an initial month and remitted in the second month, despite continuous, stable hormone administration. This suggests that luteally-confined emotional symptoms probably result from an altered neural sensitivity to hormone surges (increases) rather than an altered sensitivity to absolute high/luteal phase levels of hormones [41]. To examine potential effects of hormone withdrawal, another study used mifepristone plus placebo to induce early (midluteal) hormone withdrawal and associated bleeding in one condition, compared with using mifepristone plus human chorionic gonadotropin (hCG) in another condition to maintain the elevated midluteal hormonal profile while inducing the same midluteal menstrual bleeding. In support of the delay between the hormonal trigger and symptom expression, inducing premature E2 and P4 withdrawal did not alter the luteal symptom trajectory in PMDD [40]. This finding implicates a delayed impact of periovulatory hormone surges on the time-limited luteal symptom presentation found in PMDD and fails to support a steroid withdrawal hypothesis.
Consistent with the irritable and hypersensitive nature of these luteal-onset symptoms, effects of both P4 administration and naturally occurring surges are linked to changes in structure, responsivity, and connectivity of multiple brain regions, including the amygdala, insula, ACC, hippocampus, and OFC [46, 47]Footnote 3. In one study, P4 administration in the follicular phase (to luteal levels) increased reactivity and connectivity of amygdala in response to emotion inductions in both PMDD and controls [52], suggesting potential additional neurological factors may modulate whether this activation of negative affect and heightened stimulus salience networks translates to symptoms and impairment. Other studies suggest that in PMDD, activation of amygdala may be paired with deficits in frontal inhibition. One study on emotion processing found along with increased premenstrual (vs postmenstrual) amygdala response to negative (vs neutral) stimuli, participants with PMDD lacked the luteal increase in OFC activation typical of those without the disorder [53, 54]. Similarly, PMDD was linked to increased luteal phase-specific reactivity to negative social cues in the amygdala and insula, coupled with decreased dACC response, relative to controls; these changes were correlated with changes in P4 [55]. This increased response was paired with alterations in connectivity between the ACC and both amygdala and insula, suggesting increased reactivity in social processing coupled with decreased reactivity in regulation. Conversely, inhibition of P4 fluctuations during the cycle, and consequent anovulation, appears to improve top-down emotion regulation in patients with PMDD. One placebo-controlled study found that treatment with a progesterone receptor antagonist was associated with greater fronto-cingulate reactivity in response to provocation stimuli during an fMRI task in patients with PMDD [56]. These neural patterns of increased affective reactivity paired with decreased regulatory activation overlap considerably with those associated with impulsive aggression [57], rejection sensitivity [58, 59], and misophonia [60], all symptoms observed in luteal-onset hormone sensitivity.
A substantial number of preclinical and human studies indicate this surge sensitivity is influenced by (1) altered luteal effects of GABAergic P4 metabolites (e.g., ALLO) at the GABAAR, and (2) altered luteal phase serotonergic function. Since the bulk of the human work in this area comes from studies of luteal-onset symptoms in PMDD, these studies will be featured below; however, we expect that these findings likely generalize to P4 metabolite sensitivities broadly, including luteally-confined PME.
Altered sensitivity to surges in the neuroactive steroid metabolites of P4
The altered luteal emotional response to midcycle steroid surges is mediated by sensitivity to the typical postovulatory surge specifically in 5α-reduced metabolites of P4, such as ALLO [61]. Although ALLO acts as a potent positive allosteric modulator at the GABA-A Receptor (GABAAR), typically leading to anxiolytic, sedative, antidepressant, and prosocial effects [62], it causes opposite responses in PMDD. An RCT in PMDD found that blockade of P4-to-ALLO metabolism using dutasteride, a 5a-reductase inhibitor, reduces luteal symptoms relative to placebo [61], suggesting that ALLO (or other 5a-reduced metabolites of P4) play a causal role in luteal phase symptom onset. Two other RCTs in PMDD found that daily injections of isoallopregnanolone, thought to antagonize effects of ALLO at the GABAAR, show promise for reducing luteally-confined symptoms [63, 64]. Finally, metabolism of P4 to ALLO (and other 5a-reduced metabolites of P4) appears undisturbed in PMDD, undermining the hypothesis that altered levels produce these adverse effects of ALLO [63, 64]. While it is unclear whether luteal-onset symptoms triggered by E2 add-back alone [40] share the same pathophysiology as those triggered by P4, E2 can directly increase synthesis of P4 (and therefore ALLO) in the brain [65]. Therefore, even E2-initiated symptoms could be mediated by the ability of E2 to increase local production of P4 and its metabolites.
The exact mechanisms by which ALLO surges cause luteal-onset affective symptoms remain unknown; however, it has been repeatedly hypothesized (though never directly tested in humans) to be driven by altered subunit configuration (and associated function) of the pentameric GABAAR, which in turn alters sensitivity to typical ALLO flux across the cycle (reviewed in [66]). In rodent models of hormone-sensitive behavior change, a failure to adaptively reorganize the subunit composition—and related functional response—of GABAAR across the cycle undermines or even reverses the typical anxiolytic, antidepressant, prosocial effects of ALLO [67]. Therefore, altered steroid regulation of GABAAR subunits across the cycle may play a role in the luteal-onset phenotype, and represent a potential target for intervention specific to this symptom set.
Reduced luteal phase serotonin function
In addition to potential GABAergic processes described above, several lines of research emphasize a critical role for deficits in luteal phase serotonergic function in luteally-confined PMDD symptoms. Studies comparing patients with luteally-confined emotional symptoms to healthy controls have demonstrated lower whole-blood serotonin levels [68], blunted luteal serotonin production in response to an L-tryptophan challenge [69], and increased luteal serotonin transporter binding [70]. Each of these deficiencies appeared specifically in the luteal phase. Given the well-documented role of serotonin in regulation of irritability, aggression, and general mood states [71, 72], the large number of clinical trials documenting rapid efficacy of serotonergic agents such as SSRIs in the treatment of luteally-confined symptoms [73], and the experimental evidence that serotonergic agents provide relief in PMDD via serotonergic mechanisms [74], the impact of luteal P4 (and perhaps E2) on serotonin function represents a likely component of luteal-onset hormone sensitivity [75].
Dimension 2: Perimenstrual-onset sensitivity to low or falling E2
Phenomenology of perimenstrual-onset symptoms
In contrast to the luteal-onset pattern described above, we hypothesize the presence of a second hormone sensitivity characterized broadly by perimenstrual depressed mood and cognitive dysfunction. This can present with symptoms of increased internalizing negative affect (e.g., depression, anxiety, stress, anhedonia), suicidal thoughts and behavior, and difficulties with attention, memory, and learning. Across studies reviewed below, these perimenstrual increases in cognitive and affective symptoms emerge during and following perimenstrual hormone decreases, and persist into the early follicular phase-- unlike what is typically observed with the luteally-confined surge sensitivity symptoms described previously.
In a study of symptom trajectories among PMDD patients, about two-thirds of the sample demonstrated a pattern of symptoms in which emotional and cognitive symptoms were confined primarily to the perimenstrual phase, with this pattern most pronounced for symptoms of sadness and anxiety (in contrast with irritability, for which most patients showed a full luteal phase pattern) [76]. In BPD, lower arousal and internalizing affective symptoms (e.g., hopelessness, shame, sadness) showed the lowest levels around ovulation and the early luteal phase and demonstrated worsening in the late luteal phase that extended well into the follicular phase, during hormone withdrawal and subsequent low levels [14]. Similar findings were observed in a non-clinical sample with a range of BPD features, where those with elevated BPD features demonstrated higher depression and rumination specifically when E2 was lower [13].
These perimenstrual-onset difficulties regulating emotion-related cognitive processes may also exacerbate suicidal ideation and behavior in response to elevated negative affect. Suicide risk appears to peak in the late luteal and early follicular phases in tandem with rapid decreases in—or low levels of—E2 and P4, with lower risk during the ovulatory and midluteal cycle phases. A review of studies examining cycle phase differences in hospitalization for suicidal behaviors concluded that suicidal behavior appeared most common during—and not before—menses [77]. Consistent with this, a study assessing cycle phase of females within 24 hours of presenting to the ER following a suicide attempt demonstrated increased likelihood of attempts occurring perimenstrually [78]. A prospective study of patients recruited for past month suicidal ideation further found that the daily severity of both suicidal ideation and depressive symptoms (depressed mood, hopelessness, worthlessness/guilt) peaked during menses, rather than premenstrually, and persisted significantly into the early follicular phase [79], in contrast with the typical earlier luteal onset of irritability/hypersensitivity [14, 15]. Together, these findings provide evidence for divergence of perimenstrual-onset depression pattern from the luteal irritability/hypersensitivity pattern, both in having a later onset and a later offset.
While perimenstrual-onset and early follicular symptom elevations may often represent delayed effects of the ALLO surge sensitivity described above in relation to the hypothesized luteal hypersensitivity subtype [40], the predominance of more internalizing and cognitive symptoms in the perimenstrual and early follicular phases combined with the frequent failure of these symptoms to “switch out” with menses onset suggests the possibility of an alternative form of perimenstrual steroid sensitivity. Currently, however, it remains unclear whether perimenstrual-onset symptoms are best explained by the sudden perimenstrual decrease in steroid levels (withdrawal) or the subsequent low absolute steroid levels.
Biological processes relating to perimenstrual-onset symptoms
Altered sensitivity to low/falling perimenstrual E2.
While depressive symptoms like sadness may co-occur as part of a larger set of luteally-confined symptoms resulting from sensitivity to hormone surges, increasing evidence points to a distinct, perimenstrual steroid decrease sensitivity specific to later offset depressive symptoms. Across several experimental studies, administration of GnRHa, which induces ovarian steroid surges, subsequent withdrawal, and depletion state similar to menopause, led to symptom remission (including sadness) in the first month in a series of trials with PMDD patients (i.e., luteally-confined symptoms; [4, 41, 43]). However, several studies in patients with prospectively-confirmed PMDD have reported that the ameliorative effect of GnRHa may not extend to those with predominantly depressive premenstrual symptoms [80] and may not benefit PMDD patients with comorbid depressive disorder [81] or ongoing dysphoria (e.g., PME; [82]). This suggests at least some with PMDD may experience depressive symptoms caused by an alternative sensitivity that does not benefit from the induction of withdrawal and low steroid levels. Consistent with that possibility, GnRHa-induced hormone withdrawal (or subsequent low levels or depletion) provokes depressive symptoms specifically among individuals with histories of depression or high levels of neuroticism [83], but not in healthy controls without a history of depression [83, 84].
A series of crossover randomized controlled clinical trials in transdiagnostic outpatients recruited for suicidal ideation more specifically implicates E2 withdrawal or subsequent low E2 levels as a potential trigger of perimenstrual-onset worsening of depressed mood and suicidality. Following naturally occurring post-ovulatory hormone surges, patients were administered ovarian steroids for two weeks around menses onset to offset hormone withdrawal. Perimenstrual administration of E2 either with P4 [85] or alone [86] prevented perimenstrual worsening of suicidal ideation and depressive symptoms observed at baseline and under placebo, but did not alter premenstrual increases in irritability. These findings support an E2-related mechanism of perimenstrual-onset depressive symptoms that may also be responsible for depressive symptoms that extend into the follicular phase.
E2 affects a staggering array of neurobiological factors [2]. Numerous studies (reviewed below) demonstrate facilitative effects of E2 on DA responses throughout the brain, resulting in altered cognition and reward responses. While less explored at present, alternative neurological processes underlying sensitivities to low/falling E2 are also possible; recent evidence suggests that induced reductions in E2 cause reduced serotonergic function [87].
E2 effects on cognitive functioning
Decades of research have shown that E2 affects the structure, chemistry and function of brain areas involved in cognition with E2 receptors being widespread and highly present in the prefrontal cortex [88] and E2 modulating the frontal dopaminergic system [89]. A recent systematic review of 77 neuro-imaging studies [47] revealed that the prefrontal cortex is highly sensitive to E2 fluctuations across the menstrual cycle.
Translational findings across animal and human studies have often found higher levels of within-person E2 facilitate cognitive functioning. Studies on ovariectomized rodents demonstrate addback of higher levels of E2 improves hippocampal function [90, 91], memory [90,91,92], and spatial processing [93], as well as reductions in depression-like behavior [93]. Similarly, numerous studies demonstrate improvement of several aspects of executive functioning under higher levels of E2 across the menstrual cycle, including concentration [94], response inhibition [95, 96], and verbal working memory [97,98,99]. Similar patterns of E2 enhancement are observed for fear extinction learning in rodent [100,101,102] and human experimental studies [102,103,104]. E2 reductions in the context of low P4 in the late luteal and early follicular phase may have particularly strong potential to worsen cognitive functioning [105, 106]. Experimentally, E2 supplementation [88, 107, 108] and E2 add-back following surgical menopause [109] or hormone suppression [110] normalize executive functioning declines and associated alterations in neural activity.
In contrast, some observational [111] and experimental [112] studies examining effects of E2 on cognition in healthy human samples have failed to replicate these findings. Mixed or null findings across studies of healthy human samples would be consistent with strong individual differences in hormone sensitivity, resulting in only a subset of females experiencing clinically-significant changes in cognitive function. These interindividual differences in hormone sensitivity potentially also explain inconsistent findings on the role of E2 supplementation in menopausal cognitive functioning, with some [113, 114] but not all [115, 116] studies revealing positive effects of E2 on cognition, with meta-analyses suggesting improvements are limited to those experiencing significant symptoms in response to hormone depletion [117, 118]. E2 may also only positively influence some cognitive functions, with heightened E2 levels across the menstrual cycle linked to worsened inhibition of prepotent responses in a healthy sample [119].
Individual differences in baseline levels of executive functioning could moderate whether cognition is affected by changes in E2 and/or the level and type of impairment from E2-related cognition-related changes. For example, cycle-related E2 flux may selectively affect working memory in COMTVal carriers [97], a genotype associated with lower frontal DA, greater impulsivity, and poorer executive functioning [120], particularly in females [121]. Similarly, in a non-clinical sample of adult females (N = 32), exacerbation of ADHD symptoms was linked to decreases in E2, but only for individuals with high trait impulsivity [18]. While initial studies of cycle effects on symptoms in ADHD are ongoing, these findings suggest the potential for a significant effect of reduced E2 for those with the disorder or other clinically significant impairments in executive functioning that results in PME. A greater risk of sensitivity to low or falling E2 among patients with histories of depressive disorders [83] could reflect similar vulnerabilities, given depressive disorders are associated with trait-like impairments in executive functioning [122].
Estradiol effects on reward processing
E2 may exert similar dopaminergically mediated enhancement of neural reward processes. Animal studies have demonstrated E2 effects in upregulated dopaminergic reward processing systems [123,124,125,126], with increased DA release, striatal receptor density, and related behaviors occurring in response to naturally occurring increases in E2 [127, 128] and E2 supplementation [124, 128, 129] in rats. More recent work in rats demonstrates E2 administration produces both rapid and delayed potentiation of DA in the nucleus accumbens [130, 131] and dorsal striatum [132]. This suggests both a direct, phasic effect on DA functioning enhancing responses to behaviorally salient stimuli, as well as potential slower genetically-mediated effects. Hormone-sensitive humans similarly demonstrate enhanced reward-related neural activation during the mid-follicular phase compared to the luteal phase, indicating increased reward sensitivity when E2 is elevated [133,134,135].
Given these findings, changes in reward functioning may be normative responses to E2 flux. Effects of potential E2 enhancement of reward functioning on symptoms are reviewed in the next section on ovulatory hormone surge sensitivity; however, the effects of perimenstrual low/falling E2 on dopaminergic functioning in striatal regions seem like plausible processes contributing to depressed mood or anhedonia. Decreased reward responsivity/activation in reward networks has been broadly linked to depressive/anhedonic symptoms [136, 137], and these alterations lessen with improvement in depressive symptoms [138], suggesting the potential for E2-related reward alterations to contribute to worsening mood. While links between alterations in neural reward processes and depressive/anhedonic symptoms are largely unexamined to date in the context of hormone sensitivity, a small study demonstrated decreased responsiveness to reward in the right putamen and left postcentral and supramarginal gyri during ovarian steroid withdrawal for both those experiencing postpartum withdrawal-induced anhedonia (N = 10) and those without (N = 18) [139]. Further studies are needed, especially in samples experiencing menstrually-related depressive affect, to determine if the magnitude of effects of low/falling E2 on reward functioning differ for these individuals. If not, it seems plausible that typical E2-driven changes in reward processing could drive anhedonia specifically for individuals with greater baseline depressive symptoms (i.e., PME of depression), perhaps reflecting generally impaired or vulnerable reward functioning [83].
Dimension 3: Periovulatory-onset sensitivity to surges in E2
Phenomenology of periovulatory-onset symptoms
In contrast with midluteal- and perimenstrual-onset effects, mid-cycle and ovulatory effects on psychopathology have been much less-studied. In hormone-sensitive females, the peri-ovulatory phase is characterized generally by less emotional and cognitive vulnerability [2, 140]. Some appetitive and drive-related behaviors have also been found to increase during the periovulatory window, such as assertiveness [141], regulatory focus [142], and sex drive [143, 144].
While many of these shifts may improve well-being, some individuals demonstrate peri-ovulatory increases in potentially maladaptive appetitive urges and behaviors, such as increased alcohol misuse [21, 22], linked to social (vs. coping) drinking motives [21, 145] and rises in gambling behaviors unassociated with negative affect [146]. One study of undergraduate females found that periovulatory increases in binge drinking were accounted for by day-to-day surges in E2, particularly in at-risk (i.e., impulsive) individuals on the weekend [22], and the same periovulatory increase on weekend binge drinking was replicated in a transdiagnostic sample of patients recruited for suicidal thoughts [21]. Similarly, in a sample with BPD, while most symptoms were at their lowest levels during ovulation, proactive aggression (aggression with the purpose to meet one’s needs [147]) peaked during the peri-ovulatory phase [15], in contrast to the luteal increase in negative affect-driven reactive aggression. Consistent with this phenotype, case reports in patients with bipolar disorder also suggest that some patients experience cyclical worsening of mania around ovulation [148].
Biological processes relating to periovulatory-onset symptoms
These periovulatory symptoms may be driven by the same E2 processes implicated in perimenstrual-onset symptoms, but at the opposite extreme. As reviewed above, increases in E2 facilitate upregulation of striatal DA across both animal and human studies. Greater fMRI blood-oxygen-level-dependent response in brain regions associated with the DA-mediated reward system (i.e., amygdala, orbitofrontal cortex, midbrain, striatum) has been found during the mid-follicular phase compared to the midluteal phase, potentially suggesting increased reward system responsivity just before ovulation [135]. These reward alterations may facilitate impulsive behavior, even in the presence of potentially improved cognitive functioning with increased E2, by increasing motivation and urges to act. A small human study found that the disinhibiting effect of alcohol increased nearly two-fold during late follicular E2 elevations [149].
While this may suggest that perimenstrual-onset and periovulatory symptoms arise from a shared form of hormone sensitivity, between-person factors may shape the impact of these changes on day-to-day (dys)function. Those with baseline vulnerabilities toward reward-related dysfunctional behavior (e.g., a history of substance misuse, hostile cognitive biases, or general impulsivity) may be more likely to exhibit periovulatory “impulsivity” symptoms in the context of E2 sensitivity. While all aspects of hormone sensitivity are understudied, further research on periovulatory effects in psychiatry is particularly needed, and cycle effects should be considered in any work examining these types of behavior in ovulating people. In this context, it should be noted that careful attention to cycle phasing methods is required to define the periovulatory phase in longitudinal studies; it is the most time- and resource-intensive phase to identify with precision, but such precision may prove critical for detecting effects [30].
Conclusion
Testing and expanding the DASH-MC framework
The DASH-MC framework is intended as a starting point, informed by the most rigorous current research, to guide further research likely to lead to its refinement, including potential changes and/or additions as dictated by new evidence. While existing evidence strongly supports the existence of individual differences in sensitivity to specific hormonal triggers, many open questions exist about the nature of these sensitivities. Further work is needed to identify potential underlying physiological mechanisms and biomarkers for the proposed hormonal trigger dimensions, such as examining differences in GABAAR subunit plasticity that might be specific to ALLO flux sensitivity (versus sensitivity to low or falling E2).
In particular, prospective longitudinal designs are needed to examine the DASH-MC across a greater range of disorders and symptom sets, including observational work modeling within-person changes including methods precise enough to model ovulatory effects [30] and experimental work directly testing effects of specific hormone changes. These findings would help clarify dominant symptom presentations across proposed hormonal trigger dimensions, identify underlying behavioral changes contributing to symptom expression, and determine the extent to which these dimensions of hormone sensitivity may co-occur.
Few studies of menstrual cycle effects on psychopathology examine potential ovulatory exacerbation, preventing the application of the DASH-MC to many extant datasets. Failing to incorporate periovulatory effects into models may muddy findings or completely miss phenomena of high clinical importance, such as ovarian steroid flux-induced vulnerability to maladaptive substance use. Modeling ovulatory exacerbation accurately requires methods directly testing for indicators ovulation (as opposed to estimations from backward counts from menses date) given the substantial inter- and intra-individual variability in phase lengths [30]. In addition to LH surge testing, methods such as basal temperature monitoring using a range of wearable devices that continuously obtain temperature readings show promise as reliable indicators of ovulation [150,151,152], and may be more feasible for or acceptable to participants than urine testing in some research protocols.
Potential overlap in timing of ovulatory effects with surge sensitivities further complicates modeling of cycle dynamics. While in general, peri-ovulatory sensitivity-driven symptoms would be expected to onset (on average) earlier than ALLO surge sensitivities, more work is needed to determine potential individual differences in lags from hormone flux to related behavioral symptoms and duration of these effects, including potential differences in lags/duration specific to each hormone (e.g., does E2 exert neural effects with similar timing and variability in timing as P4?)[153]. These complexities extend into clinical applications; for example, if an individual has symptoms that respond to SSRI therapy, but with remaining difficulties that fit the peri-ovulatory timeframe and likely symptom types, applying the DASH-MC would suggest the likelihood of multiple sensitivities and need for additional treatment approaches.
Little is known about the etiology of hormone sensitivity. There is some evidence of genetic [154] and epigenetic [155] risk for reproductive mood disorders, though little of it is specific to distinct forms of hormone sensitivity. Both intra- and interpersonal contextual factors, like personality traits [156], increased life stress [157], trauma history [158], and adverse childhood experiences [159], are associated with increased risk for PMDD diagnosis or more severe symptoms in patients with PMDD [160, 161]. One study in a sample with PMDD found that prior trauma history predicted stronger covariation specifically of P4 and many affective and behavioral symptoms, with strongest effects on interpersonal symptoms [158]. Further work, including experimental designs, is needed to understand the potential impact of trauma and whether it differs across hormone sensitivity types or functions as a broader risk factor. Additionally, patient-informed work is needed to examine potential factors commonly reported but as of yet not rigorously studied. For example, patients frequently report onset of PMDD or PME or increased severity following pregnancy; however, no prospective research at this point examines effects of pregnancy on hormone sensitivity, let alone effects on specific dimensions. Within-person, prospective studies across developmental changes, particularly reproductive milestones (e.g., puberty, pregnancy, menopause) are needed to study potential developmental factors, such as exposure to higher doses of reproductive hormones, underlying development or exacerbation of sensitivity to ovarian steroid flux.
Similarly, while challenging, longer duration studies that extend beyond two cycles (the typical number assessed in research on PMDD and PME [30]) are necessary to understand intraindividual variability in hormone sensitivities and potential contextual moderators (e.g., potential impact of factors like current stress levels, inflammatory processes, and interpersonal dynamics on the degree of symptom change in a given cycle). Factors that influence intraindividual shifts in severity of cyclical symptoms could be distinct from those related to risk of having hormone sensitivities at all.
Diagnostic implications
The diagnostic criteria for PMDD best align with the luteally-confined phenotype, consistent with irritability and affective reactivity (i.e., mood swings) being the most commonly-endorsed PMDD symptoms [35]. Patients with perimenstrual-onset symptoms may also meet PMDD criteria, especially if paired with luteal sensitivity and resolving relatively early in the follicular phase; however, for some, symptoms may extend further into the follicular phase than allowed by DSM-5 PMDD. Ovulatory effects are omitted from the diagnosis entirely. As further research on these differing sensitivities emerges, it may be useful to implement subtypes of a broader diagnosis of menstrually-related affective disorder, with these common patterns of exacerbation of distinct symptom sets as specifiers.
Additionally, even though elevated baseline symptoms predict greater cyclical change [162], no diagnosis or specifier currently captures a pattern of hormone sensitivity without full follicular clearance. Adding PME as a diagnostic specifier might work well for PME of symptoms not typically observed in PMDD, such as psychosis. However, if PMDD and PME result from the same set of underlying mechanisms, amending the existing PMDD diagnosis to include specifiers such as clearance—e.g., full, partial, or mixed clearance—or timing—e.g., luteal vs. perimenstrual onset—would be more parsimonious.
Given the substantial prevalence of hormone sensitivity across many clinical populations (e.g, [12, 14, 16, 18, 19, 21,22,23]), the vulnerabilities described in the DASH-MC could underlie or contribute to the well-documented increases in risk for emotional disorders in females starting in puberty [163,164,165] and lasting through menopause [166, 167]. Similarly, while the diagnoses of PMDD and post-partum depression are not consistently correlated [168, 169], if the potential overlap is examined through this more nuanced lens of distinct hormone effects, common mechanisms might emerge (e.g., E2 sensitivity specifically contributing to risk for both perimenstrual-onset and post-partum symptoms, and ALLO surge sensitivity specifically contributing to risk for both luteal-onset and first-trimester-onset symptoms [170]).
Precision psychiatry applications
This transdiagnostic understanding of distinct dimensions of hormone sensitivity can advance precision psychiatry treatment approaches. Through careful assessment of not only the presence of menstrual cycle effects on symptoms but also the dynamics of symptoms and exact cycle timing, pharmacological approaches can be tailored to likely underlying mechanisms. The DASH-MC has particularly relevance for the development of new medications and technologies targeting any neurological pathways specific to a single dimension of hormone sensitivity. If the construct of PMDD or even PME in fact represents multiple distinct underlying biological mechanisms, but PMDD diagnosis is used to determine eligibility for clinical trials, contradictory findings and failed results are to be expected from research efforts into biological mechanisms and pharmacological interventions. For example, if a candidate pharmacological intervention works via a mechanism specific to one potential form of hormone sensitivity (e.g., sensitivity to ALLO, such as isoallopregnanolone or Sepranolone), recruiting a sample with a pattern of symptoms correlated with the sensitivity of interest could dramatically increase power to detect effects. In contrast, these effects could be missed if the sample includes individuals with a superficially similar (cycle-related increases in some form of negative affect) but mechanistically distinct form of hormone sensitivity. This is consistent with findings from the recent clinical trial of isoallopregnanolone, which failed to demonstrate effects in the overall sample, but effects were observed in post-hoc analyses when the sample was limited to individuals with an immediate offset of symptoms with menses [63]. Without a dimensional framework, effective treatments specific to one form of sensitivities may be erroneously labeled ineffective.
Summary
The Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC) framework proposes a multidimensional model of how alterations in neural responses to ovarian steroid flux produce three distinct sets of symptoms for subsets of menstruating people. Given that approximately half of females with psychiatric difficulties appear to experience some form of prospectively-confirmed hormone sensitivity [11, 14, 37], bringing a nuanced and biologically informed lens to ovarian steroid effects on psychopathology is essential for effective psychiatric care and long overdue. Additionally, given the high rates of hormone sensitivity across affective disorders (and potentially psychopathology generally), as well as the presence of and fluctuations in neurosteroids in all humans, the DASH-MC may provide insight broadly into psychiatric neural vulnerabilities. Further development of this model has the potential to contribute to accurate, nuanced biological models of psychopathology with great potential for advancing precision psychiatry and reducing human suffering.
Notes
This includes any cisgender women, transgender men, and AFAB nonbinary individuals who ovulate (i.e., excluding those who are pre-menarche, pregnant or breastfeeding, post-menopausal, using medications such as oral contraceptives and other hormonal therapies that suppress ovulation, have undergone bilateral oophorectomy)[1].
This estimate reflects the largest study of confirmed diagnoses using prospective daily ratings in a representative community sample; while initially estimated at 1.3% using DSM-IV-TR criteria, estimated rates from this data that reflect the current DSM-5 PMDD diagnostic criteria (with optional impairment) are 5.5% [3]; see [5] for review.
Of note, a number of neuroimaging studies have also found stable (non-phase-specific) differences between participants with PMDD and controls, e.g. [48], [49], [50]; see [51] for a recent review on neuroimaging of PMDD broadly. While it is possible that these stable neural differences relate to vulnerability to hormone sensitivity dimensions, we focus primarily on neural changes across the cycle that may underlie dimension-specific affective and behavioral symptoms.
References
Peters JR, Stumper A, Schmalenberger KM, Taubman AJ, Eisenlohr-Moul TA. Improving rigor through gender inclusivity in reproductive psychiatric science. Compr Psychoneuroendocrinol. 2023;16:100194. https://doi.org/10.1016/j.cpnec.2023.100194.
Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6:1135–60. https://doi.org/10.1002/cphy.c150014.
Gehlert S, Song IH, Chang C-H, Hartlage SA. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol Med 2009;39:129–36. https://doi.org/10.1017/S003329170800322X.
Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N. Engl J Med. 1998;338:209–16. https://doi.org/10.1056/NEJM199801223380401.
Reilly TJ, Patel S, Unachukwu IC, Knox CL, Wilson CA, Craig MC, et al. The prevalence of premenstrual dysphoric disorder: Systematic review and meta-analysis. J Affect Disord. 2024;349:534–40. https://doi.org/10.1016/j.jad.2024.01.066.
American Psychiatric Association, DSM-5-TR classification. Washington, DC: American Psychiatric Association Publishing, 2022.
World Health Organization, International statistical classification of diseases and related health problems (11th ed.). 2019. [Online]. Available: https://icd.who.int/.
Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, et al. Toward the reliable diagnosis of DSM-5 premenstrual dysphoric disorder: The Carolina Premenstrual Assessment Scoring System (C-PASS). Am J Psychiatry. 2017;174:51–59. https://doi.org/10.1176/appi.ajp.2016.15121510.
Nevatte T, O’Brien PMS, Bäckström T, Brown C, Dennerstein L, Endicott J, et al. ISPMD consensus on the management of premenstrual disorders. Arch Women’s Ment Health. 2013;16:279–91. https://doi.org/10.1007/s00737-013-0346-y.
Nolan LN, Hughes L. Premenstrual exacerbation of mental health disorders: A systematic review of prospective studies. Arch Women’s Ment Health. 2022;25:831–52. https://doi.org/10.1007/s00737-022-01246-4.
Hartlage SA, Brandenburg DL, Kravitz HM. Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom Med. 2004;66:698–706. https://doi.org/10.1097/01.psy.0000138131.92408.b9.
Rasgon N, Bauer M, Glenn T, Elman S, Whybrow PC. Menstrual cycle related mood changes in women with bipolar disorder. Bipolar Disord. 2003;5:48–52. https://doi.org/10.1034/j.1399-5618.2003.00010.x.
Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC. Ovarian hormones and borderline personality disorder features: Preliminary evidence for interactive effects of estradiol and progesterone. Biol Psychol. 2015;109:37–52. https://doi.org/10.1016/j.biopsycho.2015.03.016.
Eisenlohr-Moul TA, Schmalenberger KM, Owens SA, Peters JR, Dawson DN, Girdler SS. Perimenstrual exacerbation of symptoms in borderline personality disorder: Evidence from multilevel models and the Carolina Premenstrual Assessment Scoring System. Psychol Med 2018;48:2085–95. https://doi.org/10.1017/S0033291718001253.
Peters JR, Owens SA, Schmalenberger KM, Eisenlohr‐Moul TA. Differential effects of the menstrual cycle on reactive and proactive aggression in borderline personality disorder. Aggr Behav. 2020;46:151–61. https://doi.org/10.1002/ab.21877.
Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychol Med 2008;38:1749–57. https://doi.org/10.1017/S0033291708002997.
Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol. 2013;122:131–7. https://doi.org/10.1037/a0029524.
Roberts B, Eisenlohr-Moul T, Martel MM. Reproductive steroids and ADHD symptoms across the menstrual cycle. Psychoneuroendocrinology. 2018;88:105–14. https://doi.org/10.1016/j.psyneuen.2017.11.015.
Reilly TJ, Sagnay De La Bastida VC, Joyce DW, Cullen AE, McGuire P. Exacerbation of psychosis during the perimenstrual phase of the menstrual cycle: systematic review and meta-analysis. Schizophr Bull. 2020;46:78–90. https://doi.org/10.1093/schbul/sbz030.
Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Women’s Ment Health. 2020;23:113–22. https://doi.org/10.1007/s00737-019-0952-4.
Barone JC, Ross JM, Nagpal A, Guzman G, Berenz E, Pang RD, et al. Alcohol use and motives for drinking across the menstrual cycle in a psychiatric outpatient sample. Alcohol Clin Exp Res. 2023;47:127–42. https://doi.org/10.1111/acer.14971.
Martel MM, Eisenlohr-Moul T, Roberts B. Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J Abnorm Psychol. 2017;126:1104–13. https://doi.org/10.1037/abn0000304.
Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, Rasmusson AM. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress. 2015;28:1–7. https://doi.org/10.1002/jts.21984.
Stenbæk DS, Budtz-Jørgensen E, Pinborg A, Jensen PS, Frokjaer VG. Neuroticism modulates mood responses to pharmacological sex hormone manipulation in healthy women. Psychoneuroendocrinology. 2019;99:251–6. https://doi.org/10.1016/j.psyneuen.2018.10.016.
Rubinow DR. One small step for PMDD, one large step for affective disorders. AJP. 2021;178:215–7. https://doi.org/10.1176/appi.ajp.2020.20121793.
Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: The lessons of premenstrual syndrome. Front Neuroendocrinol. 2006;27:210–6. https://doi.org/10.1016/j.yfrne.2006.02.003.
Rubinow DR, Schmidt PJ. Is there a role for reproductive steroids in the etiology and treatment of affective disorders? Dialogues Clin Neurosci. 2018;20:187–96.
Peters JR, Eisenlohr-Moul TA. Ovarian hormones as a source of fluctuating biological vulnerability in borderline personality disorder. Curr Psychiatry Rep. 2019;21:109. https://doi.org/10.1007/s11920-019-1096-y.
Eisenlohr-Moul TA. Commentary on Joyce et al.: Studying menstrual cycle effects on behavior requires within-person designs and attention to individual differences in hormone sensitivity. Addiction. 2021;116:2759–60. https://doi.org/10.1111/add.15576.
Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, et al. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology. 2021;123:104895. https://doi.org/10.1016/j.psyneuen.2020.104895.
Sundström-Poromaa I, Comasco E, Sumner R, Luders E. Progesterone – Friend or foe? Front Neuroendocrinol. 2020;59:100856. https://doi.org/10.1016/j.yfrne.2020.100856.
Beddig T, Reinhard I, Kuehner C. Stress, mood, and cortisol during daily life in women with premenstrual dysphoric disorder (PMDD). Psychoneuroendocrinology. 2019;109:104372 https://doi.org/10.1016/j.psyneuen.2019.104372.
Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacol. 2007;32:2190–8. https://doi.org/10.1038/sj.npp.1301351.
Hartlage SA, Arduino KE. Toward the content validity of premenstrual dysphoric disorder: Do anger and irritability more than depressed mood represent treatment-seekers’ experiences? Psychol Rep. 2002;90:189–202. https://doi.org/10.2466/pr0.2002.90.1.189.
Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85:275–82. https://doi.org/10.1016/j.jad.2004.10.004.
Schmalenberger KM, Eisenlohr-Moul TA, Surana P, Rubinow DR, Girdler SS. Predictors of premenstrual impairment among women undergoing prospective assessment for premenstrual dysphoric disorder: A cycle-level analysis. Psychol Med. 2017;47:1585–96. https://doi.org/10.1017/S0033291716003524.
Owens SA, Schmalenberger KM, Bowers S, Rubinow DR, Prinstein MJ, Girdler SS, et al., Cyclical exacerbation of suicidal ideation in female outpatients: Prospective evidence from daily ratings in a transdiagnostic sample. J Psychopathol Clin Sci, 2023, https://doi.org/10.1037/abn0000838.
Kaspi SP, Otto MW, Pollack MH, Eppinger S, Rosenbaum JF. Premenstrual exacerbation of symptoms in women with panic disorder. J Anxiety Disord. 1994;8:131–8. https://doi.org/10.1016/0887-6185(94)90011-6.
International Association for Premenstrual Disorders, “A new light on PMDD research: A strategic plan to advance patient-centered PMDD research,” 2022.
Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N. Engl J Med. 1991;324:1174–9. https://doi.org/10.1056/NEJM199104253241705.
Schmidt PJ, Martinez PE, Neiman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. AJP. 2017;174:980–9. https://doi.org/10.1176/appi.ajp.2017.16101113.
Lopez LM, Kaptein AA, Helmerhorst FM, Oral contraceptives containing drospirenone for premenstrual syndrome Cochrane Database Syst Rev, 2012, https://doi.org/10.1002/14651858.CD006586.pub4.
Wyatt KM, Dimmock PW, Ismail KMK, Jones PW, O’Brien PMS. The effectiveness of GnRHa with and without ‘add-back’ therapy in treating premenstrual syndrome: A meta analysis. BJOG. 2004;111:585–93. https://doi.org/10.1111/j.1471-0528.2004.00135.x.
Wagner-Schuman M, Kania A, Barone JC, Ross JM, Mulvihill A, Eisenlohr-Moul TA, What’s stopping us? Using GnRH agonists with stable hormone addback in treatment-resistant premenstrual dysphoric disorder: practical guidelines and risk/benefit analysis for long-term therapy. J Clin Psychiatry, In Press.
Comasco E, Kallner HK, Bixo M, Hirshberg AL, Nyback S, de Grauw H, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: A proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178:256–65. https://doi.org/10.1176/appi.ajp.2020.20030286.
Stiernman L, Dubol M, Comasco E, Sundström-Poromaa I, Boraxbekk CJ, Johansson M, et al. Emotion-induced brain activation across the menstrual cycle in individuals with premenstrual dysphoric disorder and associations to serum levels of progesterone-derived neurosteroids. Transl Psychiatry. 2023;13:124 https://doi.org/10.1038/s41398-023-02424-3.
Dubol M, Epperson CN, Sacher J, Pletzer B, Derntl B, Lanzenberger R, et al. Neuroimaging the menstrual cycle: A multimodal systematic review. Front Neuroendocrinol. 2021;60:100878. https://doi.org/10.1016/j.yfrne.2020.100878.
Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. AJP. 2013;170:305–14. https://doi.org/10.1176/appi.ajp.2012.12030385.
Petersen N, Ghahremani DG, Rapkin AJ, Berman SM, Wijker N, Liang L, et al. Resting-state functional connectivity in women with PMDD. Transl Psychiatry. 2019;9:1–8. https://doi.org/10.1038/s41398-019-0670-8.
Comasco E, Hahn A, Ganger S, Gingnell M, Bannbers E, Oreland L, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35:4450–8. https://doi.org/10.1002/hbm.22486.
Monteiro DC, Ramos CDS, Alves LENN, Cantilino A, Sougey EB. Functional and structural neuroimaging in premenstrual dysphoric disorder: A systematic review. J Psychiatr Res. 2024;175:205–10. https://doi.org/10.1016/j.jpsychires.2024.05.024.
van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2007;13:325–33. https://doi.org/10.1038/sj.mp.4002030.
Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108:87–94. https://doi.org/10.1016/j.jad.2007.09.015.
Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA. 2005;102:16060–5. https://doi.org/10.1073/pnas.0502818102.
Gingnell M, Ahlstedt V, Bannbers E, Wikström J, Sundström-Poromaa I, Fredrikson M. Social stimulation and corticolimbic reactivity in premenstrual dysphoric disorder: a preliminary study. Biol Mood Anxiety Disord. 2014;4:3 https://doi.org/10.1186/2045-5380-4-3.
Kaltsouni E, Fisher PM, Dubol M, Hustad S, Lanzenberger R, Frokjaer VG, et al. Brain reactivity during aggressive response in women with premenstrual dysphoric disorder treated with a selective progesterone receptor modulator. Neuropsychopharmacology. 2021;46:1460–7. https://doi.org/10.1038/s41386-021-01010-9.
Da Cunha-Bang S, Knudsen GM. The Modulatory Role of Serotonin on Human Impulsive Aggression. Biol Psychiatry. 2021;90:447–57. https://doi.org/10.1016/j.biopsych.2021.05.016.
Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. https://doi.org/10.1093/scan/nsp007.
Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19:945–56. https://doi.org/10.1162/jocn.2007.19.6.945.
Neacsiu AD, Szymkiewicz V, Galla JT, Li B, Kulkarni Y, Spector CW. The neurobiology of misophonia and implications for novel, neuroscience-driven interventions. Front Neurosci, 2022;16:893903.
Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacol. 2016;41:1093–102. https://doi.org/10.1038/npp.2015.246.
Paul SM, Pinna G, Guidotti A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol Stress. 2020;12:100215 https://doi.org/10.1016/j.ynstr.2020.100215.
Bäckström T, Ekberg K, Hirschberg AL, Bixo M, Epperson CN, Briggs P, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2021;133:105426. https://doi.org/10.1016/j.psyneuen.2021.105426.
Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, et al. Treatment of premenstrual dysphoric disorder with the GABA A receptor modulating steroid antagonist Sepranolone (UC1010)—A randomized controlled trial. Psychoneuroendocrinology. 2017;80:46–55. https://doi.org/10.1016/j.psyneuen.2017.02.031.
Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. https://doi.org/10.1016/j.mce.2008.04.016.
Hantsoo L, Payne JL. Towards understanding the biology of premenstrual dysphoric disorder: From genes to GABA. Neurosci Biobehav Rev. 2023;149:105168. https://doi.org/10.1016/j.neubiorev.2023.105168.
Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: A possible model of premenstrual dysphoric disorder. Psychopharmacol (Berl). 2006;186:323–33. https://doi.org/10.1007/s00213-005-0168-3.
Rapkin AJ, Edelmuth E, Chang LC, Reading AE, McGuire MT, Su TP. Whole-blood serotonin in premenstrual syndrome. Obstet Gynecol. 1987;70:533–7.
Rasgon N, McGuire M, Tanavoli S, Fairbanks L, Rapkin A. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73:144–9. https://doi.org/10.1016/s0015-0282(99)00452-5.
Sacher J, Zsido RG, Barth C, Zientek F, Rullmann M, Luthardt J, et al. Increase in Serotonin transporter binding in patients with premenstrual dysphoric disorder across the menstrual cycle: a case-control longitudinal neuroreceptor ligand positron emission tomography imaging study. Biol Psychiatry. 2023;93:1081–8. https://doi.org/10.1016/j.biopsych.2022.12.023.
Duke AA, Bègue L, Bell R, Eisenlohr-Moul T. Revisiting the serotonin-aggression relation in humans: A meta-analysis. Psychol Bull. 2013;139:1148–72. https://doi.org/10.1037/a0031544.
Russo S, Kema IP, Haagsma EB, Boon JC, Willemse PHB, den Boer JA, et al. Irritability rather than depression during interferon treatment is linked to increased tryptophan catabolism. Psychosom Med. 2005;67:773. https://doi.org/10.1097/01.psy.0000171193.28044.d8.
Marjoribanks J, Brown J, O’Brien PMS, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD001396.pub3.
Roca CA, Schmidt PJ, Smith MJ, Danaceau MA, Murphy DL, Rubinow DR. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159:1876–81. https://doi.org/10.1176/appi.ajp.159.11.1876.
Rapkin AJ, Akopians AL. Pathophysiology of premenstrual syndrome and premenstrual dysphoric disorder. Menopause Int. 2012;18:52–59. https://doi.org/10.1258/mi.2012.012014.
Eisenlohr-Moul TA, Kaiser G, Weise C, Schmalenberger KM, Kiesner J, Ditzen B, et al. Are there temporal subtypes of premenstrual dysphoric disorder?: Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med 2020;50:964–72. https://doi.org/10.1017/S0033291719000849.
Saunders KEA, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901–12. https://doi.org/10.1017/S0033291706007392.
Baca-Garcia E, Diaz-Sastre C, Ceverino A, Perez-Rodriguez MM, Navarro-Jimenez R, Lopez-Castroman J, et al. Suicide attempts among women during low estradiol/low progesterone states. J Psychiatr Res. 2010;44:209–14. https://doi.org/10.1016/j.jpsychires.2009.08.004.
Ross JM, Barone JC, Tauseef H, Schmalenberger KM, Nagpal A, et al. Predicting acute changes in suicidal ideation and planning: a longitudinal study of symptom mediators and the role of the menstrual cycle in female psychiatric outpatients with suicidality. AJP. 2024;181:57–67. https://doi.org/10.1176/appi.ajp.20230303.
Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: Effect of symptom severity and type in a controlled trial. Obstet Gynecol. 1994;84:779.
Freeman EW, Sondheimer SJ, Rickels K, Albert J. Gonadotropin-releasing hormone agonist in treatment of premenstrual symptoms with and without comorbidity of depression: a pilot study. J Clin Psychiatry. 1993;54:192–5.
Freeman EW, Sondheimer SJ, Rickels K. Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacol Bull. 1997;33:303–9.
Frokjaer VG. Pharmacological sex hormone manipulation as a risk model for depression. J Neurosci Res. 2020;98:1283–92. https://doi.org/10.1002/jnr.24632.
Ben Dor R, Harsh VL, Fortinsky P, Koziol DE, Rubinow DR, Schmidt PJ. Effects of Pharmacologically Induced Hypogonadism on Mood and Behavior in Healthy Young Women. Am J Psychiatry. 2013;170:426–33. https://doi.org/10.1176/appi.ajp.2012.12010117.
Eisenlohr-Moul TA, Bowers SM, Prinstein MJ, Schmalenberger KM, Walsh EC, Young SL, et al. Effects of acute estradiol and progesterone on perimenstrual exacerbation of suicidal ideation and related symptoms: A crossover randomized controlled trial. Transl Psychiatry. 2022;12:528. https://doi.org/10.1038/s41398-022-02294-1.
Eisenlohr-Moul TA, Effects of acute estradiol or progesterone administration on perimenstrual exacerbation of suicidal ideation, depression, and perceived stress: A preliminary analysis of a three-period crossover randomized controlled trial. presented at the 61st Annual Meeting of the American College of Neuropsychopharmacology, Phoenix, Arizona, 2022.
Frokjaer VG, Pinborg A, Holst KK, Overgaard A, Henningsson S, Heede M, et al. Role of Serotonin transporter changes in depressive responses to sex-steroid hormone manipulation: a positron emission tomography study. Biol Psychiatry. 2015;78:534–43. https://doi.org/10.1016/j.biopsych.2015.04.015.
Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–90. https://doi.org/10.1016/S0306-4530(01)00013-0.
Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. https://doi.org/10.1016/j.yfrne.2007.07.003.
Luine V. Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. J Steroid Biochem Mol Biol. 2016;160:189–95. https://doi.org/10.1016/j.jsbmb.2015.07.022.
Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: The role of estradiol and its interaction with dopamine. Hormones Behav. 2008;53:185–91. https://doi.org/10.1016/j.yhbeh.2007.09.015.
Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–90. https://doi.org/10.1016/j.psyneuen.2003.08.001.
Kiss Á, Delattre AM, Pereira SIR, Carolino RG, Szawka RE, Anselmo-Franci JA, et al. 17β-Estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–8. https://doi.org/10.1016/j.bbr.2011.10.047.
Lord T, Taylor K. Monthly fluctuation in task concentration in female college students. Percept Mot Skills. 1991;72:435–9. https://doi.org/10.2466/pms.1991.72.2.435.
Gogos A. Natural and synthetic sex hormones: Effects on higher-order cognitive function and prepulse inhibition. Biol Psychol. 2013;93:17–23. https://doi.org/10.1016/j.biopsycho.2013.02.001.
Howard R, Gifford M, Lumsden J. Changes in an electrocortical measure of impulsivity during the menstrual cycle. Pers Individ Differ. 1988;9:917–8. https://doi.org/10.1016/0191-8869(88)90010-4.
Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. J Neurosci 2011;31:5286–93. https://doi.org/10.1523/JNEUROSCI.6394-10.2011.
Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–41. https://doi.org/10.1016/S0306-4530(01)00083-X.
Vranić A, Hromatko I. Content-specific activational effects of estrogen on working memory performance. J Gen Psychol. 2008;135:323–36. https://doi.org/10.3200/GENP.135.3.323-336.
Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–50. https://doi.org/10.1101/lm.034926.114.
Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebrón-Milad K. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res. 2017;95:163–75. https://doi.org/10.1002/jnr.23826.
Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–7. https://doi.org/10.1016/j.biopsych.2011.05.016.
Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. https://doi.org/10.1016/j.neuroscience.2010.04.030.
Wen Z, Hammoud MZ, Scott JC, Jimmy J, Brown L, Marin MF, et al., Impact of exogenous estradiol on task-based and resting-state neural signature during and after fear extinction in healthy women Neuropsychopharmacology, vol. 46, Art. no. 13, Dec. 2021, https://doi.org/10.1038/s41386-021-01158-4.
Solis-Ortiz S, Guevara MA, Corsi-Cabrera M. Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: An electroencephalographic study. Psychoneuroendocrinology. 2004;29:1047–57. https://doi.org/10.1016/j.psyneuen.2003.10.007.
Solís-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology. 2008;33:989–98. https://doi.org/10.1016/j.psyneuen.2008.04.003.
Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–83. https://doi.org/10.1016/S0197-4580(00)00123-8.
Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Hormones Behav. 1998;34:171–82. https://doi.org/10.1006/hbeh.1998.1476.
Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. https://doi.org/10.1016/0306-4530(88)90060-1.
Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, et al. Modulation of cognition-specific cortical activity by gonadal steroids: A positron-emission tomography study in women. Proc Natl Acad Sci USA 1997;94:8836–41. https://doi.org/10.1073/pnas.94.16.8836.
Leeners B, Kruger THC, Geraedts K, Tronci E, Mancini T, F Ille, et al. Lack of associations between female hormone levels and visuospatial working memory, divided attention and cognitive bias across two consecutive menstrual cycles. Front Behav Neurosci. 2017:11:120.
Schmidt PJ, Keenan PA, Schenkel LA, Berlin K, Gibson C, Rubinow DR. Cognitive performance in healthy women during induced hypogonadism and ovarian steroid addback. Arch Women’s Ment Health. 2013;16:47–58. https://doi.org/10.1007/s00737-012-0316-9.
Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411 https://doi.org/10.1097/01.gme.0000189618.48774.7b.
Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. AJP. 2001;158:227–33. https://doi.org/10.1176/appi.ajp.158.2.227.
Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s health initiative memory study. JAMA. 2004;291:2959–68,. https://doi.org/10.1001/jama.291.24.2959.
Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, et al. Effects of ultra–low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63:945–50. https://doi.org/10.1001/archneur.63.7.945.
LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: Systematic review and meta-analysis. JAMA. 2001;285:1489–99,. https://doi.org/10.1001/jama.285.11.1489.
Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. JAMA. 1998;279:688–95,. https://doi.org/10.1001/jama.279.9.688.
Colzato LS, Hertsig G, van den Wildenberg WPM, Hommel B. Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience. 2010;167:709–15. https://doi.org/10.1016/j.neuroscience.2010.02.029.
Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, et al. COMT Val158Met genotype and individual differences in executive function in healthy adults. J Int Neuropsychol Soc. 2011;17:174–80.https://doi.org/10.1017/S1355617710001402.
Lang UE, Bajbouj M, Sander T, Gallinat J. Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology. 2007;32:1950–5. https://doi.org/10.1038/sj.npp.1301335.
Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med. 2014;44:2029–40. https://doi.org/10.1017/S0033291713002535.
Becker JB. Direct effect of 17B-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990;5:157–64.
Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–12. https://doi.org/10.1016/S0091-3057(99)00168-9.
Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones Behav. 2004;45:330–8. https://doi.org/10.1016/j.yhbeh.2004.01.005.
Windels F, Kiyatkin EA. Modulatory action of acetylcholine on striatal neurons: Microiontophoretic study in awake, unrestrained rats. Eur J Neurosci. 2003;17:613–22. https://doi.org/10.1046/j.1460-9568.2003.02492.x.
Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. https://doi.org/10.1016/S0031-9384(01)00593-5.
Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. https://doi.org/10.1159/000126507.
Di Paolo T, Poyet P, Labrie F. Prolactin and estradiol increase striatal dopamine receptor density in intact, castrated and hypophysectomized rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 1982;6:377–82. https://doi.org/10.1016/S0278-5846(82)80111-5.
Shimizu H, Bray GA. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Res. 1993;621:200–6. https://doi.org/10.1016/0006-8993(93)90107-X.
Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: Genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–6. https://doi.org/10.1046/j.1471-4159.1994.62051750.x.
Shams WM, Cossette M-P, Shizgal P, Brake WG. 17β-estradiol locally increases phasic dopamine release in the dorsal striatum. Neurosci Lett. 2018;665:29–32. https://doi.org/10.1016/j.neulet.2017.11.039.
Bayer J, Bandurski P, Sommer T. Differential modulation of activity related to the anticipation of monetary gains and losses across the menstrual cycle. Eur J Neurosci. 2013;38:3519–26. https://doi.org/10.1111/ejn.12347.
Dreher J-C, Neuroimaging evidences of gonadal steroid hormone influences on reqard processing and social decision-making in humans. in Brain Mapping, Elsevier, 2015, pp. 1011-8.
Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 2007;104:2465–70. https://doi.org/10.1073/pnas.0605569104.
Geugies H, Groenewold NA, Meurs M, Doornbos B, de Klerk-Sluis JM, van Eijndhoven P, et al. Decreased reward circuit connectivity during reward anticipation in major depression. NeuroImage: Clin. 2022;36:103226 https://doi.org/10.1016/j.nicl.2022.103226.
Rupprechter S, Romaniuk L, Series P, Hirose Y, Hawkins E, Sandu AL, et al. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain. 2020;143:1946–56. https://doi.org/10.1093/brain/awaa106.
Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020;2:fcaa196 https://doi.org/10.1093/braincomms/fcaa196.
Schiller CE, Walsh E, Eisenlohr-Moul TA, Prim J, Dichter GS, Schiff L, et al. Effects of gonadal steroids on reward circuitry function and anhedonia in women with a history of postpartum depression. J Affect Disord. 2022;314:176–84. https://doi.org/10.1016/j.jad.2022.06.078.
Owens SA, Eisenlohr-Moul T. Suicide risk and the menstrual cycle: A review of candidate RDoC mechanisms. Curr Psychiatry Rep. 2018;20:106 https://doi.org/10.1007/s11920-018-0962-3.
Blake KR, Bastian B, O’Dean SM, Denson TF. High estradiol and low progesterone are associated with high assertiveness in women. Psychoneuroendocrinology. 2017;75:91–99. https://doi.org/10.1016/j.psyneuen.2016.10.008.
Blake KR, McCartney M, Arslan RC. Menstrual cycle and hormonal contraception effects on self-efficacy, assertiveness, regulatory focus, optimism, impulsiveness, and risk-taking. J Exp Soc Psychol. 2022;103:104382 https://doi.org/10.1016/j.jesp.2022.104382.
Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Hormones Behav. 2013;63:636–45. https://doi.org/10.1016/j.yhbeh.2013.02.013.
Roney JR, Simmons ZL. Within-cycle fluctuations in progesterone negatively predict changes in both in-pair and extra-pair desire among partnered women. Hormones Behav. 2016;81:45–52. https://doi.org/10.1016/j.yhbeh.2016.03.008.
Joyce KM, Hudson A, O’Connor R, Thompson K, Hodgin M, Perrot T, et al. Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depress Anxiety. 2018;35:313–20. https://doi.org/10.1002/da.22699.
Joyce KM, Hudson A, O’Connor RM, Goldstein AL, Ellery M, McGrath DS, et al. Retrospective and prospective assessments of gambling-related behaviors across the female menstrual cycle. J Behav Addict. 2019;8:135–45. https://doi.org/10.1556/2006.7.2018.133.
Poulin F, Boivin M. Reactive and proactive aggression: Evidence of a two-factor model. Psychol Assess. 2000;12:115–22. https://doi.org/10.1037/1040-3590.12.2.115.
Teatero ML, Mazmanian D, Sharma V. Effects of the menstrual cycle on bipolar disorder. Bipolar Disord. 2014;16:22–36. https://doi.org/10.1111/bdi.12138.
Griffith AK, Martel MM, Eisenlohr-Moul T, Fillmore MT, Heightened sensitivity to the disinhibiting effect of alcohol in women during the late follicular phase of the menstrual cycle. Exp Clin Psychopharmacol, 2022, https://doi.org/10.1037/pha0000611.
Alzueta E, de Zambotti M, Javitz H, Dulai T, Albinni B, Simon KC, et al. Tracking sleep, temperature, heart rate, and daily symptoms across the menstrual cycle with the oura ring in healthy women. Int J Women’s Health. 2022;14:491–503. 10.2147/IJWH.S341917.
Uchida Y, Izumizaki M. The use of wearable devices for predicting biphasic basal body temperature to estimate the date of ovulation in women. J Therm Biol. 2022;108:103290 https://doi.org/10.1016/j.jtherbio.2022.103290.
Zhu TY, Rothenbühler M, Hamvas G, Hofmann A, Welter J, Kahr M, et al. The Accuracy of Wrist Skin Temperature in Detecting Ovulation Compared to Basal Body Temperature: Prospective Comparative Diagnostic Accuracy Study. J Med Internet Res. 2021;23:e20710 https://doi.org/10.2196/20710.
Andersen EH, Nagpal A, Eisenlohr-Moul TA, Gordon JL. A novel method for quantifying affective sensitivity to endogenous ovarian hormones. Psychoneuroendocrinology. 2024;167:107095 https://doi.org/10.1016/j.psyneuen.2024.107095.
McEvoy K, Osborne LM, Nanavati J, Payne JL. Reproductive affective disorders: a review of the genetic evidence for premenstrual dysphoric disorder and postpartum depression. Curr Psychiatry Rep. 2017;19:94 https://doi.org/10.1007/s11920-017-0852-0.
Kaminsky Z, Hantsoo L, Payne J. 103. Luteal Phase Epigenetic Biomarkers Identify Premenstrual Dysphoric Disorder (PMDD) and Selective Serotonin Reuptake Response in PMDD. Biol Psychiatry. 2023;93:S135–S136. https://doi.org/10.1016/j.biopsych.2023.02.343.
Miller A, Vo H, Huo L, Roca C, Schmidt PJ, Rubinow DR. Estrogen Receptor Alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44:788–94. https://doi.org/10.1016/j.jpsychires.2010.01.013.
Namavar Jahromi B, Pakmehr S, Hagh-Shenas H. Work stress, premenstrual syndrome and dysphoric disorder: are there any associations? Iran Red Crescent Med J. 2011;13:199–202.
Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–13. https://doi.org/10.1037/0278-6133.26.2.201.
Kulkarni J, Leyden O, Gavrilidis E, Thew C, Thomas EHX. The prevalence of early life trauma in premenstrual dysphoric disorder (PMDD). Psychiatry Res. 2022;308:114381 https://doi.org/10.1016/j.psychres.2021.114381.
Gollenberg AL, Hediger ML, Mumford SL, Whitcomb BW, Hovey KM, Wactawski-Wende J, et al. Perceived stress and severity of perimenstrual symptoms: the biocycle study. J Women’s Health. 2010;19:959–67. https://doi.org/10.1089/jwh.2009.1717.
Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology. 2016;67:142–52. https://doi.org/10.1016/j.psyneuen.2016.01.026.
Kiesner J, Eisenlohr-Moul TA, Vidotto G. Affective risk associated with menstrual cycle symptom change. Front Glob Women’s Health. 2022;3:896924 https://doi.org/10.3389/fgwh.2022.896924.
Patton GC, Olsson C, Bond L, Toumbourou JW, Carlin JB, Hemphill SA, et al. Predicting female depression across puberty: a two-nation longitudinal study. J Am Acad Child Adolesc Psychiatry. 2008;47:1424–32. https://doi.org/10.1097/CHI.0b013e3181886ebe.
Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E, Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry, 7, 5, 2017, https://doi.org/10.1038/tp.2017.105.
Salk RH, Petersen JL, Abramson LY, Hyde JS. The contemporary face of gender differences and similarities in depression throughout adolescence: Development and chronicity. J Affect Disord. 2016;205:28–35. https://doi.org/10.1016/j.jad.2016.03.071.
Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C. Gender differences in depression and anxiety: The role of age. Psychiatry Res. 2013;210:1301–3. https://doi.org/10.1016/j.psychres.2013.09.027.
Freeman EW, Sammel MD, Boorman DW, Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71:36–43. https://doi.org/10.1001/jamapsychiatry.2013.2819.
Kepple AL, Lee EE, Haq N, Rubinow DR, Schmidt PJ. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77:e415–e420. https://doi.org/10.4088/JCP.15m09779.
Pereira D, Pessoa AR, Nuno M, António M, Pereira AT. Association between premenstrual dysphoric disorder and perinatal depression: a systematic review. Arch Women’s Ment Health. 2022;25:61–70. https://doi.org/10.1007/s00737-021-01177-6.
Eisenlohr-Moul T, Swales DA, Rubinow DR, Schiff L, Schiller CE. Temporal dynamics of neurobehavioral hormone sensitivity in a scaled-down experimental model of early pregnancy and parturition. Neuropsychopharmacology. 2024;49:414–21. https://doi.org/10.1038/s41386-023-01687-0.
Acknowledgements
The authors thank the many patients, study participants, and advocates (especially the International Association for Premenstrual Disorders) whose invaluable contributions have allowed us to develop this framework. The authors are supported by the following funding: National Institute of Mental Health R01 MH126940 (JRP, TEM), K23 MH112889 (JRP), RF1MH120843 (TEM), R01 MH122446 (TEM), T32 MH019927 (AS), R01 MH119119 (MMM); National Institute on Alcohol Abuse and Alcoholism T32 AA027488 (AGE); German Research Foundation (DFG) SCHM 3732/1-1, 470147139 (KS).
Author information
Authors and Affiliations
Contributions
JRP and TAEM co-developed the DASH-MC theoretical framework and drafted the majority of manuscript. KMS assisted in developing and refining the framework, and KMS, AS, AEG, and MMM assisted with manuscript drafting, editing, and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peters, J.R., Schmalenberger, K.M., Eng, A.G. et al. Dimensional Affective Sensitivity to Hormones across the Menstrual Cycle (DASH-MC): A transdiagnostic framework for ovarian steroid influences on psychopathology. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02693-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02693-4
- Springer Nature Limited