Abstract
The treatment of acute promyelocytic leukemia (APL) has evolved with the introduction of all-trans retinoic acid (ATRA) and subsequent arsenic trioxide (ATO), particularly in standard-risk APL with an initial white blood cell count (WBC) < 10,000/μL, where a high cure rate can now be achieved. However, for some patients with risk factors, early death or relapse remains a concern. Insights from the analysis of patients treated with ATRA and chemotherapy have identified risk factors such as WBC, surface antigens, complex karyotypes, FLT3 and other genetic mutations, p73 isoforms, variant rearrangements, and drug resistance mutations. However, in the ATRA + ATO era, the significance of these risk factors is changing. This article provides a comprehensive review of APL risk factors, taking into account the treatment approach, and explores the challenges associated with APL treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
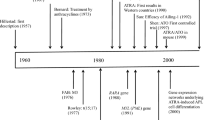
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML), which accounts for approximately 5–15% of all AML cases [1, 2]. Almost all cases of APL have PML::RARA genetic rearrangement resulted from chromosomal translocation t(15;17)(q24.1;q21.2). Clinically, APL typically presents with disseminated intravascular coagulation (DIC) requiring early intervention to prevent severe bleeding. It is also a unique clinical feature that APL is highly responsive to differentiation therapy with all-trans retinoic acid (ATRA) or arsenic trioxide (ATO). Introduction of ATRA drastically improved the outcomes of APL, and treatment with ATRA and chemotherapy (ATRA + Chemo) had been the standard treatment of APL [3,4,5,6,7,8,9,10,11,12,13]. The combination therapy of ATRA and ATO (ATRA + ATO) has further improved the prognosis of APL, and now APL with initial white blood cell count (WBC) < 10,000/μL (standard-risk APL) has become a curable disease by treatment with ATRA + ATO [14,15,16]. However, approaches to the cases with high-risk backgrounds should still be improved. In addition, the significances of risk factors can change according to the progression in treatment and diagnostic modalities. This review focuses on the risk factors and remaining challenges in APL treatment.
ATRA + Chemo
ATRA binds to PML-RARα fusion protein through the ligand binding domain (LBD) on RARα. Without the binding of ATRA, PML-RARα acts as a transcriptional repressor. Binding of ATRA to PML-RARα induces activation of downstream transcription and also causes degradation of the fusion protein, which leads to the differentiation of leukemic cells [2, 17, 18]. In the treatment of APL, ATRA can induce a complete remission (CR), but a high relapse rate was a problem with ATRA monotherapy [19]. Use of chemotherapy with ATRA (ATRA + Chemo) improved the prognosis of APL drastically [3,4,5,6,7,8,9,10,11,12,13]. In ATRA + Chemo regimens, ATRA, anthracyclines, and cytarabine are commonly used as induction and consolidation therapy, and maintenance therapy is often administered after consolidation. Representative ATRA + Chemo regimens and their long-term outcomes are listed in Table 1. Various ATRA + Chemo regimens have been reported from different groups, and they demonstrate comparable long-term survivals exceeding 80%. From these results, ATRA + Chemo had been the standard treatment of newly diagnosed APL, and thus, risk factors had been analyzed in patients treated with ATRA + Chemo. The advent of ATRA + ATO has led to improvement in treatment outcomes, consequently affecting the significance of risk factors elucidated in ATRA + Chemo regimens. Therefore, when considering risk factors in APL, it is important to be aware of whether the patients received the ATRA + Chemo or ATRA + ATO.
ATRA + ATO
Physiologically, PML forms nuclear bodies (NBs) and controls cell senescence through p53 signaling [2, 18, 20]. In APL, PML-RARα makes heterodimers with PML and disrupts its NB formation, leading to uncontrolled proliferation and block of differentiation [2, 20, 21]. ATO binds to PML and PML-RARα through B2 domain on PML and facilitates the degradation of PML-RARα and reformation of PML NBs [2, 17, 18, 20]. Clinically, single-agent ATO has been shown to be effective against relapsed/refractory or untreated APL cases [22,23,24]. Since 2004, the efficacy of ATRA + ATO for untreated APL was reported [25, 26], and finally, for the treatment of untreated, standard-risk APL, the superiority of ATRA + ATO to ATRA + Chemo was demonstrated in 2 randomized controlled trials, APL0406 and AML17 [14, 15, 27]. Outcomes of representative studies for standard-risk APL treated with ATRA + ATO are listed in Table 2. Very favorable long-term survivals of more than 90% were achieved in these studies.
Fatal bleeding, differentiation syndrome, and early death
It is a characteristic feature of APL that almost all cases exhibit DIC concomitantly and that differentiation syndrome (DS) can be induced by differentiation therapy with ATRA and/or ATO. Given the low relapse rate of APL, the success of APL treatment largely hinges on the appropriate management of severe complications such as DIC, DS, and infections to prevent early death (ED) during the remission induction. This remains consistent whether patients are treated with ATRA + Chemo or ATRA + ATO, and risk factors for ED are also analyzed as well, not only for survival.
Prognostic factors in APL treatment
White blood cell count
Among the risk factors in APL, the most potent one is the pre-treatment WBC. For patients treated with ATRA + Chemo regimens, Sanz et al. reported the predictive model for relapse-free survival (RFS) designating patients with initial WBC ≤ 10,000/μL and platelet counts > 40,000/μL as low risk, WBC ≤ 10,000/μL and platelet counts ≤ 40,000/μL as intermediate risk, and WBC > 10,000/μL as high risk [29]. In the Japanese JALSG APL92 study, patients with an initial WBC < 10,000/μL exhibited a favorable 4-year disease-free survival (DFS) rate of 67.6%, as opposed to 42.1% for those with WBC ≥ 10,000/μL [30]. Considering that WBC serves as a prognostic factor, some trials of ATRA + Chemo adopted stratified treatment according to WBC at diagnosis [5,6,7,8,9, 31].
Being based on the insights from ATRA + Chemo, ATRA + ATO designates WBC < 10,000 as standard risk and WBC > 10,000 as high risk. GIMEMA APL0406 study, which was a randomized controlled trial comparing ATRA + ATO and ATRA + Chemo, only included standard-risk patients [14], but some other studies incorporated high-risk participants [15, 16, 28]. In ATRA + ATO for high-risk patients, gemtuzumab ozogamicin (GO) or idarubicin is administered in the early phase of induction therapy to effectively suppress elevated WBC. The treatment outcomes of ATRA–ATO for high-risk cases are presented in Table 3. The survival rates for high-risk cases were approximately 85%, and these rates appear to be lower than those observed in standard-risk cases (Table 2). To date, there have been no randomized trials demonstrating the superiority of ATRA + ATO over ATRA + Chemo in high-risk patients [15].
The high WBC at diagnosis was also one of the independent risk factors of ED in both ATRA + Chemo and ATRA + ATO [32,33,34,35].
Surface antigens
Aberrant expression of CD56 is observed in 10–15% of APL cases, and is known to correlate with the expression of CD2, CD7, CD34, and HLA-DR [36,37,38,39,40]. CD56 positivity is also associated with bcr3 isoform (short form) of PML::RARA [36, 39, 40]. An analysis of 651 cases that underwent ATRA + Chemo treatment through PETHEMA and HOVON trials LPA96, 99, and 2005 showed that CD56 positivity, along with elevated WBC, was an independent risk factor for relapse with a hazard ratio (HR) of 2.3 compared to CD56-negative cases [36]. In the analysis of JALSG APL97, CD56-positive cases showed a tendency towards unfavorable event-free survival (EFS). Particularly, among patients with WBC > 3000/μL, a significantly worse prognosis of CD56-positive cases was demonstrated (9-year EFS 30.8% vs 63.6%) [37]. JALSG APL204 is a randomized study that compared ATRA and tamibarotene as maintenance therapies in ATRA + Chemo treatment [8] and revealed that, together with high WBC and ATRA maintenance, expression of CD56 was still an unfavorable prognostic factor for RFS (HR 3.19) [38]. Analyses of GIMEMA AIDA0493 and 2000 suggested that, in addition to CD56, CD15-positive cases also had poor outcomes [39]. Along with high WBC (HR 2.4) and PML::RARA bcr3 isoform (HR 2.2), expression of CD56 or CD15 was an independent adverse factor for overall survival (OS) (HR 1.9). Thus, in the ATRA + Chemo era, expression of CD56 is considered an unfavorable prognostic factor.
Reports on differences in prognosis based on surface antigens are limited for patients treated with ATRA + ATO. Improved outcomes achieved with ATRA + ATO may have contributed to overcoming the risk associated with surface antigens such as CD56. Nonetheless, a report of 184 cases who underwent ATRA + ATO induction in China indicated that CD56 positivity was an independent prognostic factor for RFS (HR 4.7) [40]. However, it is important to note that this group received chemotherapy-based treatment as post-remission therapy, which differs from the current ATRA + ATO regimen where ATRA and ATO are administered without chemotherapy agents in the post-remission phase. Administering an appropriate dosage of ATO as post-remission therapy might potentially help in overcoming the risk associated with CD56 expression.
Additional chromosomal abnormalities
Additional chromosomal abnormalities (ACAs) aside from t(15;17) are observed in approximately 30% of APL cases [41,42,43,44,45,46,47]. The most commonly observed ACA is trisomy 8, accounting for 30–50%, followed by abnormalities in chromosomes 7, 9, or 17 [41,42,43,44,45,46]. The presence of ACAs does not appear to impact prognosis, regardless of the treatment, whether it is ATRA + Chemo [41,42,43, 45, 47] or ATO-containing regimen [44,45,46]. Meanwhile, some of the studies focused on the number of ACAs and found that complex karyotype with ≥ 2 or ≥ 3 ACAs could adversely affect the prognosis of the patients treated with ATRA + Chemo or ATRA + ATO [45,46,47].
FLT3 and other genetic abnormalities
In addition to the disease-defining PML::RARA fusion gene resulting from chromosomal translocation t(15;17)(q24;q21), approximately 70% of APL patients have at least one additional genetic mutation at the time of diagnosis [48]. FLT3 mutations are most commonly observed, with FLT3-internal tandem duplication (ITD) accounting for 20–40% and FLT3-tyrosine kinase domain mutations (TKD) for 10–20% of cases [48,49,50,51]. Following FLT3 mutations, WT1, NRAS, KRAS, ARID1A/B are recurrently mutated, whereas mutations in genes frequently observed in other subtypes of AML, such as DNMT3A, TET2, IDH1/2, ASXL1, GATA2, and NPM1, are rare or absent in APL [48, 49]. Except for FLT3 mutations, roles and impacts of individual mutated gene in the pathogenesis and prognosis of APL are not well understood. Nevertheless, in the analysis of 44 patients, most of whom underwent ATRA + Chemo treatment, cases with ≥ 2 additional mutations had an increased risk of relapse compared to those with fewer mutations [50].
FLT3-ITD mutation is known to be associated with increased WBC, morphological microgranular variant, and bcr3 isoform of PML::RARA [52,53,54,55,56,57,58]. Impact of FLT3-ITD on the prognosis of the patients treated with ATRA + Chemo is controversial. Several studies indicated in univariate analysis that FLT3-ITD have a significant adverse impact on ED and survival [10, 55,56,57,58]. Some of these studies demonstrated that FLT3-ITD mutation remained an independent adverse prognostic factor for survival in multivariate analysis [10, 57, 58], while in others, the significance was lost [55, 56]. This discrepancy could be attributed to the strong correlation between FLT3-ITD and elevated WBC, which could complicate the assessment of the prognostic impact of FLT3-ITD due to the potent effect of WBC as a risk factor.
In the ATO era, a report that analyzed 134 patients who received ATO alone as induction therapy demonstrated that KRAS and GATA2 mutations were independent risk factors for ED, while FLT3-ITD was not [59]. Prospective and retrospective studies incorporating ATO as part of induction and/or post-remission therapy alongside ATRA + Chemo also failed to demonstrate the significance of FLT3-ITD for survival [16, 40, 45, 60,61,62]. Furthermore, in the sub-analysis of the APL0406 trial, FLT3-ITD did not have a significant effect on EFS in the ATRA + ATO arm, although there was a non-significant trend of worse EFS in positive FLT3-ITD cases in the ATRA + Chemo arm [63]. According to these reports, it appears that FLT3-ITD has a diminished impact on survival outcomes in patients who received an ATO-containing regimen as initial therapy.
p73 isoforms
p73, a member of the p53 family encoded by TP73, has a closely related structure with p53 and functions as a tumor suppressor regulating apoptosis and cell cycle [64, 65]. Using the alternative promoters or transcription start sites, full-length p73 (TAp73) containing the N-terminal transactivation domain (TAD) or truncated inactive form (ΔNp73) without entire or part of the N-terminal TAD are transcribed [64, 65]. ΔNp73 dominant-negatively inhibits the activities of p53 and TAp73, and thus overweighed expression of ΔNp73 can contribute to tumorigenesis or resistance to chemotherapy [64, 65]. As for AML, it was reported that ΔNp73 mRNA expression was observed in 96.7% of non-APL AML patients, whereas it was present in only 31.7% of APL cases [66]. This finding may partially explain the better prognosis of APL. Indeed, analysis of 129 patients who were enrolled in the IC-APL study and received ATRA + Chemo demonstrated that a high ΔNp73/TAp73 mRNA ratio (≥ 1.6) was an independent unfavorable factor for OS along with higher age and WBC and lower albumin [67]. A scoring system incorporating ΔNp73/TAp73, FLT3-ITD, and expression levels of ID1, BAALC, ERG, and KMT2E genes was also proposed [68]. While the importance of ΔNp73/TAp73 ratio has been examined in patients treated with ATRA + Chemo, there is a possibility that ΔNp73/TAp73 may also carry some significance in the context of ATRA + ATO due to the potential involvement of the p73 pathway in the therapeutic mechanisms of both ATRA and ATO [65, 69].
Variant rearrangements, and drug resistance-associated mutations in PML::RARA
The therapeutic effects of ATRA and ATO are mediated through their binding to LBD in RARα and B2 domain in PML, respectively [2, 17, 18, 20]. Therefore, it is anticipated that mutations in or lack of LBD or B2 domain could result in resistance to ATRA or ATO.
The vast majority of APL patients have PML::RARA fusion gene, but approximately 2% of the cases harbor variant rearrangements, with ZBTB16::RARA being the most common, followed by STAT5B::RARA [70, 71]. Recently, rearrangements involving RARG or RARB, instead of RARA, are also known [70, 71]. These variant cases exhibit resistance or do not show a clear response to ATO, whereas sensitivity to ATRA is variable, although LBD is retained in RARα even in variant rearrangements [17, 70, 71]. The treatment of patients with variant rearrangements primarily relies on chemotherapy, while the role of hematopoietic stem cell transplantation is not well-defined due to the limited number of patients and requires future investigation [17, 70,71,72]. Recent case reports implied the benefits of hypomethylating agents and venetoclax [73,74,75].
Mutations in PML::RARA are common in relapsed APL patients. The frequency of these mutations is 15–40% at the first relapse and reaches 40–70% at the second relapse [50, 76, 77]. In relapse after treatment with ATRA- and/or ATO-containing regimens, mutations are found in the LBD region of RARA and/or B2 domain region of PML, respectively, which contribute to the resistance to each drug [17, 50, 76,77,78]. Interestingly, the hotspot mutation can occur in unrearranged PML, which may also be involved the mechanism of resistance against ATO [79, 80]. Information about these drug-resistant mutations is important for the choice of salvage therapy.
Future challenges in APL treatment
The introduction of ATRA + ATO has led to a high cure rate for standard-risk APL. In addition, analysis of the patients enrolled in the APL0406 trial has demonstrated that a better quality of life was maintained for a long time among patients treated with ATRA + ATO than those treated with ATRA + Chemo [81]. Further, oral arsenic has been shown to be as effective as intravenous ATO [82], allowing standard-risk APL patients to access less invasive and more convenient treatments. However, cases with risk factors such as high WBC face challenges like ED and relapse even in the ATRA + ATO era. Risk factors may shift as treatment and diagnostic methods advance. It is important to evaluate risk factors appropriately, and, especially when patients are considered as high-risk, careful monitoring of measurable residual disease using PML::RARA is essential. There is also a strong need for the development of more effective treatments tailored to high-risk patients.
References
Kayser S, Schlenk RF, Platzbecker U. Management of patients with acute promyelocytic leukemia. Leukemia. 2018;32(6):1277–94.
de The H, Pandolfi PP, Chen Z. Acute promyelocytic leukemia: a paradigm for oncoprotein-targeted cure. Cancer Cell. 2017;32(5):552–60.
Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716–25.
Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–6.
Asou N, Kishimoto Y, Kiyoi H, Okada M, Kawai Y, Tsuzuki M, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110(1):59–66.
Ades L, Chevret S, Raffoux E, de Botton S, Guerci A, Pigneux A, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10.
Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–9.
Shinagawa K, Yanada M, Sakura T, Ueda Y, Sawa M, Miyatake J, et al. Tamibarotene as maintenance therapy for acute promyelocytic leukemia: results from a randomized controlled trial. J Clin Oncol. 2014;32(33):3729–35.
Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46.
Iland H, Bradstock K, Seymour J, Hertzberg M, Grigg A, Taylor K, et al. Results of the APML3 trial incorporating all-trans-retinoic acid and idarubicin in both induction and consolidation as initial therapy for patients with acute promyelocytic leukemia. Haematologica. 2012;97(2):227–34.
de Botton S, Chevret S, Coiteux V, Dombret H, Sanz M, San Miguel J, et al. Early onset of chemotherapy can reduce the incidence of ATRA syndrome in newly diagnosed acute promyelocytic leukemia (APL) with low white blood cell counts: results from APL 93 trial. Leukemia. 2003;17(2):339–42.
Ades L, Chevret S, Raffoux E, Guerci-Bresler A, Pigneux A, Vey N, et al. Long-term follow-up of European APL 2000 trial, evaluating the role of cytarabine combined with ATRA and Daunorubicin in the treatment of nonelderly APL patients. Am J Hematol. 2013;88(7):556–9.
Takeshita A, Asou N, Atsuta Y, Sakura T, Ueda Y, Sawa M, et al. Tamibarotene maintenance improved relapse-free survival of acute promyelocytic leukemia: a final result of prospective, randomized, JALSG-APL204 study. Leukemia. 2019;33(2):358–70.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–305.
Iland HJ, Collins M, Bradstock K, Supple SG, Catalano A, Hertzberg M, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. 2015;2(9):e357–66.
Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int J Hematol. 2013;97(6):717–25.
Dos Santos GA, Kats L, Pandolfi PP. Synergy against PML-RARa: targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. J Exp Med. 2013;210(13):2793–802.
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, et al. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990;76(9):1704–9.
de The H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol. 2012;198(1):11–21.
Adams J, Nassiri M. Acute promyelocytic leukemia: a review and discussion of variant translocations. Arch Pathol Lab Med. 2015;139(10):1308–13.
Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–24.
Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Arsenic trioxide therapy for relapsed or refractory Japanese patients with acute promyelocytic leukemia: need for careful electrocardiogram monitoring. Leukemia. 2002;16(4):617–22.
Yanada M, Tsuzuki M, Fujita H, Fujimaki K, Fujisawa S, Sunami K, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood. 2013;121(16):3095–102.
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101(15):5328–35.
Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107(9):3469–73.
Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, Vignetti M, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35(6):605–12.
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129(10):1275–83.
Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53.
Asou N, Adachi K, Tamura J, Kanamaru A, Kageyama S, Hiraoka A, et al. Analysis of prognostic factors in newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Japan Adult Leukemia Study Group. J Clin Oncol. 1998;16(1):78–85.
Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43.
Minamiguchi H, Fujita H, Atsuta Y, Asou N, Sakura T, Ueda Y, et al. Predictors of early death, serious hemorrhage, and differentiation syndrome in Japanese patients with acute promyelocytic leukemia. Ann Hematol. 2020;99(12):2787–800.
Cai P, Wu Q, Wang Y, Yang X, Zhang X, Chen S. An effective early death scoring system for predicting early death risk in de novo acute promyelocytic leukemia. Leuk Lymphoma. 2020;61(8):1989–95.
Gill H, Yung Y, Chu HT, Au WY, Yip PK, Lee E, et al. Characteristics and predictors of early hospital deaths in newly diagnosed APL: a 13-year population-wide study. Blood Adv. 2021;5(14):2829–38.
Zhu HH, Ma YF, Yu K, Ouyang GF, Luo WD, Pei RZ, et al. Early death and survival of patients with acute promyelocytic leukemia in ATRA plus arsenic era: a population-based study. Front Oncol. 2021;11: 762653.
Montesinos P, Rayon C, Vellenga E, Brunet S, Gonzalez J, Gonzalez M, et al. Clinical significance of CD56 expression in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based regimens. Blood. 2011;117(6):1799–805.
Ono T, Takeshita A, Kishimoto Y, Kiyoi H, Okada M, Yamauchi T, et al. Expression of CD56 is an unfavorable prognostic factor for acute promyelocytic leukemia with higher initial white blood cell counts. Cancer Sci. 2014;105(1):97–104.
Takeshita A, Asou N, Atsuta Y, Furumaki H, Sakura T, Ueda Y, et al. Impact of CD56 continuously recognizable as prognostic value of acute promyelocytic leukemia: results of multivariate analyses in the Japan Adult Leukemia Study Group (JALSG)-APL204 study and a review of the literature. Cancers (Basel). 2020;12(6).
Breccia M, De Propris MS, Minotti C, Stefanizzi C, Raponi S, Colafigli G, et al. Aberrant phenotypic expression of CD15 and CD56 identifies poor prognostic acute promyelocytic leukemia patients. Leuk Res. 2014;38(2):194–7.
Lou Y, Ma Y, Suo S, Ni W, Wang Y, Pan H, et al. Prognostic factors of patients with newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide-based frontline therapy. Leuk Res. 2015;39(9):938–44.
De Botton S, Chevret S, Sanz M, Dombret H, Thomas X, Guerci A, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: results of APL 93 trial. Br J Haematol. 2000;111(3):801–6.
Cervera J, Montesinos P, Hernandez-Rivas JM, Calasanz MJ, Aventin A, Ferro MT, et al. Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica. 2010;95(3):424–31.
Ono T, Takeshita A, Iwanaga M, Asou N, Naoe T, Ohno R, et al. Impact of additional chromosomal abnormalities in patients with acute promyelocytic leukemia: 10-year results of the Japan Adult Leukemia Study Group APL97 study. Haematologica. 2011;96(1):174–6.
Lou Y, Suo S, Tong H, Ye X, Wang Y, Chen Z, et al. Characteristics and prognosis analysis of additional chromosome abnormalities in newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide as the front-line therapy. Leuk Res. 2013;37(11):1451–6.
Poire X, Moser BK, Gallagher RE, Laumann K, Bloomfield CD, Powell BL, et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma. 2014;55(7):1523–32.
Epstein-Peterson ZD, Derkach A, Geyer S, Mrozek K, Kohlschmidt J, Park JH, et al. Effect of additional cytogenetic abnormalities on survival in arsenic trioxide-treated acute promyelocytic leukemia. Blood Adv. 2022;6(11):3433–9.
Labrador J, Luno E, Vellenga E, Brunet S, Gonzalez-Campos J, Chillon MC, et al. Clinical significance of complex karyotype at diagnosis in pediatric and adult patients with de novo acute promyelocytic leukemia treated with ATRA and chemotherapy. Leuk Lymphoma. 2019;60(5):1146–55.
Fasan A, Haferlach C, Perglerova K, Kern W, Haferlach T. Molecular landscape of acute promyelocytic leukemia at diagnosis and relapse. Haematologica. 2017;102(6):e222–4.
Madan V, Shyamsunder P, Han L, Mayakonda A, Nagata Y, Sundaresan J, et al. Comprehensive mutational analysis of primary and relapse acute promyelocytic leukemia. Leukemia. 2016;30(8):1672–81.
Iaccarino L, Ottone T, Alfonso V, Cicconi L, Divona M, Lavorgna S, et al. Mutational landscape of patients with acute promyelocytic leukemia at diagnosis and relapse. Am J Hematol. 2019;94(10):1091–7.
Picharski GL, Andrade DP, Fabro A, Lenzi L, Tonin FS, Ribeiro RC, et al. The impact of Flt3 gene mutations in acute promyelocytic leukemia: a meta-analysis. Cancers (Basel). 2019;11(9).
Kiyoi H, Naoe T, Yokota S, Nakao M, Minami S, Kuriyama K, et al. Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho). Leukemia. 1997;11(9):1447–52.
Noguera NI, Breccia M, Divona M, Diverio D, Costa V, De Santis S, et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia. 2002;16(11):2185–9.
Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106(12):3768–76.
Au WY, Fung A, Chim CS, Lie AK, Liang R, Ma ES, et al. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004;125(4):463–9.
Barragan E, Montesinos P, Camos M, Gonzalez M, Calasanz MJ, Roman-Gomez J, et al. Prognostic value of FLT3 mutations in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy. Haematologica. 2011;96(10):1470–7.
Yoo SJ, Park CJ, Jang S, Seo EJ, Lee KH, Chi HS. Inferior prognostic outcome in acute promyelocytic leukemia with alterations of FLT3 gene. Leuk Lymphoma. 2006;47(9):1788–93.
Lucena-Araujo AR, Kim HT, Jacomo RH, Melo RA, Bittencourt R, Pasquini R, et al. Internal tandem duplication of the FLT3 gene confers poor overall survival in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based chemotherapy: an International Consortium on Acute Promyelocytic Leukemia study. Ann Hematol. 2014;93(12):2001–10.
Chen X, Fan S, Zhao Y, Zhou J. Gene mutations in acute promyelocytic leukemia early death in patients treated with arsenic trioxide alone. Clin Transl Oncol. 2021;23(10):2171–80.
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342–7.
Song X, Hu X, Lu S, Gao L, Chen L, Yang J, et al. Incorporation of arsenic trioxide in induction therapy improves survival of patients with newly diagnosed acute promyelocytic leukaemia. Eur J Haematol. 2014;93(1):54–62.
Yang S, Ma R, Yuan X, Jiang L, Shi J, Yang J, et al. Improved outcomes of all-trans-retinoic acid and arsenic trioxide plus idarubicin as a frontline treatment in adult patients with acute promyelocytic leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(7):e382–91.
Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, et al. PML-RARalpha kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30(10):1987–92.
Ramos H, Raimundo L, Saraiva L. p73: From the p53 shadow to a major pharmacological target in anticancer therapy. Pharmacol Res. 2020;162: 105245.
Humbert M, Federzoni EA, Tschan MP. Distinct TP73-DAPK2-ATG5 pathway involvement in ATO-mediated cell death versus ATRA-mediated autophagy responses in APL. J Leukoc Biol. 2017;102(6):1357–70.
Rizzo MG, Giombini E, Diverio D, Vignetti M, Sacchi A, Testa U, et al. Analysis of p73 expression pattern in acute myeloid leukemias: lack of DeltaN-p73 expression is a frequent feature of acute promyelocytic leukemia. Leukemia. 2004;18(11):1804–9.
Lucena-Araujo AR, Kim HT, Thome C, Jacomo RH, Melo RA, Bittencourt R, et al. High DeltaNp73/TAp73 ratio is associated with poor prognosis in acute promyelocytic leukemia. Blood. 2015;126(20):2302–6.
Lucena-Araujo AR, Coelho-Silva JL, Pereira-Martins DA, Silveira DR, Koury LC, Melo RAM, et al. Combining gene mutation with gene expression analysis improves outcome prediction in acute promyelocytic leukemia. Blood. 2019;134(12):951–9.
Momeny M, Zakidizaji M, Ghasemi R, Dehpour AR, Rahimi-Balaei M, Abdolazimi Y, et al. Arsenic trioxide induces apoptosis in NB-4, an acute promyelocytic leukemia cell line, through up-regulation of p73 via suppression of nuclear factor kappa B-mediated inhibition of p73 transcription and prevention of NF-kappaB-mediated induction of XIAP, cIAP2. BCL-XL and survivin Med Oncol. 2010;27(3):833–42.
Wen L, Xu Y, Yao L, Wang N, Wang Q, Liu T, et al. Clinical and molecular features of acute promyelocytic leukemia with variant retinoid acid receptor fusions. Haematologica. 2019;104(5):e195–9.
Guarnera L, Ottone T, Fabiani E, Divona M, Savi A, Travaglini S, et al. Atypical rearrangements in APL-like acute myeloid leukemias: molecular characterization and prognosis. Front Oncol. 2022;12: 871590.
Sobas M, Talarn-Forcadell MC, Martinez-Cuadron D, Escoda L, Garcia-Perez MJ, Mariz J, et al. PLZF-RAR(alpha), NPM1-RAR(alpha), and other acute promyelocytic leukemia variants: the PETHEMA registry experience and systematic literature review. Cancers (Basel). 2020;12(5).
Liu M, Zhao X, Pan W, Qian Z, Du M, Wang LM, et al. A novel HNRNPC-RARA fusion in acute promyelocytic leukaemia lacking PML-RARA rearrangement, sensitive to venetoclax-based therapy. Br J Haematol. 2021;195(2):e123–8.
Song B, Wang X, Kong X, Wang M, Yao L, Shen H, et al. Clinical response to venetoclax and decitabine in acute promyelocytic leukemia with a novel RARA-THRAP3 fusion: a case report. Front Oncol. 2022;12: 828852.
Ding W, Weng G, Wang Z, Guo Y, Wang M, Shen H, et al. Case report: identification of a novel HNRNPC::RARG fusion in acute promyelocytic leukemia lacking RARA rearrangement. Front Oncol. 2022;12:1028651.
Lou Y, Ma Y, Sun J, Ye X, Pan H, Wang Y, et al. Evaluating frequency of PML-RARA mutations and conferring resistance to arsenic trioxide-based therapy in relapsed acute promyelocytic leukemia patients. Ann Hematol. 2015;94(11):1829–37.
Gallagher RE, Moser BK, Racevskis J, Poire X, Bloomfield CD, Carroll AJ, et al. Treatment-influenced associations of PML-RARalpha mutations, FLT3 mutations, and additional chromosome abnormalities in relapsed acute promyelocytic leukemia. Blood. 2012;120(10):2098–108.
Zhu HH, Qin YZ, Huang XJ. Resistance to arsenic therapy in acute promyelocytic leukemia. N Engl J Med. 2014;370(19):1864–6.
Lehmann-Che J, Bally C, de The H. Resistance to therapy in acute promyelocytic leukemia. N Engl J Med. 2014;371(12):1170–2.
Iaccarino L, Ottone T, Divona M, Cicconi L, Cairoli R, Voso MT, et al. Mutations affecting both the rearranged and the unrearranged PML alleles in refractory acute promyelocytic leukaemia. Br J Haematol. 2016;172(6):909–13.
Efficace F, Platzbecker U, Breccia M, Cottone F, Carluccio P, Salutari P, et al. Long-term quality of life of patients with acute promyelocytic leukemia treated with arsenic trioxide vs chemotherapy. Blood Adv. 2021;5(21):4370–9.
Zhu HH, Wu DP, Du X, Zhang X, Liu L, Ma J, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19(7):871–9.
Funding
Japan Agency for Medical Research and Development, 23ck0106715h0003, Yasuhisa Yokoyama.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yokoyama, Y. Risk factors and remaining challenges in the treatment of acute promyelocytic leukemia. Int J Hematol (2024). https://doi.org/10.1007/s12185-023-03696-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12185-023-03696-7