Abstract
Acute promyelocytic leukemia (APL) is a subtype of acute leukemia characterized by a unique t(15;17) translocation generating the PML/RARA fusion gene and hybrid oncoprotein. Besides its critical role in leukemogenesis, this genetic aberration serves as a disease-specific biomarker for rapid diagnosis and monitoring of minimal residual disease (MRD). Moreover, PML/RARA is specifically targeted by All-trans retinoic acid (ATRA) and arsenic trioxide (ATO), two agents that synergistically act to induce degradation of the oncoprotein. Large clinical studies including two randomized trials conducted in newly diagnosed APL patients have shown that the ATRA–ATO combination is superior to conventional ATRA and chemotherapy both in terms of efficacy and safety. Preliminary studies using oral formulations of arsenic and ATRA suggest that oral arsenic is as effective and manageable as intravenous ATO. Following early retrospective studies indicating the prognostic relevance of PML/RARA monitoring, several prospective studies were conducted in large cohorts of APL patients enrolled in clinical trials with the aim of better assessing the prognostic value of longitudinal PCR testing. The results consistently showed that molecular remission (defined as negativization of the PCR test for PML/RARA) correlates with a significantly decreased risk of relapse, whereas persistence of PCR positivity for PML/RARA after consolidation or conversion from negative to positive during follow-up is strongly associated with hematologic relapse. Based on these data, various groups started using pre-emptive salvage therapy for patients who persisted PCR-positive after frontline consolidation or converted from negative to positive PCR during follow-up. Finally, several expert panels have recommended that molecular remission should be considered a therapeutic objective in APL, and molecular response has been adopted as a study endpoint in modern clinical trials.
Similar content being viewed by others
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia characterized by particular clinical and biological features. These include bone marrow infiltration by dysplastic promyelocytes, a unique genetic hallmark in leukemic cells (namely, i.e., the t(15;17) chromosome translocation), and a frequent coagulopathy associated with hemorrhagic diathesis. The disease may occur abruptly and is associated with high risk of early death (up to 30% according to population-based studies) mostly due to severe hemorrhages [1].
Once regarded as the most rapidly fatal leukemia, APL is nowadays curable in the majority of patients using targeted therapy only. Critical for favorable outcome are: (i) an early therapeutic intervention with aggressive supportive care (platelets and fibrinogen transfusions) to counteract the coagulopathy; (ii) initiation of specific anti-leukemic therapy with All-trans retinoic acid (ATRA); and iii) correct diagnostic assessment through identification of specific genetic aberration [2]. The APL unique t(15;17) translocation fuses together the promyelocytic leukemia (PML) and retinoic acid receptor alpha (RARA) genes, located on chromosomes 15 and 17, respectively, resulting in the PML/RARA hybrid gene and oncoportein. This fusion gene is readily detectable by modern PCR-based techniques, allowing rapid diagnosis and accurate assessment of the response to treatment [3].
Until recently, standard frontline treatment of APL has relied on the simultaneous administration of All-trans retinoic acid (ATRA) and anthracycline-based chemotherapy for induction and consolidation, and low-dose chemotherapy and intermittent ATRA for maintenance. As shown by several large multicentre studies, this approach results in the achievement of molecular remission and long-term disease-free survival in nearly 80% of patients. However, ATRA and chemotherapy are associated with significant negative side effects, including cardiotoxicity, death in remission, and the occurrence of secondary malignancies [4,5,6].
More recently, i.v. arsenic trioxide (ATO) and other arsenic formulations have been introduced in APL therapy. Both clinical and laboratory studies have shown that ATO is the most effective agent against this disease because it induces molecular remission and prolonged survival in a high proportion of patients when used as (a) single agent [7,8,9]. Furthermore, ATO has been shown to act synergistically with ATRA to induce the degradation of the PML/RARA oncoprotein [10]. Recent studies conducted in frontline therapy, including two large independent randomized trials, have clearly shown that the ATRA–ATO combination is superior to ATRA and chemotherapy, resulting in significantly improved EFS, DFS, and CIR [11,12,13]. Moreover, this approach is associated with considerably less toxicity, particularly in terms of myelosuppression and infections, occurrence of death in remission, and development of secondary malignancies. These results have led to a paradigm shift in the treatment of newly diagnosed APL: the chemo-free ATRA–ATO approach is nowadays regarded as the first treatment choice for patients with non-high-risk APL [14, 15].
The PML/RARA oncoprotein: functions and clinical relevance
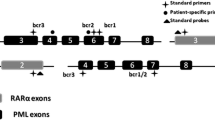
The PML-RARA fusion gene is generated by the breakpoint in RARA intron 2 and breakpoints in the PML gene, which may occur in one of three different regions (intron 6, intron 3, and exon 6). When rearranged with RARA exon 2, these distinct breakpoint locations on PML give rise to long isoform, short isoform, and variable PML/RARA isoforms, respectively [3]. The resulting PML/RARA hybrid protein impairs the physiological functions of both PML and RARA, acting as an aberrant retinoic acid receptor with altered DNA-binding properties. Compared with wild-type RARA multimers, the PML/RARA multimeric protein has an increased affinity for a co-repressor complex which recruits histone deacetylase (HDAC) and ultimately induces transcriptional repression of genes critical for myeloid differentiation [16]. Moreover, PML/RARA induces gene hypermethylation by recruiting DNA methyltransferases to target promoters, further contributing to transcriptional repression. PML/RARA also disrupts the physiological function of PML by delocalizing the protein from normal nuclear bodies to microspeckled nuclear particles [17].
Pharmacological doses of ATRA (10–6 M) can release the differentiation block of APL blasts by degrading the PML/RARA oncoprotein and thus permitting the release of the co-repressor complex and recruitment of a coactivator complex with histone acetylase (HAT) activity [16]. Clinically, ATRA-induced differentiation is insufficient to cure APL. In fact, almost all patients induced into hematologic remission with ATRA alone show persistence of residual disease and ultimately undergo hematological relapse [18, 19]. By contrast, long-term remission of the disease is invariably associated with an absence of PML/RARA-detectable transcripts in patients’ blood and marrow [20, 21].
PML/RARA degradation is also mediated by arsenic trioxide, the most active single agent in APL. Unlike ATRA, ATO targets the PML moiety of PML/RARA and induces sumoylation of specific cysteine residues located on PML, resulting in the proteasome-mediated degradation of the oncoprotein. ATO also exerts its anti-leukemic efficacy by inducing apoptosis of leukemic cells through caspase activation and production of ROS [10, 22].
The APL unique t(15;17) aberration and the resulting PML/RARA fusion transcript and oncoprotein are of utmost importance for the genetic diagnosis of APL and are readily detectable at the chromosome-, DNA-level, RNA-level, or protein-level using conventional karyotyping, fluorescence in situ hybridization (FISH), and RT-PCR and anti-PML monoclonal antibodies [2, 3, 17]. All these specific diagnostic techniques are valuable for diagnostic purposes and are accepted for qualifying patients into APL-tailored clinical studies; however PCR-based methods offer the additional advantage of defining the precise PML/RARA isoform, which in turn enables more accurate and sensitive detection of residual disease (PML/RARA transcripts) during patient follow-up [3].
MRD monitoring in APL: early studies
Initial monitoring studies were carried out retrospectively in patients treated with ATRA combined or not with chemotherapy [18, 19]. These studies showed that treatment with ATRA alone was almost invariably associated with persistence of PML/RARA transcripts and subsequent disease relapse. By contrast, when combined with chemotherapy, ATRA induced molecular remissions in majority of patients, which translated into a high probability of long-term remission and cure, while RT-PCR positivity at the end of consolidation or conversion from PCR-negative to positive during follow-up were both strongly associated with disease relapse [20, 23, 24]. Subsequent prospective studies consistently confirmed the prognostic impact of monitoring minimal residual disease in APL [25, 26]. Diverio et al. reported on 163 patients enrolled in the Italian GIMEMA AIDA trial and regularly monitored at pre-established time points using a PCR assay with sensitivity of 10–4. The authors found that 20 out of the 21 patients who converted from PCR-negative to PCR-positive during follow-up underwent hematologic relapse at a median time of 3 months from PCR positivity, while only eight of the 142 patients who tested >2 times as negative post consolidation (i.e., after 4 total cycles of therapy) underwent relapse during follow-up (Fig. 1a) [25]. In the UK-based MRC prospective study on ATRA and chemotherapy for newly diagnosed APL, a total of 239 patients were monitored once again adopting a PCR assay with sensitivity of 10–4. The relapse risk was 57% vs. 27% for patients testing PCR-positive or negative after consolidation, respectively (P = .006, Fig. 1b) [26]. In both studies, PML–RARA positivity was frequently detected at the end of the induction therapy (40–64% of patients) but was not predictive of relapse, being most probably related to slower maturation kinetics under the action of ATRA. Conversely, positivity of RT-PCR at the end of the consolidation therapy was highly predictive of the impending relapse, and conversion from PCR-negative to PCR-positive during follow-up almost invariably preceded the overt disease recurrence [25, 26].
In another study conducted in the US, Jurcic et al. [27] evaluated serial bone marrow samples of APL patients treated with ATRA and chemotherapy. Forty of the 47 patients who were induced using ATRA alone had residual disease detectable by RT-PCR before additional therapy. After three cycles of consolidation therapy, residual disease was found in only four of 40 evaluable patients. Among the newly diagnosed patients who had two or more negative RT-PCR assays, only three of 41 suffered a relapse, whereas all four patients who had two or more positive results underwent relapse. These data confirmed that two or more negative RT-PCR assays on bone marrow, performed at least 1 month apart after completing therapy, are strongly associated with long-term remissions. Conversely, a confirmed positive test was highly predictive of the relapse [26].
Taken together, the above studies contributed to the establishment of a new surrogate end point for improved survival in APL (i.e., molecular remission, defined as undetectable PML/RARA transcripts using tests with sentitivity of 10–4), as recommended in 2003 by an international working group (IWG) who revised the criteria for diagnosis, standardization of response assessments, and outcome definitions for AML [28]. Also, the above studies provided a rationale for the administration of pre-emptive therapy in patients experiencing molecular relapse. Indeed, most clinical trials on newly diagnosed APL conducted worldwide since the publication of the IWG criteria have included molecular response as a study end point and, in some instances, the administration of pre-emptive therapy for patients with persistent or recurrent MRD during follow-up.
Two studies conducted in the pre-ATO era evaluated the survival benefit for patients treated either in molecular or in hematologic relapse with the combination of ATRA and various chemotherapy regimens [29, 30]. The first study performed by the GIMEMA group on 14 patients in molecular relapse showed a 2-year survival estimate from the time of first molecular relapse of 92%, compared with the 44% 2-year survival rate of the historical series of 37 APL patients treated at the time of hematologic relapse [29]. A subsequent study conducted by the Spanish PETHEMA group substantially confirmed the GIMEMA data with the survival outcome of patients treated during molecular relapse comparing favorably to the outcome of those treated during hematologic relapse (5-year survival: 64% vs. 24%, P = 0.01) [30]. More recently, a registry study conducted by the European LeukemiaNet on relapsed APL analyzed the outcome of APL patients in first relapse treated with ATO-based salvage therapy followed or not by transplant procedures [31]. In this study, there was no difference in the overall survival and cumulative incidence of relapse at 3 years, in comparison with patients treated at hematologic or molecular relapse (3-year OS: 68% vs. 66%, respectively). However, patients treated at molecular relapse had better OS in the first year after ATO salvage due to the absence of deaths in induction and other severe complications (e.g., differentiation syndrome, bleeding). Notwithstanding the loss of OS advantage at a longer follow-up, the lower rates of early deaths and treatment side effects may still argue in favor of the policy of molecular monitoring, allowing the institution of pre-emptive treatment [31].
Technical considerations: quantitative RQ-PCR
The routine use of RT-PCR tests for MRD monitoring has some important limitations: (i) poor-quality samples (e.g., degraded RNA) may sometimes not be identified, generating false-negative results; (ii) the prolonged post-PCR handling required in the two-step nested RT-PCR procedure carries a high risk of contamination (false positive); (iii) unlike RQ-PCR, RT-PCR is unable to distinguish between decreasing and increasing levels of leukemia-specific transcripts, which would provide relevant information on MRD kinetics. These caveats were in part resolved in the late 90′s by the advent of real-time quantitative PCR assays and their application to leukemia-associated aberrations. An international effort to standardize RQ-PCR for leukemia fusion transcripts, including PML/RARA, was finalized by Gabert and co-workers in 2003, with the development of standardized protocols for RQ-PCR analysis [32]. A total of 26 European laboratories from ten countries collaborated to establish a standardized protocol for TaqMan-based RQ-PCR. This collaborative work established primers and probes design, experimental conditions, standards for control gene, and result interpretation and became a highly quoted reference in MRD studies for leukemia. For the PML/RARA hybrid, the assay described by Gabert et al. had a sensitivity of 10–4 [32]. As for nested PCR, a status of molecular remission when using RQ-PCR assay is conventionally defined at undetectable PML/RARA transcripts using a test yielding a sensitivity of 10–4.
Prospective studies using RQ-PCR
A prospective study using RQ-PCR was undertaken by investigators of the UK NCRI trial involving a large cohort of 406 newly diagnosed APL patients treated with ATRA and chemotherapy [33]. A total of 6727 serial peripheral blood (PB) and bone marrow (BM) samples were analyzed by RQ-PCR for the PML/RARA transcript, with a median assay sensitivity of 10–4 [2]. MRD monitoring according to the recommended schedule (i.e., after each cycle of therapy and at 3-month intervals during follow-up) successfully identified the majority of patients subjected to relapse and proved the most powerful predictor of relapse-free survival (RFS) in multivariable analysis (hazard ratio, 17.87; 95% CI, 6.88–46.41; P < .0001); MRD monitoring was far superior to presenting WBC (hazard ratio, 1.02; 95% CI, 1.00–1.03; P = .02), which is currently widely used to guide the therapy. In patients predicted to experience relapse on the basis of MRD monitoring, early treatment intervention with arsenic trioxide prevented progression to overt relapse in the majority of cases. In the same study, the authors also compared the predictive value of BM vs. PB monitoring using paired patient samples. They reported that molecular conversion from RQ-PCR-negative to positive in BM preceded that in PB in 7/12 patients evaluated by a median of 29 days (range 14–72 days), indicating BM as the preferred sampling source in this disease [33]. In addition to highlighting the capacity of MRD monitoring in detecting early relapse before overt clinical recurrence, this prospective study also identified the challenges of an effective MRD monitoring. In fact, the rapid kinetics of APL relapse imposes a stringent sampling schedule in order to timely deliver salvage therapy, which implies optimal compliance to MRD sampling from either physicians or patients. In addition, the rarity of the disease and the need of high-level expertize suggest that monitoring should be performed using central reference laboratories, which in turn may pose logistic and organizational challenges.
Another prospective study employing RQ-PCR conducted by the PETHEMA cooperative group in the context of ATRA and chemotherapy found no correlation between molecular status after induction and relapse risk, whereas after the third consolidation course, two out of three cases (66%) with positive RQ-PCR relapsed compared with 16 out of 119 (13%) patients in the post-consolidation RQ-PCR-negative group. In addition, the value of RQ-PCR testing in relapse prediction was confirmed during maintenance therapy and out-of treatment assessments, in which all patients with >10 PML/RARA normalized copy numbers (NCN) (n = 19) underwent relapse and all patients with <1 NCN at the end of the study remained in hematologic remission (P < 0.0001) [34].
To monitor the dynamics of PML/RARA transcript in patients treated with ATO and ATRA, Ia prospective Chinese study by Hu et al. [35] employed RQ-PCR to analyze the kinetics of molecular response in newly diagnosed APL patients. A total of 31 patients received ATO–ATRA induction followed by three cycles of consolidation chemotherapy. The authors reported significantly decreased PML/RARA transcripts (i.e., an average reduction of greater than 2-log) after induction therapy and further reduced levels below 10–4 at the end of consolidation therapy, with an average 5-logs reduction (Fig. 2). During maintenance therapy and throughout follow-up, those patients in continuous remission displayed consistently low or undetectable levels of PML/RARA (5-log reduction).
PML–RARA transcript clearance (RQ-PCR) after ATO–ATRA induction and CHT consolidation (Hu et al. [35])
More recently, two independent randomized clinical trials were carried out in the UK, Italy, and Germany to compare ATRA–ATO vs. ATRA and chemotherapy in newly diagnosed APL patients [12, 13]. In both studies, a protocol-predefined MRD assessment was planned upfront, and the molecular response represented a study objective. As for the APL0406 trial, EFS was chosen as a primary study objective, and a persistence of detectable PML/RARA transcripts at the end of consolidation (i.e., after a total of 4 cycles in each arm) was counted as an event. The results of MRD monitoring for 184 Italian patients enrolled in the Italian–German APL0406 study have recently been reported [36]. In keeping with the previous observations, a high proportion of patients (>60%) tested positive for PML/RARA after induction therapy (Fig. 3). Log-reduction of PML/RARA transcripts after induction was significantly greater in patients receiving ATRA–CHT compared with those treated with ATRA–ATO (3.4 vs. 2.9 logs; P = 0.0182). Conversely, at the end of the consolidation, only one patient tested positive in the ATRA–CHT group, and a greater log-reduction of PML/RARA transcripts was observed in the ATRA–ATO group compared with ATRA–CHT (6.3 vs. 5.3 logs; P = 0.0024). In line with the data published in studies using ATRA plus chemotherapy, PML/RARA levels at the time point of post-induction were not predictive of subsequent relapse, likely reflecting more delayed blast cell maturation in the ATRA–ATRO setting, in comparison with ATRA and chemotherapy. This study further confirmed that post-consolidation assessment is the most appropriate and informative time point for evaluating molecular response to treatment in patients with APL treated with ATRA and ATO [36].
In the Italian–German and UK randomized trials [12, 13], the cumulative incidence of molecular relapse in patients treated with ATRA–ATO was extremely low (1.5 and 2%, respectively). Together with other experiences [11, 37], these studies demonstrate that nearly all low-intermediate risk APL patients treated with ATRA plus ATO who achieve molecular remission after consolidation are likely cured of their disease. These data obviously question the cost-effectiveness of prolonged MRD monitoring beyond the achievement of molecular remission, also in light of a potentially negative impact on patients’ quality of life. Hence, the role of MRD monitoring will be probably redefined in the near future, at least in the setting of low-intermediate risk patients.
Zhu and collaborators analyzed the kinetics of PML/RARA transcripts also in 93 patients receiving oral arsenic (realgar indigo naturalis formula or RIF) and ATRA [38]. In this randomized, non-inferiority study, comparing induction therapy with i.v. ATO–ATRA or oral RIF and ATRA, patients were given three cycles of chemotherapy after induction and then maintenance with ATRA–ATO or ATRA–RIF. As shown in Fig. 4, in both groups, the levels of PML/RARA transcripts decreased only slightly after induction and were undetectable after consolidation with no significant difference between the treatment arms. The same authors subsequently reported the results of a pilot study on 20 patients with non-high-risk APL treated with oral arsenic and ATRA and without any chemotherapy for a total of 7 months [39]. Interestingly, the primary end point in this study was complete molecular response, defined as a negative test using quantitative PCR to detect PML/RARA transcripts. The rate of complete molecular remission was 65% at 3 months and 100% at 6 months. After a median follow-up of 48 months (range 42–53 months), all patients remained alive and disease-free (Dr. H.H. Zhu, personal communication).
PML–RARA transcript reduction after RIF (oral tetra-arsenic tetra-sulfide) and i.v. ATO (Reprinted with permission from Zhu et al. [38])
The time points for minimal residual disease monitoring and correlation with clinical outcomes described in previous trials are summarized in Table 1.
Summary and conclusions
Several lines of evidence indicate that molecular response is a valuable end point for clinical trials in APL. In fact: (i) a disease-specific biomarker, the PML/RARA fusion gene, is available in 100% of patients. Not only does this represents an APL unique lesion with well-established pathogenetic role, but also the target of specific agents active in this leukemia; (ii) molecular assays to sensitively measure residual PML/RARA transcripts (including RQ-PCR tests) have been standardized and used for many years in the context of controlled clinical trials; (iii) the results of such studies have led to the establishment of the predictive role of residual disease in relation to risk of relapse; to the adoption early pre-emptive therapy as means to avoid overt relapse; and to the identification of molecular remission after induction and consolidation as a surrogate early endpoint for improved survival; (iv) expert panels including the US IWG and NCCN, Canadian consensus, and the European LeukemiaNet have recommended to assess molecular response after consolidation as a therapeutic objective in APL. As a result, most completed or ongoing controlled studies in APL front-line therapy include molecular response among secondary or even primary study objective(s). Finally, it is important to note that a rapidly achievable surrogate end point, such as molecular remission at post-consolidation, would allow the scientific community to obtain clear answers related to drug efficacy in a timely manner.
References
Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic Leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015. https://doi.org/10.1038/bcj.2015.25.
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–91.
Lo-Coco F, Ammatuna E. The biology of acute promyelocytic leukemia and its impact on diagnosis and treatment. Hematology. 2006;2006:156–61.
Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503.
Montesinos P, González JD, González J, Rayón C, de Lisa E, Amigo ML, et al. Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic Acid and anthracycline-based chemotherapy. J Clin Oncol. 2010;28:3872–9.
Fenaux P, Wang ZZ, Degos L. Treatment of acute promyelocytic leukemia by retinoids. Curr Top Microbiol Immunol. 2007;313:101–28.
Mathews V, George B, Chendamarai E, Lakshmi KM, Desire S, Balasubramanian P, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28:3866–71.
Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60.
Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29:2753–7.
de The H, Pandolfi PP, Chen Z. Acute promyelocytic leukemia: a paradigm for oncoprotein-targeted cure. Cancer Cell. 2017;32:552–60.
Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–73.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–21.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–305.
Seftel MD, Barnett MJ, Couban S, Leber B, Storring J, Assaily W, et al. A Canadian consensus on the management of newly diagnosed and relapsed acute promyelocytic leukemia in adults. Curr Oncol. 2014;21:234–50.
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2017;15:926–57.
Di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Asp Med. 2015;41:1–115.
Falini B, Flenghi L, Fagioli M, Lo Coco F, Cordone I, Diverio D, et al. Immunocytochemical diagnosis of acute promyelocytic leukemia (M3) with the monoclonal antibody PG-M3 (anti-PML). Blood. 1997;90:4046–53.
Miller WHJ, Kakizuka A, Frankel SR, Warrell RPJ, DeBlasio A, Levine K, et al. Reverse transcription polymerase chain reaction for the rearranged retinoic acid receptor alpha clarifies diagnosis and detects minimal residual disease in acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1992;89:2694–8.
Lo Coco F, Diverio D, Pandolfi PP, Biondi A, Rossi V, Avvisati G, et al. Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukaemia. Lancet. 1992;340:1437–8.
Diverio D, Pandolfi PP, Biondi A, Avvisati G, Petti MC, Mandelli F, et al. Absence of reverse transcription-polymerase chain reaction detectable residual disease in patients with acute promyelocytic leukemia in long-term remission. Blood. 1993;82:3556–9.
Martinelli G, Remiddi C, Visani G, Farabegoli P, Testoni N, Zaccaria A, et al. Molecular analysis of PML-RAR alpha fusion mRNA detected by reverse transcription-polymerase chain reaction assay in long-term disease-free acute promyelocytic leukaemia patients. Br J Haematol. 1995;90:966–8.
Lallemand-Breitenbach V, Zhu J, Chen Z, de The H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42.
Laczika K, Mitterbauer G, Korninger L, Knobl P, Schwarzinger I, Kapiotis S, et al. Rapid achievement of PML-RAR alpha polymerase chain reaction (PCR)-negativity by combined treatment with all-trans-retinoic acid and chemotherapy in acute promyelocytic leukemia: a pilot study. Leukemia. 1994;8:1–5.
Huang W, Sun GL, Li XS, Cao Q, Lu Y, Jang GS, et al. Acute promyelocytic leukemia: clinical relevance of two major PML-RAR alpha isoforms and detection of minimal residual disease by retrotranscriptase/polymerase chain reaction to predict relapse. Blood. 1993;82:1264–9.
Diverio D, Rossi V, Avvisati G, De Santis S, Pistilli A, Pane F, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter ‘AIDA’ trial. GIMEMA-AIEOP Multicent. Blood. 1998;92:784–9.
Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood. 1999;93:4131–43.
Jurcic JG. Prognostic significance of minimal residual disease detection and PML/RAR-alpha isoform type: long-term follow-up in acute promyelocytic leukemia. Blood. 2001;98:2651–6.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, et al. Therapy of molecular relapse in acute promyelocytic leukemia. Blood. 1999;94:2225–9.
Esteve J, Escoda L, Martin G, Rubio V, Diaz-Mediavilla J, Gonzalez M, et al. Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia. 2007;21:446–52.
Lengfelder E, Lo-Coco F, Ades L, Montesinos P, Grimwade D, Kishore B, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet. Leukemia. 2015;29:1084–91.
Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17:2318–57.
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–8.
Santamaria C, Chillon MC, Fernandez C, Martin-Jimenez P, Balanzategui A, Garcia Sanz R, et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica. 2007;92:315–22.
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106:3342–7.
Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, et al. PML-RARalpha kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30:1987–92.
Iland HJ, Collins M, Bradstock K, Supple SG, Catalano A, Hertzberg M, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. 2015;2:e357–66.
Zhu H, Wu D-P, Jin J, Li J, Ma J, Wang J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31:4215–22.
Zhu H-H, Huang X-J. Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. N Engl J Med. 2014;371:2239–41.
Chendamarai E, Balasubramanian P, George B, Viswabandya A, Abraham A, Ahmed R, et al. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood. 2012;119:3413–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cicconi, L., Fenaux, P., Kantarjian, H. et al. Molecular remission as a therapeutic objective in acute promyelocytic leukemia. Leukemia 32, 1671–1678 (2018). https://doi.org/10.1038/s41375-018-0219-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0219-5
- Springer Nature Limited
This article is cited by
-
Anti-tumor activity of all-trans retinoic acid in gastric-cancer: gene-networks and molecular mechanisms
Journal of Experimental & Clinical Cancer Research (2023)
-
Mineral medicine: from traditional drugs to multifunctional delivery systems
Chinese Medicine (2022)
-
Adefovir dipivoxil inhibits APL progression through degradation of the oncoprotein PML-RARA
Experimental Hematology & Oncology (2022)
-
Role of cardiolipins, mitochondria, and autophagy in the differentiation process activated by all-trans retinoic acid in acute promyelocytic leukemia
Cell Death & Disease (2022)
-
PRMT5-mediated RNF4 methylation promotes therapeutic resistance of APL cells to As2O3 by stabilizing oncoprotein PML-RARα
Cellular and Molecular Life Sciences (2022)