Abstract
Premenstrual dysphoric disorder (PMDD) is characterized by the predictable onset of mood and physical symptoms secondary to gonadal steroid fluctuation during the luteal phase of the menstrual cycle. Although menstrual-related affective dysfunction is responsible for considerable functional impairment and reduction in quality of life worldwide, currently approved treatments for PMDD are suboptimal in their effectiveness. Research over the past two decades has suggested that the interaction between allopregnanolone, a neurosteroid derivative of progesterone, and the gamma-aminobutyric acid (GABA) system represents an important relationship underlying symptom genesis in reproductive-related mood disorders, including PMDD. The objective of this narrative review is to discuss the plausible link between changes in GABAergic transmission secondary to the fluctuation of allopregnanolone during the luteal phase and mood impairment in susceptible individuals. As part of this discussion, we explore promising findings from early clinical trials of several compounds that stabilize allopregnanolone signaling during the luteal phase, including dutasteride, a 5-alpha reductase inhibitor; isoallopregnanolone, a GABA-A modulating steroid antagonist; and ulipristal acetate, a selective progesterone receptor modulator. We then reflect on the implications of these therapeutic advances, including how they may promote our knowledge of affective regulation more generally. We conclude that these and other studies of PMDD may yield critical insight into the etiopathogenesis of affective disorders, considering that (1) symptoms in PMDD have a predictable onset and offset, allowing for examination of affective state kinetics, and (2) GABAergic interventions in PMDD can be used to better understand the relationship between mood states, network regulation, and the balance between excitatory and inhibitory signaling in the brain.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
The relationship between gamma-aminobutyric acid (GABA) and allopregnanolone is an important therapeutic target in premenstrual dysphoric disorder (PMDD). |
Early findings from studies of dutasteride, isoallopregnanolone, and ulipristal support this notion, and provide optimism for expansion of a treatment armamentarium currently characterized by a small number of suboptimal options. |
PMDD research has potential not only to reduce substantial global symptom burden, but also to provide critical insights into the pathophysiology underlying affective state dysregulation. |
1 Introduction
Premenstrual dysphoric disorder (PMDD) is a cyclical mood disorder characterized by emergence of affective symptoms near the onset of the luteal phase of the menstrual cycle and subsequent improvement of those symptoms within 3–4 days of onset of menstruation. The diagnosis is heterogeneous in terms of its symptomatology; to meet the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for PMDD, one must have a total of at least five of eleven possible symptoms, one of which must be mood changes manifested as either depression, anxiety, affective lability, or irritability [1]. At least one associated symptom, such as decreased interest, impairment in concentration, lethargy, change in appetite, hypersomnia/insomnia, feeling overwhelmed, or physical symptoms such as breast tenderness or joint pain, must also be present [1]. PMDD is also characterized by its temporal rather than symptom-based presentation. Symptoms must be present in a majority of menstrual cycles, and luteal phase worsening, improvement with menses, and absence of symptoms during the week following menses must all occur for a diagnosis to be made. Additionally, prospective daily documentation of mood in relation to menses is necessary to establish whether temporal criteria are met [1], as patient self-report is subject to recall bias and poor reliability. It is necessary to distinguish whether significant affective symptoms are present during menses and/or the follicular phase, as this would be suggestive of different or perhaps coincident affective pathology, and the approach to treatment of PMDD differs (at least, to an extent) from that for other affective disorders. Detection and examination of pathophysiologic mechanisms specific to PMDD can be challenging given that women with PMDD are predisposed to developing major depression [2], and luteal phase mood worsening of an active affective disorder (with which PMDD can be confounded) appears to occur in about 60% of women [3].
Why certain women are susceptible to menstrual cycle-related mood dysregulation has been a fundamental question in PMDD research. Early studies investigating hormone levels showed no difference between healthy women and those with PMDD [4], but the link between estrogen/progesterone fluctuation and changes in mood states was definitively established with paradigms that suppressed the hypothalamic–pituitary–gonadal (HPG) axis and normal ovarian cycling (typically via gonadotropin-releasing hormone agonism) [5,6,7,8,9]. These latter studies demonstrated in women with PMDD that affective symptoms are alleviated by eliminating menstrual cycles and that subsequent exogenous administration of estrogen or progesterone (“add back”) results in recurrence [9, 10]. Further, the last study showed that the change in hormone levels rather than the levels themselves trigger the PMDD symptoms [10]. Notably, identical hormone manipulations in women without a history of PMDD result in no symptom appearance. Taken together, these findings suggest that there is a subgroup of women who are differentially vulnerable to experiencing behavioral changes associated with otherwise normal changes in reproductive steroids. The attendant consideration is that there are uniquely aberrant signaling effects that must occur in the brain due to steroid hormone fluctuation in PMDD, a hypothesis for which there is mounting support both from studies of PMDD specifically [11] as well as from work on peripartum and perimenopausal mood disorders (which are similarly characterized by maladaptive responses to steroid hormone changes) [12, 13]. This paper reviews our current understanding of the (patho)physiology of luteal-phase mood worsening and subsequent recovery with menses, specifically focusing on the relationship between neuroactive steroid hormones and the gamma-amino butyric acid (GABA)-ergic system. Examples of novel pharmacologic approaches with early positive findings will be used to illustrate how modulating GABAergic function via progesterone-derived neurosteroids represents a promising avenue for furthering our understanding of, and developing effective treatments for, PMDD.
2 Neurobiological Basis of Hormone-Related Affective Switching
Reproductive steroids such as estrogen and progesterone act extensively throughout the central nervous system, exerting profound effects at nearly every level of signaling (reviewed in [14]). Full disclaimer: to describe hormone-related mood changes in terms of one neurotransmitter system is undeniably reductionistic, with any such description conveying, at most, the function of a single contributing component within a complex, multifaceted system. Nonetheless, there is increasing evidence that the interplay between GABA receptors and neuroactive steroids is central to the regulation of mood in the context of PMDD and other hormone-related mood disorders.
2.1 GABA and Mood Regulation
GABA is the major inhibitory neurotransmitter in the central nervous system (CNS). It has been shown to be important not only for regulation of the balance of excitatory/inhibitory signaling at local and global levels [15,16,17] but as well for the spatiotemporal organization of network activity [18]. GABAergic interneurons, for example, can be thought of as choreographing or “sculpting” the network activity underlying most emergent brain functions (e.g., attention, salience determination, affect regulation). Given that neural circuit dysfunction is a core feature of affective disorders [19], it is not surprising that dysregulation of the GABAergic system has been consistently observed in depression, anxiety, and other stress-related pathology [20,21,22]. Factors that have been shown to increase the risk for GABAergic dysregulation include chronic stress [23], glucocorticoid exposure [24], female sex [25], and age [26], among others. Patients with MDD have been observed to have decreased levels of GABA in cerebrospinal fluid [22, 27], with postmortem analyses showing decreases in both enzymes that synthesize GABA [28] as well somatostatin (SST)-expressing GABAergic interneurons, particularly in prefrontal cortex (PFC) [29, 30].
How and to what extent these changes in GABA function affect observed network changes in MDD, which include increases in default mode network (DMN) activity and decreases in salience network and central executive network activity [31,32,33,34,35], are not entirely understood. GABA’s central role in signal processing appears to begin at the level of cortical microcircuits [22, 36], widely distributed multicellular units consisting of excitatory glutamatergic pyramidal neurons and various subtypes of GABAergic inhibitory interneurons (including those that express somatostatin, those that express serotonin 3a receptors/vasoactive intestinal peptide, and those that express parvalbumin; the distribution and individual function of each of these interneuron subtypes is beyond the scope of this review but is discussed in [37]). Neocortical pyramidal neurons within a given microcircuit receive synaptic excitatory input from thalamus (feed-forward signals) and other cortical regions (feedback signals), information which is “tuned” by inhibitory signals from GABAergic interneurons before being propagated via action potentials to other areas of the brain; the pattern of activity transmitted by these microcircuits represents a critical component of neural coding [38]. When GABA’s inhibitory function is compromised, as is seen following stress and in depressive disorders, the resultant shift toward excitatory activity can produce deficits in information processing and subsequent effects on behavior and cognition. For example, it has been demonstrated that optogenetically increasing the ratio of excitatory:inhibitory activity in adult mouse medial prefrontal cortex results in profound impairments in fear conditioning and social behavior, which are associated with alterations in local electroencephalographic (EEG) patterns [39]. Similarly, increasing the relative excitatory balance via blockade of SST+ neurons in the frontal cortex of mice affects the expression of anxiety and depressive behaviors, with acute inhibition producing an increase in these behaviors and chronic inhibition/ablation producing a decrease (likely reflecting a compensatory effect within the associated network) [40]. Region-specific changes in GABA function have been shown to affect the functional connectivity of these regions with others in their network [41,42,43,44], linking microcircuit level changes to observed macro-level associations between GABA, brain networks, and behavior.
2.2 Allopregnanolone and the GABAergic System
Allopregnanolone, a 3-alpha-reduced neurosteroid metabolite of progesterone, exerts effects on GABA signaling through positive allosteric modulation of the GABA-A receptor [45] (by convention, a neurosteroid is a steroid hormone that is both made and acts in the brain; a neuroactive steroid, by contrast, acts in the brain but is made in the periphery [46, 47]). Allopregnanolone appears to bind to at least two distinct sites on the GABA-A receptor [48, 49] to modulate both tonic and phasic activity, potentiating the effects of GABA at low (nM) concentrations [50] and directly opening receptor channels at higher (μM) ones [51, 52]. Unlike benzodiazepines, which also modulate the GABA-A receptor, allopregnanolone binds to and modulates extrasynaptic alpha-5 and delta subunit containing GABA receptors (which mediate tonic inhibition) [53, 54] and additionally plays a role in receptor trafficking [55]. Consequent to its pharmacologic profile, allopregnanolone administration produces anesthetic, analgesic, anxiolytic, and antiseizure effects [56,57,58,59]. At the circuit level, allopregnanolone—through both phasic (synaptic) and tonic inhibition [60]—appears to exert significant effects on networks and regions underlying mood regulation [61,62,63,64,65]. Allopregnanolone has been shown to modulate oscillations in basolateral amygdala via its interaction with GABA receptors, an effect that mitigates stress-induced changes in salience network function and promotes behavioral resilience [61]. Similar effects have been observed for allopregnanolone on reward processing regions [nucleus accumbens, prefrontal cortex(PFC)] through regulation of dopamine release by the GABA-A receptor [63, 66,67,68]. There is also evidence that allopregnanolone can acutely alter resting-state functional connectivity between amygdala and other brain regions important for mood regulation, including dorsomedial PFC, hippocampus, and insula [62]; these connectivity effects may be menstrual cycle-phase dependent [64], consistent with elevations of progesterone and allopregnanolone during the luteal phase.
Allopregnanolone has received particular attention for its putative role in the affective changes that can occur following childbirth [69]. Postpartum depression (PPD) is hypothesized to be caused by dysregulation of GABAergic signaling following the precipitous decline in allopregnanolone levels after delivery, a speculation supported by clinical trials demonstrating the efficacy of intravenous synthetic allopregnanolone (Brexanolone) in PPD [70, 71]. Consistent with this hypothesis, PPD is characterized by changes in default mode, salience, and reward network function [72, 73], systems that are modulated by allopregnanolone, as above. Unlike the decrease in allopregnanolone preceding PPD, allopregnanolone levels increase rather than decrease prior to the symptomatic portion of the luteal phase in PMDD. This apparent inconsistency could be partially resolved by the fact that the onset of PPD in many cases is during the third trimester [74, 75], when allopregnanolone levels are high and increasing rather than decreasing. Nonetheless, observations of changes in GABA-A receptor subunit composition and subsequent paradoxical behavioral effects (e.g., anxiogenesis rather than anxiolysis) following allopregnanolone administration [76, 77] have suggested that the behavioral consequence of allopregnanolone modulation of GABA receptors cannot solely be explained by absolute allopregnanolone levels (consistent with [10]).
2.3 Mediators of Differential Sensitivity to Ovarian Steroids
Though the biological mediators underlying differential sensitivity to ovarian hormone fluctuation are far from being definitively understood, research has begun to shed light on factors that may play a role. At the systems level, there appear to be alterations in hypothalamic–pituitary–adrenal (HPA) axis function in women with premenstrual mood dysregulation, including a heightened sensitivity to stress during the luteal phase [78, 79]. Though findings are mixed [80] and may represent effects present only in a subgroup of women [81], higher baseline cortisol concentrations [82] and blunted glucocorticoid responses to mental stress [83, 84], as well as decreased allopregnanolone responses to both exogenous stress and adrenocorticotropic hormone administration [83, 85], have been demonstrated during the luteal phase in women with PMDD. Allopregnanolone serves an important allostatic role in the stress response. This occurs primarily via potentiation of GABA signaling at the GABA-A receptor, which plays a critical role in negative modulation of the HPA axis [86,87,88,89,90] (of note, allopregnanolone has also been shown to directly downregulate expression of the corticotropin-releasing hormone gene [91]). GABAergic afferents to the paraventricular nucleus of the hypothalamus mediate HPA responses to stress [88], and administration of GABA antagonists and mimetics (the latter of which include allopregnanolone) amplify and attenuate, respectively, glucocorticoid responses following stressful stimuli [88, 91]. The above HPA-axis changes in PMDD therefore suggest that the diminished restraint of axis function (and subsequent consequences on affective regulation) may be in part due to impaired allopregnanolone upregulation of GABA activity following stress [92]. Decreased responsivity to allopregnanolone and other GABAergic agents have also been observed in animals following chronic social isolation [93], as well as during pregnancy [94, 95], conditions that are characterized by similar HPA-axis dysregulation [96, 97]. As with PMDD, response reduction appears to be mediated by changes in GABA-receptor subunit composition [95, 98, 99].
Recent work has examined molecular factors that may underlie vulnerability to behavioral changes secondary to steroid hormone fluctuation. Genetic differences between patients with PMDD and healthy controls have been observed for the estrogen receptor 1 (ESR1) gene [100, 101], with current evidence suggesting a complex relationship whereby ESR1 may affect behavioral traits that influence PMDD and vulnerability to PMDD independently [101]. The ESR1 gene has also been implicated in peripartum depression, suggesting the possibility of a common genetic vulnerability to reproductive steroid-related mood disorders [102,103,104]. An association has been observed for the ESC/Z complex [105], a family of genes responsible for ovarian-steroid-regulated epigenetic gene silencing. The possible involvement of genes modulating epigenesis is intriguing, as it suggests a mechanism for the transduction of environmental events into enduring alterations in transcriptional and hence neural responses to hormonal changes. Preliminary findings have implicated differential expression of genes coding for BDNF, estrogen-dependent calcium homeostasis, and the endoplasmic reticular stress response in the pathogenesis of PMDD, as well [106, 107]. A single nucleotide polymorphism in the BDNF gene (Val66Met) has been shown in preclinical experiments and a small clinical study, to be associated with cycle-dependent behavioral fluctuations [108, 109]; PMDD patients with the Val66Met genotype demonstrated reduced fronto-cingulate activity in response to an emotional processing task during the luteal phase compared with healthy controls [109]. Genetic associations for GABA-related genes are sparser, though it was recently shown that copy number variations in a gene encoding the GABA-A receptor B2 subunit are enriched in individuals with a PMDD diagnosis [110]. Overall, the evidence for molecular factors associated with PMDD is promising, but at this juncture, provides limited information about the mechanisms producing vulnerability or degree of clinical relevance.
Increased levels of several markers of immune activation, including pro-inflammatory interleukins (e.g., IL-4, IL-10, IL-12) and interferon-gamma, have been observed in PMDD [111, 112], and the interaction between GABA and the immune system represents a complex reciprocal relationship [113, 114]. GABA and GABA agonists (including allopregnanolone) can downregulate inflammatory function of several types of immune cells (e.g., peripheral macrophages, T cells), many of which contain GABA receptors [114, 115] and are able to metabolize GABA [114]; inflammation has conversely been strongly implicated in the structural and functional changes in GABAergic circuitry secondary to stress that are associated with affective dysregulation [113, 116,117,118,119]. Inflammation/inflammatory conditions have been observed to reverse the polarity of the typical GABA signal (i.e., normally inhibitory GABAergic signals become excitatory) through alterations in chloride homeostasis [120]. Accordingly, one hypothesis is that inflammatory responses in PMDD may mediate paradoxical GABAergic responses to increasing allopregnanolone levels [121, 122].
2.4 Targeting the Allopregnanolone–GABA Relationship in PMDD
Irrespective of how differential sensitivity arises, dysfunction of GABA signaling secondary to fluctuations in allopregnanolone levels represents both a potential pathophysiologic mechanism underlying behavioral changes in PMDD and an appealing target for therapeutic intervention. In the following section, we examine approaches that stabilize progesterone/allopregnanolone function during the luteal phase either through direct blockade or decreased biosynthesis. We first discuss preclinical experiments and comment on mechanisms of currently approved treatments, then review early phase findings for three compounds, each of which interferes with allopregnanolone signaling via a distinct mechanism: dutasteride, a 5-alpha reductase inhibitor; isoallopregnanolone, a GABA-A modulating steroid antagonist; and ulipristal acetate, a selective progesterone receptor modulator.
3 Evidence and Rationale for Stabilizing GABA Function in PMDD
Both preclinical and clinical findings have pointed toward a key role for GABA-receptor plasticity in hormone-related affective regulation. In animal-based experiments of hormone withdrawal, knockout of the delta-subunit of GABA-A receptor has been shown to mitigate the anxiogenic effect of allopregnanolone withdrawal [123] and produce increased levels of depression- and anxiety-like behaviors during the postpartum drop in progesterone levels [99]. Studies have shown that exposure to progesterone-derived neurosteroids, though anxiolytic with acute exposure, can provoke anxiety-like behavior responses in rodents if administered over a slightly longer time frame (e.g., if administered continuously for 48 h), an effect that is mediated by a change in the subunit composition of GABA-A receptors [76, 77, 124]. Though human data are scarce, women with PMDD appear to have altered sensitivity to the GABAergic effects of allopregnanolone. While healthy women experience luteal phase GABA-mediated sedation [as manifested by decreased saccadic eye velocity (SEV)] when administered allopregnanolone, women with PMDD demonstrate the opposite effect (increased luteal phase SEV) [125]. Additionally, women with PMDD demonstrate higher degrees of luteal phase anxiety-potentiated startle [126] and acoustic startle [127], measures of physiologic arousal that have their neural basis in GABA function [128, 129]. This suggests a causal relationship between steroid hormone fluctuation, GABA signaling, and hyperarousal-associated symptoms of PMDD, including anxiety [130], sleep disturbance [131], and exaggerated response to perceived threat [132].
Currently approved treatments for PMDD may act by altering progesterone metabolism and stabilizing allopregnanolone levels. Selective serotonin reuptake inhibitors (SSRIs), the gold standard of treatment [133,134,135], exert rapid effects on PMDD symptomatology at relatively low doses compared with major depression [136, 137]. This therapeutic difference may reflect the impact of SSRIs on the enzymes responsible for the conversion of progesterone to allopregnanolone. SSRIs have been shown to promote formation of allopregnanolone from its precursor 5-alpha-dihydroprogesterone by inducing 3-alpha hydroxysteroid dehydrogenase [138, 139], which may in turn produce a downstream effect on GABA signaling through this pathway [140, 141]. One open-label study demonstrated differential effects of SSRIs on peripheral luteal phase allopregnanolone depending on basal levels (i.e., increasing if baseline levels were low and decreasing if baseline levels were high), suggesting that the effects of SSRIs in PMDD may be achieved by normalizing steroid metabolism [142]. SSRIs may also increase GABA signaling through mechanisms unrelated to allopregnanolone; for example, increased brain GABA levels have been shown to occur acutely following SSRI treatment [143]. Ultimately, more work is needed to fully understand the mechanism of action of SSRIs in PMDD. Combined estradiol–progestin oral contraceptives (OCPs), also used in the treatment of PMDD, may derive their effect by stabilizing neurosteroid signaling through suppression of gonadotropin production which, by preventing ovulation, prevents the luteal phase-related surge in progesterone. This hypothesis is supported by two observations: OCPS are effective when given continuously but not conventionally (with one hormone free week to precipitate menstruation) [144,145,146,147] and shorter hormone-free intervals (and thus less variation in hormone levels) have been associated with greater benefit compared with placebo [146, 147]. It should be noted however that the findings for OCPs are both scant and inconsistent [148, 149].
Recent experiments have investigated compounds that stabilize allopregnanolone signaling following ovulation in a more targeted fashion[150,151,152]. Martinez and colleagues explored the effects of dutasteride, a 5-alpha reductase inhibitor currently US Food and Drug Administration (FDA) approved for treatment of benign prostatic hyperplasia, in two double-blind, placebo-controlled crossover studies of PMDD (one using low-dose, 0.5 mg/day dutasteride, the other using high dose, 2.5 mg/day dutasteride) [150]. In the low-dose study, increases in allopregnanolone levels were observed from the follicular phase to the luteal phase irrespective of treatment group, and there was no effect on mood cycling in women with PMDD, suggesting that the dutasteride 0.5 mg/day had minimal effect on progesterone metabolism and resultant allopregnanolone signaling. In the high-dose study, however, dutasteride successfully blunted the luteal-phase rise in plasma allopregnanolone and was associated with a significant decrease in PMDD symptoms, with six of eight participants who received high-dose dutasteride no longer meeting criteria for PMDD after treatment. Consistent with observations of differential sensitivity described above, dutasteride had no effect on mood in healthy control women at either dose, despite similar effects on allopregnanolone levels.
Another means of blocking allopregnanolone’s effect is via isoallopregnanolone, an isomer of allopregnanolone that has been shown to antagonize the former’s effects at the GABA receptor while exerting minimal influence on baseline GABA-mediated Cl− current [153]. Isoallopregnanolone, marketed as Sepranolone by Asarina Pharmaceuticals, is currently in phase II of its development for treatment of PMDD. The first phase II study of isoallopregnanolone (NCT01875718) [151] was unfortunately hampered by two major methodological flaws. First, participants with non-luteal phase mood symptoms were included, meaning non-PMDD mood pathology (MDD, bipolar disorder) may have confounded the results. Second, a large percentage of women with pure PMDD (31%) had ovulation identified either too early or too late due to a technical error with the luteinizing hormone (LH) assay, which resulted in treatment occurring outside of the luteal phase (in some cases, treatment started early and was terminated before the end of the luteal phase; in others, treatment started late and extended into menses). Despite these issues, the study found significant treatment effects both in the whole sample as well as in the subgroup of women with confirmed PMDD who received treatment as intended [151]. A subsequent set of parallel studies of Sepranolone conducted by Backstrom et al. (NCT03697265) found that, while no significant difference was observed between two different doses (10 and 16 mg) of isoallopregnanolone and placebo for total symptom scores for the five worst premenstrual days, perceived distress scores were significantly lower in the isoallopregnanolone group and there was a trend toward less subjective impairment [154]. Additionally, when the analysis was extended to include nine luteal phase days rather than five, the 10 mg dose of isoallopregnanolone was found to be significantly better than placebo for total daily symptom scores, with significantly fewer participants experiencing minimal or no symptoms. It was unclear why the 16 mg dose failed to perform similarly, though the investigators speculated that differences in group composition (a higher percentage of previous non-PMDD affective disorders in the 16 mg group) and potential nonlinear dose-response characteristics may have contributed to this outcome.
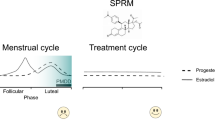
Finally, there is emerging evidence suggesting that signaling can be stabilized indirectly through modulation of progesterone receptor function. A recent randomized-controlled trial of ulipristal acetate (UPA), a negative selective progesterone receptor modulator (SPRM) approved for the treatment of uterine fibroids, found significant reduction in PMDD symptoms for UPA relative to placebo across 3 months of treatment [152]. Interestingly, this effect appeared to be mediated exclusively by improvements in mood, as there was no significant difference in physical symptoms (e.g., breast tenderness, bloating) between UPA and placebo groups. A concurrent substudy [155] demonstrated similar benefit of UPA relative to placebo (93% response rate for ulipristal versus 53% for placebo) and found enhanced functional magnetic resonance imaging (fMRI) responses in regions implicated in top-down emotional control (dorsomedial prefrontal cortex and dorsal anterior cingulate cortex) in the treatment group during a task designed to measure reactive aggression. Deficits in cortical regulation of limbic reactivity have been suggested as physiologic markers of decreased tolerance to stressful stimuli in PMDD [156], and the findings from the UPA substudy provide evidence that these changes may be ameliorated by progesterone-modulating treatment.
Ulipristal was hypothesized a priori to have benefit in PMDD in large part due to its inhibitory effect on progesterone (and consequently, allopregnanolone) synthesis, mediated primarily by reduction and stabilization of luteinizing hormone release by the pituitary [157]. It was therefore surprising that most participants in the first UPA study of PMDD (approximately 75%) continued to cycle, given that prior studies of uterine fibroid treatment demonstrated an ~80% rate of amenorrhea with ulipristal administration [158]. This suggests that the effect of ulipristal was not mediated wholly by decreased, stabilized allopregnanolone levels. A possible explanation is that ulipristal may exert its effect, in part, by antagonizing intracellular and membrane-bound progesterone receptors (PR) within the CNS [158]. Though the relationship between progesterone receptors and brain networks underlying affect is not fully understood, PR are widely distributed in regions critical for emotion regulation [159], and manipulations of PR function have been shown to influence mood [160]. These effects appear to be mediated by genomic and nongenomic actions of PR on classical neurotransmitter systems [161, 162] and neuroendocrine function [163].
UPA and other selective hormone receptor modulators are particularly appealing due to their tissue specificity, addressing the major problem of hormone receptor ubiquity and off-target effects. Tissue specificity is dependent on several factors, including receptor distribution [164], conformational changes induced by ligand binding [165], posttranslational receptor modifications [166], and interaction with tissue-specific coregulators [167]. Tamoxifen, a selective estrogen receptor modulator (SERM), has been an important first line treatment for estrogen-receptor positive breast cancer for over 30 years; it exhibits anti-estrogenic activity in breast tissue, but estrogenic activity in liver, endometrium, vagina, and bone, avoiding typical anti-estrogenic consequences such as vulvovaginal atrophy and osteoporosis [168]. Clomiphene, another SERM used for treatment of infertility, stimulates gonadotropin-releasing hormone by blocking estrogen receptors in the hypothalamus while exerting estrogenic/protective effects on bone [169, 170]. Ulipristal’s benefit for uterine fibroids lies in its relative selectivity for endometrial tissue [171,172,173] but, as above, exerts its effect in part via interaction with CNS progesterone receptors. Future research may identify other receptor modulators that act selectively in the brain, thereby augmenting the psychotropic armamentarium without accompanying undesirable side effects.
4 Clinical Perspectives/Discussion
Currently utilized treatments for PMDD are only modestly effective. Conservative estimates of SSRI treatment response rates appear to be around 60% [133, 174,175,176], and SSRIs may produce untoward side effects that limit their use [e.g., sexual dysfunction, gastrointestinal (GI) side effects]. Beyond SSRIs, there are few empirically validated treatments. Oral contraceptives have mixed evidence, as above, and the trials are notable for their significant placebo effects [148, 177, 178]. Lithium and quetiapine may offer some benefit when first-line treatments fail [179,180,181], and there is a small amount of evidence for supplementation with calcium, magnesium, and/or vitamin B6 [182,183,184,185,186,187]. GnRH agonists such as leuprolide can prevent PMDD symptoms [9, 188,189,190] by suppressing pituitary-stimulated ovarian steroid synthesis and release altogether, but this approach essentially induces menopause (albeit reversibly), resulting in vasomotor symptoms, increased risk of osteoporosis and cardiovascular disease, and an inability to conceive. These treatment limitations, in conjunction with the observation that PMDD affects about 5% of women and produces morbidity on par with major depressive disorder [191, 192], make clear the need for the development of targeted treatments with greater efficacy and fewer adverse effects.
This article reviews emerging evidence for the significance of the relationship between the neurosteroid allopregnanolone and GABA in PMDD, with clinical trials of dutasteride, isoallopregnanolone, and ulipristal serving as proof-of-concept for stabilizing GABA function via allopregnanolone antagonism. Though our article focuses specifically on the etiopathogenesis of PMDD, other considerations for future study exist, including further delineation of neural markers that characterize the disorder. Readers interested in commentary on how brain imaging techniques can facilitate understanding of PMDD are encouraged to explore another review recently published in this journal [193].
Subsequent large-scale randomized control trials (RCTs) are necessary to replicate initial findings for the above-described interventions that stabilize allopregnanolone signaling. Additionally, studies designed to address outstanding questions may provide further insight into the optimal treatment and underlying pathophysiology of PMDD. For instance, what about ulipristal makes it ineffective for physical symptoms associated with PMDD, in contrast to Sepranolone and dutasteride? How can we better characterize the relationship between steroid hormone fluctuation and neural circuit function as it relates to symptom genesis? Are there factors outside of the allopregnanolone–GABA relationship that contribute to pathogenesis? Patients with PMDD appear to have a reduced response to traditional GABAergic modulators like benzodiazepines [194]; can allopregnanolone-modulating treatments be used to restore sensitivity to other treatments that affect the GABAergic system, allowing for a combined treatment effect in patients who do not respond to monotherapy?
The idea that interfering with allopregnanolone’s action can result in symptom improvement may seem counterintuitive at first glance, given the clear benefit of administration of allopregnanolone in postpartum depression [69]. In the case of PPD, the therapeutic effect of allopregnanolone appeared consistent with the speculation that the precipitous decline in progesterone (and therefore allopregnanolone) following delivery results in a deficit in GABAergic signaling and subsequent symptoms [69]. What could not be explained by this hypothesis, however, was the frequent onset of perinatal depression during pregnancy (when levels of progesterone are high) [74, 75] and the persistence of remission following the withdrawal of brexanolone [70]. Similarly, PMDD is characterized by mood disturbances precipitated during times when progesterone and allopregnanolone are high and/or increasing (during the mid-luteal phase), suggesting that “withdrawal” from allopregnanolone cannot explain the mood dysregulation seen in PPD or PMDD. Nonetheless, these ostensibly paradoxical observations are consistent with a fundamental principle that exists throughout the endocrine system; namely, that changes in hormone levels are regulatory signals that can be as important as the levels themselves. For instance, the frequency at which gonadotropin-releasing hormone is secreted is the major determinant of subsequent gonadotropin production: rapid pulses stimulate transcription of alpha and LH-beta subunits, slow pulses stimulate follicle-stimulating hormone (FSH)-beta subunits, and continuous secretion will shut off gonadotropin secretion [195]. Similarly, glucocorticoid feedback inhibition of stress-related adrenocorticotropic hormone (ACTH) elevation occurs on several different timescales: a fast, rate-dependent mechanism and a slow, proportional or dose/concentration-dependent mechanism [196, 197]. Therefore, PPD and PMDD can be thought of as analogous phenomena in the sense that they are the product of maladaptive responses to a change in the level of steroid hormone present, irrespective of the direction of the change (further discussion of the complex behavioral effects of progesterone and its metabolites can be found elsewhere) [198].
As with PPD, the putative link between GABA, allopregnanolone, and mood in PMDD represents a testable neurobiological hypothesis that has the potential to generate interventions that are specific, rapidly acting, effective, and well tolerated. This line of investigation also bears promise for the understanding of affective disorders more broadly, given both GABA’s role in non-hormone-related affective pathology as well as the uniquely predictable decompensation/recovery pattern in PMDD that allows for investigation of mood state kinetics. Affective disorders can be broadly thought of as disorders of affective state, meaning that associated symptoms (sadness, anhedonia, guilt, etc.) occur as part of a self-organized, replicable combination of psychological and physiological variables with associated characteristic cognitions (e.g., self and object relations). Disorders such as depression are heterogeneous in presentation (for instance, 227 different symptom permutations may lead to a diagnosis of depression according to DSM-5 [199]), but their unifying feature is the persistence and relative resistance to perturbation of the affective states themselves. This suggests that state kinetics—the processes that contribute to initiation, maintenance, and termination of behavioral states—are as critical to understanding mood disorders as the individual symptoms. Studying state kinetics is difficult in disorders such as major depression and bipolar disorder, as symptoms cannot be reliably induced or terminated. PMDD solves this problem, as the onset and offset of symptoms are linked to the menstrual cycle, providing multiple, repeatable opportunities to study and manipulate mood-state dynamics.
From a physiologic perspective, affective states emerge from the interplay of neural networks that regulate attention, hedonic response, determination of salience, and assignment of affective valence [200]. The dynamic processes by which states appear and then disappear are disrupted in depressive disorders, suggesting that depression emerges in part as a consequence of disturbed choreography between neural networks, a process largely mediated by GABAergic interneurons that pace pyramidal neuron excitability and the cortical oscillations that represent coordinated neuronal firing [24]. The observed efficacy of GABAergic modulation in PMDD overlays nicely with state-based frameworks, as it indicates that restoration or enhancement of GABAergic tonic inhibition may destabilize the dominant network activity that ostensibly underlies the persistence of the depressed state. PMDD also offers the opportunity to examine the exact nature of transitions from healthy to dysregulated network function, utilizing predictable switches into and out of behavioral states to study changes in GABA activity and how they produce associated network states and behavior.
Taken together, the above factors suggest that PMDD represents a model illness for studying the critical physiologic processes responsible for affective dysregulation. Though studies of hormone-mood relationships (which have focused primarily on cortisol and the HPA axis) have historically yielded little in the way of therapeutics, advancement in scientific tools and a more sophisticated understanding of neuronal function, brain network dynamics, and differential vulnerability have paved the way for treatments such as zuranolone for major depressive disorder, an orally acting version of allopregnanolone currently under priority review by the FDA [201]. Dutasteride, isoallopregnanolone, and ulipristal certainly represent important advances for PMDD, but any effectiveness they demonstrate comes with a broader implication; namely, that reproduction and reproductive hormones should be considered as potentially relevant when attempting to understand the susceptibility to and triggering of affective disorders writ large. Targeted interventions such as those described above may not only mitigate patient suffering, but can as well serve as probes that advance our understanding of the complex neural system interactions governing mood more generally.
5 Conclusions
The relationship between GABA and allopregnanolone is an important therapeutic target in PMDD. Early findings from studies of dutasteride, isoallopregnanolone, and ulipristal support this notion and provide optimism for expansion of a treatment armamentarium currently characterized by a small number of suboptimal options. PMDD research has potential not only to reduce substantial global symptom burden, but also to provide critical insights into the pathophysiology underlying affective state dysregulation.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). [Internet]. 2013.
Hartlage SA, Arduino KE, Gehlert S. Premenstrual dysphoric disorder and risk for major depressive disorder: a preliminary study. J Clin Psychol. 2001;57(12):1571–8. https://doi.org/10.1002/jclp.1119.
Kuehner C, Nayman S. Premenstrual exacerbations of mood disorders: findings and knowledge gaps. Curr Psychiatry Rep. 2021;23(11):78. https://doi.org/10.1007/s11920-021-01286-0.
Rubinow DR, Roy-Byrne P, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141(5):684–6. https://doi.org/10.1176/ajp.141.5.684.
Muse KN, Cetel NS, Futterman LA, Yen SC. The premenstrual syndrome. Effects of “medical ovariectomy.” N Engl J Med. 1984;311(21):1345–9. https://doi.org/10.1056/NEJM198411223112104.
Hammarbäck S, Bäckström T. Induced anovulation as treatment of premenstrual tension syndrome. A double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstet Gynecol Scand. 1988;67(2):159–66. https://doi.org/10.3109/00016348809004191.
Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: effect of symptom severity and type in a controlled trial. Obstet Gynecol. 1994;84(5):779–86.
West CP, Hillier H. Ovarian suppression with the gonadotrophin-releasing hormone agonist goserelin (Zoladex) in management of the premenstrual tension syndrome. Hum Reprod. 1994;9(6):1058–63. https://doi.org/10.1093/oxfordjournals.humrep.a138633.
Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–16. https://doi.org/10.1056/nejm199801223380401.
Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174(10):980–9. https://doi.org/10.1176/appi.ajp.2017.16101113.
Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305–14. https://doi.org/10.1176/appi.ajp.2012.12030385.
Duan C, Cosgrove J, Deligiannidis KM. Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep. 2017;19(10):70. https://doi.org/10.1007/s11920-017-0824-4.
Wang X, Tao J, Li L, Zhong Z, Liu S, Jiang T, et al. Alterations in white matter fractional anisotropy in subsyndromal perimenopausal depression. BMC Psychiatry. 2014;14:367. https://doi.org/10.1186/s12888-014-0367-8.
Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44(1):111–28. https://doi.org/10.1038/s41386-018-0148-z.
Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6(5):484–90. https://doi.org/10.1038/nn1043.
Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26(8–9):907–13. https://doi.org/10.1023/a:1012376215967.
Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 2010;32(8):1300–9. https://doi.org/10.1111/j.1460-9568.2010.07331.x.
Roth FC, Draguhn A. GABA metabolism and transport: effects on synaptic efficacy. Neural Plast. 2012;2012:805830. https://doi.org/10.1155/2012/805830
Spellman T, Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177(5):381–90. https://doi.org/10.1176/appi.ajp.2020.20030280.
Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75–90. https://doi.org/10.1016/j.neuron.2019.03.013.
Northoff G, Sibille E. Cortical GABA neurons and self-focus in depression: a model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19(9):959. https://doi.org/10.1038/mp.2014.108.
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82(8):549–59. https://doi.org/10.1016/j.biopsych.2017.05.024.
Fogaça MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13:87. https://doi.org/10.3389/fncel.2019.00087.
Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19(9):966–77. https://doi.org/10.1038/mp.2014.68.
Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. 2013;4:104. https://doi.org/10.3389/fpsyt.2013.00104.
Pandya M, Palpagama TH, Turner C, Waldvogel HJ, Faull RL, Kwakowsky A. Sex- and age-related changes in GABA signaling components in the human cortex. Biol Sex Differ. 2019;10(1):5. https://doi.org/10.1186/s13293-018-0214-6.
Gold BI, Bowers MBJ, Roth RH, Sweeney DW. GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry. 1980;137(3):362–4. https://doi.org/10.1176/ajp.137.3.362.
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–42. https://doi.org/10.1038/mp.2011.113.
Seney ML, Tripp A, McCune S, Lewis DA, Sibille E. Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2015;73:213–9. https://doi.org/10.1016/j.nbd.2014.10.005.
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32(2):471–82. https://doi.org/10.1038/sj.npp.1301234.
Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CAJ. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry. 2018;84(8):582–90. https://doi.org/10.1016/j.biopsych.2018.01.027.
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–37. https://doi.org/10.1016/j.biopsych.2006.09.020.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatr. 2015;72(6):603–11. https://doi.org/10.1001/jamapsychiatry.2015.0071.
Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–81. https://doi.org/10.1176/jnp.9.3.471.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–7. https://doi.org/10.1073/pnas.0812686106.
Prévot T, Sibille E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry. 2021;26(1):151–67. https://doi.org/10.1038/s41380-020-0727-3.
Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91(2):260–92. https://doi.org/10.1016/j.neuron.2016.06.033.
Murayama M, Pérez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457(7233):1137–41. https://doi.org/10.1038/nature07663.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8. https://doi.org/10.1038/nature10360.
Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39(9):2252–62. https://doi.org/10.1038/npp.2014.76.
Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–7. https://doi.org/10.1038/nn2001.
Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J Neurosci Off J Soc Neurosci. 2013;33(47):18566–73. https://doi.org/10.1523/JNEUROSCI.1973-13.2013.
Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–9. https://doi.org/10.1016/j.neuroimage.2012.09.029.
Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, et al. GABA in the insula—a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–8. https://doi.org/10.1016/j.neuroimage.2013.04.042.
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–7. https://doi.org/10.1126/science.2422758.
Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. https://doi.org/10.1016/j.ynstr.2019.100196.
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109–22. https://doi.org/10.1016/j.neubiorev.2012.10.005.
Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–9. https://doi.org/10.1038/nature05324.
Sugasawa Y, Cheng WWL, Bracamontes JR, Chen ZW, Wang L, Germann AL, et al. Site-specific effects of neurosteroids on GABAA receptor activation and desensitization. Czajkowski CM, Aldrich RW, editors. Elife [Internet]. 2020;9:e55331. https://doi.org/10.7554/eLife.55331
Turkmen S, Backstrom T, Wahlstrom G, Andreen L, Johansson IM. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br J Pharmacol. 2011;162(2):311–27. https://doi.org/10.1111/j.1476-5381.2010.01059.x.
Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–75. https://doi.org/10.1038/nrn1703.
Alvarez LD, Pecci A, Estrin DA. In search of GABA(A) receptor’s neurosteroid binding sites. J Med Chem. 2019;62(11):5250–60. https://doi.org/10.1021/acs.jmedchem.8b01400.
Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4(5):759–65. https://doi.org/10.1016/0896-6273(90)90202-q.
Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl-currents. Mol Pharmacol. 1991;39(6):691–6.
Puia G, Ravazzini F, Castelnovo LF, Magnaghi V. PKCε and allopregnanolone: functional cross-talk at the GABAA receptor level. Front Cell Neurosci. 2015;9:83. https://doi.org/10.3389/fncel.2015.00083.
Reddy DS, Apanites LA. Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Res. 2005;1033(1):96–101. https://doi.org/10.1016/j.brainres.2004.11.026.
Poisbeau P, Keller AF, Aouad M, Kamoun N, Groyer G, Schumacher M. Analgesic strategies aimed at stimulating the endogenous production of allopregnanolone. Front Cell Neurosci. 2014;8:174. https://doi.org/10.3389/fncel.2014.00174.
Kapur J, Joshi S. Progesterone modulates neuronal excitability bidirectionally. Neurosci Lett. 2021;744:135619. https://doi.org/10.1016/j.neulet.2020.135619
Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. 2014;113:79–87. https://doi.org/10.1016/j.pneurobio.2013.09.003.
Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230(2):151–88. https://doi.org/10.1007/s00213-013-3276-5.
Antonoudiou P, Colmers PLW, Walton NL, Weiss GL, Smith AC, Nguyen DP, et al. Allopregnanolone mediates affective switching through modulation of oscillatory states in the basolateral amygdala. Biol Psychiatry. 2022;91(3):283–93. https://doi.org/10.1016/j.biopsych.2021.07.017.
Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Hum Brain Mapp. 2014;35(7):3249–61. https://doi.org/10.1002/hbm.22399.
Dornellas APS, Macedo GC, McFarland MH, Gómez-A A, O’Buckley TK, Da Cunha C, et al. Allopregnanolone decreases evoked dopamine release differently in rats by sex and estrous stage. Front Pharmacol. 2020;11:608887. https://doi.org/10.3389/fphar.2020.608887
Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, et al. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril. 2017;107(5):1246-1255.e4. https://doi.org/10.1016/j.fertnstert.2017.03.021.
Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, Shaffer SA, Villamarin V, Tan Y, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2019;44(3):546–54. https://doi.org/10.1038/s41386-018-0242-2.
Beauchamp MH, Ormerod BK, Jhamandas K, Boegman RJ, Beninger RJ. Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacol Biochem Behav. 2000;67(1):29–35. https://doi.org/10.1016/s0091-3057(00)00299-9.
Fish EW, Whitman BJ, DiBerto JF, Robinson JE, Morrow AL, Malanga CJ. Effects of the neuroactive steroid allopregnanolone on intracranial self-stimulation in C57BL/6J mice. Psychopharmacology. 2014;231(17):3415–23. https://doi.org/10.1007/s00213-014-3600-8.
Peart DR, Andrade AK, Logan CN, Knackstedt LA, Murray JE. Regulation of cocaine-related behaviours by estrogen and progesterone. Neurosci Biobehav Rev. 2022;135:104584. https://doi.org/10.1016/j.neubiorev.2022.104584
Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. 2020;12:100212. https://doi.org/10.1016/j.ynstr.2020.100212
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet (London, England). 2018;392(10152):1058–70. https://doi.org/10.1016/S0140-6736(18)31551-4.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet (London, England). 2017;390(10093):480–9. https://doi.org/10.1016/S0140-6736(17)31264-3.
Nguyen AJ, Hoyer E, Rajhans P, Strathearn L, Kim S. A tumultuous transition to motherhood: Altered brain and hormonal responses in mothers with postpartum depression. J Neuroendocrinol. 2019;31(9):e12794. https://doi.org/10.1111/jne.12794
Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J Neuroendocrinol. 2014;26(10):665–84. https://doi.org/10.1111/jne.12183.
Vignato J, Perkhounkova Y, McCarthy AM, Segre LS. Pain and depression symptoms during the third trimester of pregnancy. MCN Am J Matern Nurs [Internet]. 2020;45(6).
Zhang L, Wang L, Cui S, Yuan Q, Huang C, Zhou X. Prenatal depression in women in the third trimester: prevalence, predictive factors, and relationship with maternal-fetal attachment. Front Public Health. 2020;8:602005. https://doi.org/10.3389/fpubh.2020.602005
Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910(1–2):55–66. https://doi.org/10.1016/s0006-8993(01)02565-3.
Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305(2):541–8. https://doi.org/10.1124/jpet.102.045120.
Beddig T, Reinhard I, Kuehner C. Stress, mood, and cortisol during daily life in women with premenstrual dysphoric disorder (PMDD). Psychoneuroendocrinology. 2019;109:104372. https://doi.org/10.1016/j.psyneuen.2019.104372
Petersen N, London ED, Liang L, Ghahremani DG, Gerards R, Goldman L, et al. Emotion regulation in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2016;19(5):891–8. https://doi.org/10.1007/s00737-016-0634-4.
Lee EE, Nieman LK, Martinez PE, Harsh VL, Rubinow DR, Schmidt PJ. ACTH and cortisol response to Dex/CRH testing in women with and without premenstrual dysphoria during GnRH agonist-induced hypogonadism and ovarian steroid replacement. J Clin Endocrinol Metab. 2012;97(6):1887–96. https://doi.org/10.1210/jc.2011-3451.
Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Bäckström T, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131–7. https://doi.org/10.1007/s00737-013-0327-1.
Rasgon N, McGuire M, Tanavoli S, Fairbanks L, Rapkin A. Neuroendocrine response to an intravenous l-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144–9. https://doi.org/10.1016/s0015-0282(99)00452-5.
Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788–97. https://doi.org/10.1016/s0006-3223(00)01044-1.
Huang Y, Zhou R, Wu M, Wang Q, Zhao Y. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160–8. https://doi.org/10.3109/10253890.2014.999234.
Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2004;18(2):79–87. https://doi.org/10.1080/09513590310001652955.
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci Off J Soc Neurosci. 2011;31(50):18198–210. https://doi.org/10.1523/JNEUROSCI.2560-11.2011.
Almeida FB, Pinna G, Barros HMT. The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. Int J Mol Sci. 2021;22(11). https://doi.org/10.3390/ijms22115495
Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213(1–2):63–72. https://doi.org/10.1007/s00429-008-0192-2.
Miklós IH, Kovács KJ, Herman JP, Cullinan WE, Ziegler DR, Tasker JG. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Eur J Neurosci. 2002;113(3):381–5. https://doi.org/10.1046/j.1460-9568.2002.02133.x.
Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16(3):381–5. https://doi.org/10.1046/j.1460-9568.2002.02133.x.
Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62(1):265–71. https://doi.org/10.1016/0306-4522(94)90330-1.
Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 2015;172(3):227–36. https://doi.org/10.1176/appi.ajp.2014.14070918.
Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37(1–3):110–5. https://doi.org/10.1016/s0165-0173(01)00129-1.
Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, et al. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95(22):13284–9. https://doi.org/10.1073/pnas.95.22.13284.
Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375(1–3):225–35. https://doi.org/10.1016/s0014-2999(99)00232-0.
Boero G, Pisu MG, Biggio F, Muredda L, Carta G, Banni S, et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology. 2018;133:242–53. https://doi.org/10.1016/j.neuropharm.2018.01.045.
Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo–pituitary–adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14(1):6–12. https://doi.org/10.3109/10253890.2010.482628.
Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, et al. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98(1):122–33. https://doi.org/10.1111/j.1471-4159.2006.03850.x.
Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. https://doi.org/10.1016/j.neuron.2008.06.019.
Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925–33. https://doi.org/10.1016/j.biopsych.2006.12.019.
Miller A, Vo H, Huo L, Roca C, Schmidt PJ, Rubinow DR. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788–94. https://doi.org/10.1016/j.jpsychires.2010.01.013.
Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression—a pilot study. Arch Womens Ment Health. 2013;16(6):499–509. https://doi.org/10.1007/s00737-013-0373-8.
Costas J, Gratacòs M, Escaramís G, Martín-Santos R, de Diego Y, Baca-García E, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44(11):717–24. https://doi.org/10.1016/j.jpsychires.2009.12.012.
Mehta D, Newport DJ, Frishman G, Kraus L, Rex-Haffner M, Ritchie JC, et al. Early predictive biomarkers for postpartum depression point to a role for estrogen receptor signaling. Psychol Med. 2014;44(11):2309–22. https://doi.org/10.1017/S0033291713003231.
Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, et al. The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Mol Psychiatry. 2017;22(8):1172–84. https://doi.org/10.1038/mp.2016.229.
Li HJ, Goff A, Rudzinskas SA, Jung Y, Dubey N, Hoffman J, et al. Altered estradiol-dependent cellular Ca(2+) homeostasis and endoplasmic reticulum stress response in premenstrual dysphoric disorder. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01144-8.
Marrocco J, Einhorn NR, Petty GH, Li H, Dubey N, Hoffman J, et al. Epigenetic intersection of BDNF Val66Met genotype with premenstrual dysphoric disorder transcriptome in a cross-species model of estradiol add-back. Mol Psychiatry. 2020;25(3):572–83. https://doi.org/10.1038/s41380-018-0274-3.
Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, et al. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72(6):499–504. https://doi.org/10.1016/j.biopsych.2012.03.032.
Comasco E, Hahn A, Ganger S, Gingnell M, Bannbers E, Oreland L, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450–8. https://doi.org/10.1002/hbm.22486.
Ullah A, Long X, Mat WK, Hu T, Khan MI, Hui L, et al. Highly recurrent copy number variations in GABRB2 associated with schizophrenia and premenstrual dysphoric disorder. Front psychiatry. 2020;11:572. https://doi.org/10.3389/fpsyt.2020.00572.
Bertone-Johnson ER, Ronnenberg AG, Houghton SC, Nobles C, Zagarins SE, Takashima-Uebelhoer BB, et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum Reprod. 2014;29(9):1987–94. https://doi.org/10.1093/humrep/deu170.
Yama K, Asari Y, Ono A, Machida M, Miura J. Plasma interleukin-10 levels are altered in women with severe premenstrual syndrome: a preliminary study. Women’s Heal Rep (New Rochelle, NY). 2020;1(1):73–9. https://doi.org/10.1089/whr.2019.0010.
Crowley T, Cryan JF, Downer EJ, O’Leary OF. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–77. https://doi.org/10.1016/j.bbi.2016.02.001.
Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107(6):2580–5. https://doi.org/10.1073/pnas.0915139107.
Tian J, Middleton B, Kaufman DL. GABA(A)-receptor agonists limit pneumonitis and death in murine coronavirus-infected mice. Viruses. 2021;13(6). https://doi.org/10.3390/v13060966
Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6(11):e946. https://doi.org/10.1038/tp.2016.212
Clements RJ, McDonough J, Freeman EJ. Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp Brain Res. 2008;187(3):459–65. https://doi.org/10.1007/s00221-008-1317-9.
Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav Immun. 2013;28:218–26. https://doi.org/10.1016/j.bbi.2012.11.012.
Giovanoli S, Weber L, Meyer U. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun. 2014;40:48–54. https://doi.org/10.1016/j.bbi.2014.04.005.
Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. https://doi.org/10.1038/ncpneuro0883.
Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, et al. Allopregnanolone and mood disorders. Prog Neurobiol. 2014;113:88–94. https://doi.org/10.1016/j.pneurobio.2013.07.005.
Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac Rev. 2022;11:11. https://doi.org/10.12703/r/11-11
Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186(3):323–33. https://doi.org/10.1007/s00213-005-0168-3.
Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49(5):573–86. https://doi.org/10.1016/j.neuropharm.2005.04.026.
Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck ACN, Bixo M. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacology. 2016;233(11):2109–17. https://doi.org/10.1007/s00213-016-4258-1.
Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87. https://doi.org/10.1007/s11920-015-0628-3.
Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2007;32(10):2190–8. https://doi.org/10.1038/sj.npp.1301351.
Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61(8):741–56. https://doi.org/10.1037/0003-066X.61.8.741.
Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35(1):105–35. https://doi.org/10.1038/npp.2009.109.
Kenwood MM, Kalin NH, Barbas H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology. 2022;47:1141. https://doi.org/10.1038/s41386-021-01216-x.
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. https://doi.org/10.1016/j.smrv.2009.04.002.
Aue T, Okon-Singer H. Expectancy biases in fear and anxiety and their link to biases in attention. Clin Psychol Rev. 2015;42:83–95. https://doi.org/10.1016/j.cpr.2015.08.005.
Marjoribanks J, Brown J, O’Brien PMS, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;2013(6):CD001396. https://doi.org/10.1002/14651858.CD001396.pub3
Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529–34. https://doi.org/10.1056/NEJM199506083322301
Yonkers KA, Halbreich U, Freeman E, Brown C, Endicott J, Frank E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA. 1997;278(12):983–8.
Steinberg EM, Cardoso GMP, Martinez PE, Rubinow DR, Schmidt PJ. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531–40. https://doi.org/10.1002/da.21959.
Kornstein SG, Pearlstein TB, Fayyad R, Farfel GM, Gillespie JA. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry. 2006;67(10):1624–32. https://doi.org/10.4088/jcp.v67n1020.
Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512–7. https://doi.org/10.1073/pnas.96.23.13512.
Trauger JW, Jiang A, Stearns BA, LoGrasso PV. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451–9. https://doi.org/10.1021/bi026109w.
Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9(1):24–30. https://doi.org/10.1016/j.coph.2008.12.006.
Devall AJ, Santos JM, Fry JP, Honour JW, Brandão ML, Lovick TA. Elevation of brain allopregnanolone rather than 5-HT release by short term, low dose fluoxetine treatment prevents the estrous cycle-linked increase in stress sensitivity in female rats. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25(1):113–23. https://doi.org/10.1016/j.euroneuro.2014.11.017.
Gracia CR, Freeman EW, Sammel MD, Lin H, Sheng L, Frye C. Allopregnanolone levels before and after selective serotonin reuptake inhibitor treatment of premenstrual symptoms. J Clin Psychopharmacol US 2009;403–5. https://doi.org/10.1097/JCP.0b013e3181ad8825
Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161(2):368–70. https://doi.org/10.1176/appi.ajp.161.2.368.
Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10(6):561–9. https://doi.org/10.1089/15246090152543148.
Graham CA, Sherwin BB. A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. J Psychosom Res. 1992;36(3):257–66. https://doi.org/10.1016/0022-3999(92)90090-o.
Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106(3):492–501. https://doi.org/10.1097/01.AOG.0000175834.77215.2e.
Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72(6):414–21. https://doi.org/10.1016/j.contraception.2005.08.021.
Eisenlohr-Moul TA, Girdler SS, Johnson JL, Schmidt PJ, Rubinow DR. Treatment of premenstrual dysphoria with continuous versus intermittent dosing of oral contraceptives: results of a three-arm randomized controlled trial. Depress Anxiety. 2017;34(10):908–17. https://doi.org/10.1002/da.22673.
Halbreich U, Freeman EW, Rapkin AJ, Cohen LS, Grubb GS, Bergeron R, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85(1):19–27. https://doi.org/10.1016/j.contraception.2011.05.008.
Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, et al. 5α-Reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41(4):1093–102. https://doi.org/10.1038/npp.2015.246.
Bixo M, Ekberg K, Poromaa IS, Hirschberg AL, Jonasson AF, Andréen L, et al. Treatment of premenstrual dysphoric disorder with the GABA(A) receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology. 2017;80:46–55. https://doi.org/10.1016/j.psyneuen.2017.02.031.
Comasco E, Kopp Kallner H, Bixo M, Hirschberg AL, Nyback S, de Grauw H, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178(3):256–65. https://doi.org/10.1176/appi.ajp.2020.20030286.
Bäckström T, Das R, Bixo M. Positive GABA(A) receptor modulating steroids and their antagonists: implications for clinical treatments. J Neuroendocrinol. 2022;34(2):e13013. https://doi.org/10.1111/jne.13013.
Bäckström T, Ekberg K, Hirschberg AL, Bixo M, Epperson CN, Briggs P, et al. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology. 2021;133:105426. https://doi.org/10.1016/j.psyneuen.2021.105426.
Kaltsouni E, Fisher PM, Dubol M, Hustad S, Lanzenberger R, Frokjaer VG, et al. Brain reactivity during aggressive response in women with premenstrual dysphoric disorder treated with a selective progesterone receptor modulator. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2021;46(8):1460–7. https://doi.org/10.1038/s41386-021-01010-9.
Dubol M, Epperson CN, Lanzenberger R, Sundström-Poromaa I, Comasco E. Neuroimaging premenstrual dysphoric disorder: a systematic and critical review. Front Neuroendocrinol. 2020;57:100838. https://doi.org/10.1016/j.yfrne.2020.100838.
Rabe T, Saenger N, Ebert AD, Roemer T, Tinneberg HR, De Wilde RL, et al. Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. Biomed Res Int. 2018;2018:1374821. https://doi.org/10.1155/2018/1374821.
Whitaker LHR, Williams ARW, Critchley HOD. Selective progesterone receptor modulators. Curr Opin Obstet Gynecol [Internet]. 2014;26(4).
Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–39. https://doi.org/10.1016/j.yfrne.2008.02.001.
Beckley EH, Scibelli AC, Finn DA. Progesterone receptor antagonist CDB-4124 increases depression-like behavior in mice without affecting locomotor ability. Psychoneuroendocrinology. 2011;36(6):824–33. https://doi.org/10.1016/j.psyneuen.2010.11.004.
Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville JR, Remage-Healey L, Sinchak K, et al. Actions of steroids: new neurotransmitters. J Neurosci Off J Soc Neurosci. 2016;36(45):11449–58. https://doi.org/10.1523/JNEUROSCI.2473-16.2016.
Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne). 2012;3:7. https://doi.org/10.3389/fendo.2012.00007.
Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96(2):162–71. https://doi.org/10.1159/000339822.
Paulmurugan R, Tamrazi A, Massoud TF, Katzenellenbogen JA, Gambhir SS. In vitro and in vivo molecular imaging of estrogen receptor α and β homo- and heterodimerization: exploration of new modes of receptor regulation. Mol Endocrinol. 2011;25(12):2029–40. https://doi.org/10.1210/me.2011-1145.
Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–29. https://doi.org/10.1056/NEJMra022219.
Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125(3):411–4. https://doi.org/10.1016/j.cell.2006.04.021.
Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev [Internet]. 2004;25(1):45–71. https://doi.org/10.1210/er.2003-0023.
Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–52. https://doi.org/10.2147/CIA.S66690.
Moskovic DJ, Katz DJ, Akhavan A, Park K, Mulhall JP. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110(10):1524–8. https://doi.org/10.1111/j.1464-410X.2012.10968.x.
Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action—a hypothesis revisited. Fertil Steril. 1984;42(3):331–44. https://doi.org/10.1016/s0015-0282(16)48069-6.
Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids. 2003;68(10–13):1005–11. https://doi.org/10.1016/s0039-128x(03)00130-2.
Hild SA, Reel JR, Hoffman LH, Blye RP. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000;15(4):822–9. https://doi.org/10.1093/humrep/15.4.822.
Communal L, Vilasco M, Hugon-Rodin J, Courtin A, Mourra N, Lahlou N, et al. Ulipristal acetate does not impact human normal breast tissue. Hum Reprod. 2012;27(9):2785–98. https://doi.org/10.1093/humrep/des221.
Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13(7):566–72. https://doi.org/10.1017/s1092852900016849.
Dimmock PW, Wyatt KM, Jones PW, O’Brien PM. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet (London, England). 2000;356(9236):1131–6. https://doi.org/10.1016/s0140-6736(00)02754-9.
Shah NR, Jones JB, Aperi J, Shemtov R, Karne A, Borenstein J. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder: a meta-analysis. Obstet Gynecol. 2008;111(5):1175–82. https://doi.org/10.1097/AOG.0b013e31816fd73b.
Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586. https://doi.org/10.1002/14651858.CD006586.pub4
de Wit AE, de Vries YA, de Boer MK, Scheper C, Fokkema A, Janssen CAH, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. 2021;225(6):624–33. https://doi.org/10.1016/j.ajog.2021.06.090.
Sletten IW, Gershon S. The premenstrual syndrome: A discussion of its pathophysiology and treatment with lithium ion. Compr Psychiatry [Internet]. 1966;7(3):197–206. https://doi.org/10.1016/S0010-440X(66)80015-9
Deleon-Jones FA, Val E, Herts C. MHPG excretion and lithium treatment during premenstrual tension syndrome: a case report. Am J Psychiatry. 1982;139(7):950–2. https://doi.org/10.1176/ajp.139.7.950.
Jackson C, Pearson B, Girdler S, Johnson J, Hamer RM, Killenberg S, et al. Double-blind, placebo-controlled pilot study of adjunctive quetiapine SR in the treatment of PMS/PMDD. Hum Psychopharmacol. 2015;30(6):425–34. https://doi.org/10.1002/hup.2494.
Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16(3):e407-29.
Doll H, Brown S, Thurston A, Vessey M. Pyridoxine (vitamin B6) and the premenstrual syndrome: a randomized crossover trial. J R Coll Gen Pract. 1989;39(326):364–8.
Kendall KE, Schnurr PP. The effects of vitamin B6 supplementation on premenstrual symptoms. Obstet Gynecol. 1987;70(2):145–9.
Moslehi M, Arab A, Shadnoush M, Hajianfar H. The association between serum magnesium and premenstrual syndrome: a systematic review and meta-analysis of observational studies. Biol Trace Elem Res. 2019;192(2):145–52. https://doi.org/10.1007/s12011-019-01672-z.
Arab A, Rafie N, Askari G, Taghiabadi M. Beneficial role of calcium in premenstrual syndrome: a systematic review of current literature. Int J Prev Med. 2020;11:156. https://doi.org/10.4103/ijpvm.IJPVM_243_19.
Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am J Obstet Gynecol. 1998;179(2):444–52. https://doi.org/10.1016/s0002-9378(98)70377-1.
Mortola JF, Girton L, Fischer U. Successful treatment of severe premenstrual syndrome by combined use of gonadotropin-releasing hormone agonist and estrogen/progestin. J Clin Endocrinol Metab. 1991;72(2):252A-252F. https://doi.org/10.1210/jcem-72-2-252.
Mezrow G, Shoupe D, Spicer D, Lobo R, Leung B, Pike M. Depot leuprolide acetate with estrogen and progestin add-back for long-term treatment of premenstrual syndrome. Fertil Steril. 1994;62(5):932–7.
Freeman EW, Sondheimer SJ, Rickels K. Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacol Bull. 1997;33(2):303–9.
Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. https://doi.org/10.1016/s0306-4530(03)00098-2.
Rapkin AJ, Lewis EI. Treatment of premenstrual dysphoric disorder. Women’s Heal [Internet]. 2013;9(6):537–56. https://doi.org/10.2217/WHE.13.62.
Sundström-Poromaa I, Comasco E. New pharmacological approaches to the management of premenstrual dysphoric disorder. CNS Drugs. 2023;37(5):371–9. https://doi.org/10.1007/s40263-023-01004-9.
Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1997;17(6):370–81. https://doi.org/10.1016/S0893-133X(97)00086-9.
Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. https://doi.org/10.1016/b978-0-12-571136-4.50008-5.
Carroll BJ, Ritchie JC, Rogers H, Kim DK. Fast feedback inhibition of adrenocorticotropic hormone secretion by endogenous cortisol in humans. Neuroendocrinology [Internet]. 2019;109(4):299–309. https://doi.org/10.1159/000499662.
Dallman MF, Yates FE. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969;156(2):696–721. https://doi.org/10.1111/j.1749-6632.1969.tb14008.x.
Sundström-Poromaa I, Comasco E, Sumner R, Luders E. Progesterone—friend or foe? Front Neuroendocrinol. 2020;59:100856. https://doi.org/10.1016/j.yfrne.2020.100856.
Olbert CM, Gala GJ, Tupler LA. Quantifying heterogeneity attributable to polythetic diagnostic criteria: theoretical framework and empirical application. J Abnorm Psychol. 2014;123(2):452–62. https://doi.org/10.1037/a0036068.
Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35(3):121–43. https://doi.org/10.1017/S0140525X11000446.
Carvalho T. New depression drug zuranolone one step closer to FDA ruling. Nat Med US. 2023;49:1032–3. https://doi.org/10.1038/d41591-023-00032-8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this manuscript.
Conflicts of Interest
Dr. Rubinow is on the Scientific Advisory Board of (and has received honoraria and stock options from) Sage Therapeutics. He is also on the Scientific Advisory Boards of Sensorium Therapeutics and Embarq Neuro. He also has consulted to Arrivo Therapeutics. Dr. Sikes-Keilp reports no conflicts of interest.
Ethics approval
No ethics approval for this review was required.
Consent to participate
Not applicable.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
No software application or custom code was used in the generation of this manuscript.
Author contributions
CSK and DRR both made substantial contributions to the written manuscript, including conceptualization, drafting, and critical review for important intellectual content. Both authors provided final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sikes-Keilp, C., Rubinow, D.R. GABA-ergic Modulators: New Therapeutic Approaches to Premenstrual Dysphoric Disorder. CNS Drugs 37, 679–693 (2023). https://doi.org/10.1007/s40263-023-01030-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-023-01030-7