Abstract
The chemokine receptor CCR7 is widely implicated in breast cancer pathobiology. Although recent reports correlated high CCR7 levels with more advanced tumor grade and poor prognosis, limited in vivo data are available regarding its specific function in mammary gland neoplasia and the underlying mechanisms involved. To address these questions we generated a bigenic mouse model of breast cancer combined with CCR7 deletion, which revealed that CCR7 ablation results in a considerable delay in tumor onset as well as significantly reduced tumor burden. Importantly, CCR7 was found to exert its function by regulating mammary cancer stem-like cells in both murine and human tumors. In vivo experiments showed that loss of CCR7 activity either through deletion or pharmacological antagonism significantly decreased functional pools of stem-like cells in mouse primary mammary tumors, providing a mechanistic explanation for the tumor-promoting role of this chemokine receptor. These data characterize the oncogenic properties of CCR7 in mammary epithelial neoplasia and point to a new route for therapeutic intervention to target evasive cancer stem cells.
Similar content being viewed by others
Introduction
Despite concerted efforts and significant advances, breast cancer-related mortality is still a leading cause of death in women worldwide.1 Clearly novel therapies are urgently needed. The 'cancer stem cell' theory specifies that a small subset of cells in a heterogeneous tumor (termed 'cancer stem cells' (CSCs)) possess stem cell-like properties of self-renewal and differentiation. CSCs are suggested to sustain and propagate tumors, and are inherently therapy-resistant (for the latest reviews see2 and3).
CSCs may originate from adult stem cells, but can also arise from more committed lineage progenitor cells if they acquire stem cell-like features owing to genetic or epigenetic changes.4 Multiple intrinsic and extrinsic factors are reported to have a role in CSC maintenance, regulation and support of stem-like characteristics. Most prominent are the Notch,5 Hedgehog,6 Wnt7 and TGFβ8 signaling systems. Several cytokines and chemokines have also been recently suggested as maintaining and promoting the CSC phenotype in a number of solid malignancies, including mammary tumors (reviewed in9); however, definitive in vivo data has been sparse.
Chemokine receptors and their cognate chemokine ligands have become widely accepted as important mediators of cancer growth and progression in many human neoplasms, being involved in tissue transformation, invasion, angiogenesis and resistance to chemotherapy.10 Among these, the chemokine receptor CCR7 has been implicated in metastatic spread of multiple malignancies.11 In breast carcinogenesis, it has been attributed a number of potential functions, including promotion of cell motility, migration and adhesion, regulation of matrix metalloproteinases leading to basement membrane degradation,12 and cell survival through inhibition of anoikis.13 Data obtained using cell lines has implicated CCR7 in breast cancer spread to the lymph nodes,14 and in human breast cancer its role was inferred from retrospective studies on archived tumor tissues.15 High expression levels of CCR7 were also correlated with higher grade and occurrence of secondary tumors, and poor prognosis.16, 17
Whereas all these studies point to a role for CCR7 in malignancy, a direct function for CCR7 in cancer has not yet been established. Furthermore, its role in breast cancer in particular is unclear. We show here the development of a novel bigenic mouse model combining deletion of CCR7 with the polyoma middle-T transgene, which is under control of the mouse mammary tumor virus promoter (MMTV-PyMT), to study tumor development in vivo. Using this model we show that CCR7 deletion has a striking preventative effect on PyMT-driven mammary tumors, supporting the notion that CCR7 has a major determining role in breast oncogenesis. Moreover, our data reveal that the tumor-promoting effect of CCR7 is mediated through stem-like cells in both primary mouse and human breast tumors. These results provide new insights into the role of CCR7 in breast cancer stem-like cells and have important implications for the development of future therapeutics in breast cancer.
Results
CCR7 deletion arrests mammary tumorigenesis in the PyMT transgenic breast cancer mouse model
The MMTV-PyMT transgenic breast cancer mouse model has been extensively used in recent years to study various aspects of mammary neoplasia. Expression of the PyMT protein promotes the rapid epithelial transformation of mammary cells via the corruption of various pathways including those of Src, ras and PI3 kinase. This model also results in spontaneous metastasis and has been found to closely mimic the development of human breast cancer.18, 19, 20 Representative images are shown in Supplementary Figure 1a, in which α-smooth muscle actin is used to stain myoepithelial cells.
To directly assess the role of CCR7 in the multistage process of mammary tumorigenesis in vivo, we generated bigenic MMTV-PyMT Ccr7−/− knockout mice (further referred to as CCR7 KO+) and traced the development of mammary tumors relative to MMTV-PyMT Ccr7WT mice (further referred to as WT+).
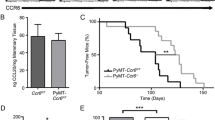
Deletion of CCR7 significantly delayed PyMT-driven primary mammary tumorigenesis (representative pictures Figure 1a). Tumor-free survival was significantly extended (Figure 1b) and total tumor burden was markedly reduced in CCR7 KO+ mice (Figure 1c) when compared with the WT+ animals. The lungs of WT+ and CCR7 KO+ females were also examined for metastatic lesions at the time of killing. CCR7 KO+ mice developed significantly fewer and smaller metastases than WT+ mice (Figures 1d–f), although the number of metastases varied largely between mice of the same genotype.
CCR7 deletion arrests mammary tumorigenesis in the PyMT transgenic breast cancer mouse model. (a–c) CCR7 ablation delays tumor onset and reduces tumor burden in the MMTV-PyMT-driven mouse model of breast cancer. (a) Representative images of MMTV-PyMT Ccr7WT (WT+) and MMTV-PyMT Ccr7−/− (CCR7 KO+) mice at 22-weeks-old, showing grossly visible tumors (demarcated by red arrows and dotted lines). (b) Kaplan–Meier analysis of tumor-free survival for WT+ (n=18) and CCR7 KO+ (n=17) mice. (c) Number of tumors in WT+ and CCR7 KO+ mice at the time of killing. (d–f) CCR7 KO+ mice developed less lung metastases than their WT+ counterparts. (d) Total cumulative area of lung metastatic lesions in WT+ (n=8) and CCR7 KO+ (n=8) mice. (e) Distribution data of lung metastases in WT+ and CCR7 KO+ mice. (f) Representative images of H&E-stained lung sections from WT+ (left) and CCR7 KO+ (right) mice with metastatic lesions (black arrowheads). Red arrowhead indicates inset magnified image. *P<0.05, **P<0.01, ***P<0.001.
As these experiments indicated a role for CCR7 in mammary gland function, we next examined normal, pre-cancerous and cancer-bearing mouse mammary glands for CCR7 expression and signaling. CCR7 was shown to be expressed on all mouse mammary epithelial cells tested, regardless of the tumor stage (Supplementary Figure 1b), and the removal of CCR7 did not affect the expression levels of its ligands CCL19 and CCL21 within the mouse mammary fat pad (Supplementary Figure 2a). CCR7 was also found to be functional in PyMT-driven mammary tumors, as tumor cells mobilized intracellular calcium, a hallmark of chemokine receptor activity, in response to stimulation with CCL21 (Supplementary Figure 2b). These data showed that CCR7 was expressed and was functional within normal and transformed mammary epithelium.
Interestingly, despite the large impact of CCR7 on overall mammary tumorigenesis, initial PyMT-driven hyperplastic growth in 8- or 11-week-old WT+ and CCR7 KO+ mice was not affected (Supplementary Figure 3a), with similar tissue architecture in glands from both genotypes (Supplementary Figure 3b). This indicated that the CCR7 KO+ mammary glands underwent the initial oncogenic transformation leading to epithelial proliferation, but further tumorigenic transition was largely blocked by CCR7 deletion.
CCR7 promotes tumorigenesis by amplifying breast cancer stem-like cells
To investigate the underlying mechanisms responsible for the tumorigenic effects seen, we examined the role of CCR7 in mammary gland development and on stem-like cells. We found that in non-PyMT wild-type (WT) pubertal mice, the epithelial tree was longer with a better developed ductal structure than that in Ccr7−/− (CCR7 KO) mice (Figures 2a and b), indicating that ablation of CCR7 had a mild inhibitory effect on pubertal growth of the mammary gland epithelium. CCR7 was robustly expressed in normal mammary epithelium (Figure 2c), making this receptor also potentially relevant to normal mammary development. However, the development of mammary epithelium in the CCR7 KO mice caught up with that of the WT mice by the age of 8 weeks, and at 12 weeks mammary glands from the two genotypes were indistinguishable (Figure 2d), demonstrating that CCR7 deletion mainly delays early mammary gland development.
The effect of CCR7 on normal mammary development. (a and b) Development of the mammary ductal tree was evaluated in Ccr7WT (WT) and Ccr7−/− (CCR7 KO) C57Bl/6 mice at 6 weeks of age. (a) Representative whole mount images of mammary glands, with apparent reduction in the size of ductal trees in CCR7 KO (right, n=6) compared with WT (left, n=7). LN= lymph node. (b) Quantitation of the length of the main epithelial duct (left), total number of mammary epithelial terminal structures (center), and branching within mammary epithelium determined by quantifying branch points per mm along three individual ducts (right). *P<0.05, **P<0.01, ***P<0.001. (c) CCR7 expression (magenta) in normal mouse mammary terminal end buds of pubertal mice. Nuclei are counterstained with DAPI (gray). (d) Representative whole mount images of mammary glands taken from adult WT (left, n=6) and CCR7 KO (right, n=6) mice at 12 weeks of age with no apparent differences in the size and architecture of the mammary tree.
Because normal development and breast cancer are believed to be linked by common regulatory mechanisms, we hypothesized that the observed promotion of PyMT-driven tumorigenesis and mammary development was due to CCR7 regulating stem/progenitor cell pools in mammary epithelium. Thus, we next assayed the stem-like cell content in mice using the lineage-negative (Lin−) CD24+CD29hi cell surface marker profile,4 which was previously functionally characterized in the MMTV-PyMT model.21, 22 CCR7 was expressed in all cell lineages in both the normal and PyMT-expressing mammary glands regardless of CD24 and CD29 status. Notably, however, higher levels of CCR7 (>90%) were observed in Lin−CD24+CD29hi normal and cancer mouse mammary stem cell-enriched populations (Figure 3a, Supplementary Figure 4a and b). Importantly, CCR7 was also expressed on human CD44+CD24− putative mammary stem cells23 from both normal and breast tumor tissue (Figure 3b, Supplementary Figure 4c).
CCR7 activity amplifies stem-like cells in both mouse and human mammary glands. (a–c) Normal mammary glands and mammary tumors were analyzed by flow cytometry for stem-like cell content. (a) Proportion of cells positive for CCR7 in Lin− stem cell-enriched populations in normal mouse mammary epithelium (left) and PyMT-expressing glands (right), as denoted by surface marker expression CD24+CD29hi. Shaded histograms=fluorescence-minus-one negative gates. (b) Proportion of cells positive for CCR7 in putative stem-like cell populations in normal human mammary epithelium (left) and breast cancer (right), as denoted by surface marker expression CD44+CD24−. Shaded histograms, fluorescence-minus-one negative gates. (c) CCR7 deletion decreases the proportion of mouse Lin− stem cell-enriched populations in normal (left) and PyMT-expressing (right) mammary glands as indicated. (d–f) The effect of CCR7 deletion/activation on primary and secondary mammosphere formation was assessed. Shown are mammosphere-forming efficiencies of cells derived from normal mouse mammary glands (d), PyMT-expressing mouse mammary glands (e), and human patient-derived breast tumors (f). WT mouse cells and primary human cells were stimulated with CCL21 and CCL19 as indicated. (a–f) Mouse data are representative of at least three independent experiments, n=6–10 mice per group. Human results are representative of two normal and four independent tumor samples. *P<0.05, **P<0.01, ***P<0.001.
Further analysis demonstrated a significantly lower content of Lin−CD24+CD29hi cells in non-PyMT CCR7 KO mice relative to WT (Figure 3c left panel). In PyMT-expressing mice at the stage of early neoplasia, when no morphological differences were found in WT+ and CCR7 KO+ glands (Supplementary Figure 3) and the stem/progenitor cell populations may, therefore, best reflect the tumor-initiating cell content, the difference in the stem cell-enriched population between WT+ and CCR7 KO+ mice was even more pronounced with the deletion of CCR7 leading to a twofold reduction in stem-like cells (Figure 3c right panel).
Recently Pece et al. have suggested a new and potentially more efficient set of markers, in which the notch ligands delta-like ligand 1 (DLL1) and delta and notch-like epidermal growth factor-related receptor (DNER) are used in combination with CD49f (Lin−CD49f+DLL1+DNER+) to delineate putative stem cells in human mammary tumors.24 We found that the stem-like cells from both human and mouse mammary glands defined by this profile also expressed high levels of CCR7 (Supplementary Figure 4d and e). Moreover, the Lin−CD49f+DLL1+DNER+ cell pools were significantly smaller in both normal and PyMT-expressing CCR7 KO murine mammary glands (Supplementary Figures 5a and b), providing further support for the findings described above.
It is generally accepted that non-adherent passaged mammosphere cultures are enriched in cells with stem-like characteristics, and secondary/tertiary mammosphere-forming efficiency (MFE) is representative of cells’ potential to exhibit stem cell traits.25, 26, 27 Stem-like activity, as measured by MFE, was then analyzed in the mammary epithelium in the presence or absence of CCR7. Primary and secondary sphere formation from normal (Figure 3d) or PyMT-expressing (Figure 3e) mammary cells was substantially reduced after CCR7 ablation and, importantly, stimulation of WT and WT+ cells with CCR7 ligands CCL19 and CCL21 significantly potentiated mammosphere growth (Figures 3d and e).
This CCR7 stimulatory function was seen exclusively on mammosphere growth, as stimulation with CCL19 and CCL21 had no detectable effect on the proliferation of bulk mammary tumor cells in adherent culture, a condition that supports a more differentiated phenotype (Supplementary Figure 5c). The addition of CCR7 ligands to sphere cultures derived from CCR7 KO+ mammary cells also had no effect on MFE (Supplementary Figure 5d), demonstrating a CCR7 receptor-mediated mechanism. The specificity of CCR7 was further shown by testing a panel of ligands for other tumor-associated chemokine receptors CCR6,16 CXCR328 and CXCR5.29 No effects were observed on MFE (Supplementary Figure 5e).
To extend these findings to human breast cancer we next examined the activity of CCR7 in human primary tumor cells from resected breast cancer tissue. The addition of CCL19 and CCL21 resulted in an increase in primary and secondary MFE of human breast cancer cells by two- to threefold (Figure 3f), consistent with results obtained in the mouse model.
To specifically link the deletion of CCR7 to depleted tumor-initiating cells, a limiting dilution transplantation approach was used24 to estimate tumor-initiating cell (TIC) frequency. Secondary mammosphere-derived cells from WT+ and CCR7 KO+ mice with early neoplasia were used in this assay to address the potential of cells in mammosphere cultures to exhibit stem cell traits of self-renewal and tumor initiation in vivo, in the context of CCR7-dependency. Cells were injected into contralateral inguinal fat pads of non-PyMT WT recipients. Analysis of grafted fat pads after 6 weeks showed that WT+ sphere cells produced much more robust growth at all dilutions (Figure 4a and Supplementary Figure 6). Most importantly, the frequency of stem-like cells capable of tumor initiation within WT+ sphere culture (1/189) was over threefold higher than in CCR7 KO+ (1/913) (Figure 4b), providing strong evidence for the critical role of CCR7 in the regulation and maintenance of stem-like cells and tumor-initiating cells in the mammary gland.
CCR7 increases in vivo tumor-initiating capacity of sphere cells. (a) Representative images of intact and respective whole-mounted contralateral mammary glands engrafted with 2500 WT+ or CCR7 KO+ sphere-derived cells. Black arrowheads indicate areas of outgrowth from engrafted cells. LN=lymph node. (b) Results of limiting dilution assay indicating frequency of tumor-initiating cells (TIC) in WT+ and CCR7 KO+ sphere cultures. Fractions indicate the number of fat pads with lesion(s) per total number of recipient fat pads.
CCR7 is required for the propagation of mammary tumors
To obtain in vivo evidence for the role of CCR7 in tumor propagation, we took advantage of the PyMT mouse model, which allows tumor formation upon transplantation.20 Expression of the PyMT oncogene results in multifocal tumors and hence can generate diverse CSC pools owing to various underlying mutations within the same gland at the late stages of tumorigenesis. Therefore, we reasoned that if taken at the early stage of pre-neoplastic tumor development, the population of CSCs should be more homogeneous. Consequently, small 1mm3 fragments of pre-neoplastic mammary tissue from 8-week-old PyMT transgenic WT+ and CCR7 KO+ mice were simultaneously transplanted into contralateral inguinal mammary fat pads of non-PyMT WT recipients. Representative histological sections from both WT+ and CCR7 KO+ 8-week-old mice, corresponding to donor tissue, are shown in Figure 5a, confirming that the glands used for transplantation were at the equivalent stage of tumorigenesis.
CCR7 is required for the propagation of mammary tumors. (a) Representative H&E-stained sections of pre-neoplastic mice at the WT+ and CCR7 KO+ donor age of 8 weeks. Bottom: magnified images of boxed area. (b) Representative whole-mount images of WT-recipient glands after transplantation of pre-neoplastic mammary tissue from WT+ (top) and CCR7 KO+ (bottom) donor mice at 8 weeks of age. Black arrowheads indicate areas of outgrowth from donor tissue. Fractions indicate the number of fat pads with lesion(s) per total number of recipient fat pads. LN=lymph node. (c) Cumulative area of transplant outgrowth in recipient mammary glands. n=6 mice per group. *P<0.05.
Analysis of tumorigenic outgrowth from transplanted tissue showed that the deletion of CCR7 almost completely blocked secondary tumor development. Only one out of six transplants from the CCR7 KO+ donors was able to give rise to a neoplastic lesion, whereas five out of six fragments from the WT+ donors produced secondary outgrowths in WT recipients (Figures 5b and c), demonstrating a key role of CCR7 in tumor propagation.
Pharmacological antagonism of CCR7 in vivo depletes the stem-like cell population and inhibits mammary tumorigenesis
A CCR7 antagonist, CCL19(8–83),30 was used to explore the potential of targeting CCR7 for CSC-directed therapeutic intervention. Initially, the ability of CCL19(8–83) to block the stimulatory activity of CCR7 ligands on mammosphere-forming capacity was tested ex vivo and found to specifically abrogate the effect of CCL21 (Figure 6a) and CCL19 (data not shown) on mammosphere growth, providing a rationale for in vivo studies.
Pharmacological antagonism of CCR7 in vivo depletes the stem-like cell population and inhibits mammary tumorigenesis. (a) MFE of Lin− mammary cells from WT+ mice (n=9), untreated or treated with CCL21 and/or the CCR7 antagonist CCL19(8–83). *P<0.05. (b–d) Treatment of mice with CCL19(8–83) reduces tumorigenesis in MMTV-PyMT WT+ mice. (b) Representative image of intact mammary glands treated with vehicle or CCL19(8–83) as indicated. (c) Cellularity of contralateral vehicle- or CCL19(8–83)-treated glands. (d) Proportions of Lin−CD24+CD29hi cells (left) and MFE (right) of cells from vehicle- or CCL19(8–83)-treated glands. (e) Treatment of mice with CCL19(8–83) reduces the stem cell-enriched population in a transplant model. Experimental strategy (left), proportion of Lin−CD24+CD29hi cells (center) and MFE (right) of cells from transplanted tumors with or without CCL19(8–83) treatment. (b–e) Data are representative of two independent experiments, n=4–6 mice per experiment. *P<0.05, ***P<0.001.
The effect of CCR7 blockade by CCL19(8–83) on tumor initiation was then examined in the context of the PyMT transgenic mouse model. CCL19(8–83) was injected for eight consecutive weeks into inguinal mammary glands of animals from the age of 4-weeks-old. Glands were then excised and examined for the extent of tumorigenesis and stem-like cell content and function. Macroscopic analysis demonstrated that CCL19(8–83)-injected glands had smaller lesions than their control counterparts (representative image Figure 6b). The total weight of fat pads was not statistically different; however, the cellularity (total cell count and cells per mg of tissue) was significantly reduced by the antagonist (Figure 6c, Supplementary Figures 7a and b).
Treatment with CCL19(8–83) also resulted in a significant decrease in the proportion of stem-like cells (Lin−CD24+CD29hi Figure 6d left panel, and Lin−CD49f+DLL1+DNER+ Supplementary Figure 7c) and the function of stem and early progenitor cells (Figure 6d right panel), without affecting the level of CCR7 receptor expression (Supplementary Figure 7d). PyMT transgenic mice on both FVB and C57Bl/6 (not shown) backgrounds were tested, with similar results.
To determine whether treatment with CCL19(8–83) has an inhibitory effect on established and/or advanced later stage tumors, 1mm3 size fragments of MMTV-PyMT WT+ tumors from 16-week-old mice, corresponding to the invasive ductal carcinoma stage of human breast cancer (Supplementary Figure 1a), were transplanted into inguinal glands of WT recipients followed by eight weekly injections of CCL19(8–83) or vehicle control (Figure 6e left panel). Although no significant differences were seen between CCL19(8–83)- or vehicle-treated tumors in size or cellularity (Supplementary Figure 7e) as was observed in primary tumors, the proportions of stem-like cells determined by both conventional (Lin−CD24+CD29hi Figure 6e center panel) or novel (Lin−CD49f+DLL1+DNER+ Supplementary Figure 7f) marker sets, as well as mammosphere growth (Figure 6e right panel), were significantly reduced in antagonist-treated glands, demonstrating that the CCR7 axis can be blocked in vivo to target stem-like cells in mammary tumors.
Discussion
The contribution of CSCs to tumor initiation is a major issue in tumor biology, yet one of the least understood processes.3 We show here that ablating CCR7 using a bigenic MMTV-PyMT Ccr7−/− model significantly depleted the breast CSC-enriched pool. Using the surface marker profiles CD24+CD29hi 4 and CD49f+DLL1+DNER+ 24 we showed that the underlying mechanism involves a decrease in the ability of stem-like cells and early progenitor cells to self-renew and initiate neoplasia. Significantly, exogenously targeting CCR7 with a peptide antagonist led to a decrease in tumorigenesis.
CCR7 has been extensively studied for its role in adaptive immunity and secondary lymphoid organogenesis, and CCR7-null mice display disrupted architecture of the thymus and lymph node, as well as a reduced ability to mount a primary immune response.31 The role of CCR7 in mediating anti-tumor immunity is also slowly emerging.32 In this context, the fact that abrogation of CCR7 severely affected mammary tumorigenesis provides definitive evidence of CCR7 as a pro-tumorigenic driver. Furthermore, numerous transplantation approaches used in this study underscore an immune system-independent role of this chemokine receptor in maintaining stem-like cell pools in breast cancer.
Interestingly, hyperplastic outgrowth, widely believed to be a precursor of mammary tumors,20 was found in 100% of WT and CCR7-null PyMT-carrying glands examined. However, the majority of CCR7 KO+ glands were unable to sustain this initial proliferative burst of tumor cells and progress to the next stage in tumor development. Therefore, the delay in mammary tumorigenesis appears to be due to CCR7 maintaining specialized hierarchical sub-populations of cancer stem and progenitor-like cells that are thought to be crucial for tumor initiation and advanced tumorigenesis.2
The fact that both CCL19 and CCL21 stimulated mammosphere growth from both human and mouse tumor cells strongly suggests that CCR7 has a global role in sustaining properties of stemness in mammary epithelium. The specificity of CCR7 in this process was validated by testing a panel of chemokine receptor ligands, where only CCL19 and CCL21 showed an ability to significantly increase MFE.
Stimulation of CXCR4, the chemokine receptor that is consistently found to be upregulated together with CCR7 in a number of cancers,17 did potentiate sphere formation but to a lesser extent (Supplementary Figure 5e). Recently, Clarke and colleagues demonstrated that stimulation of CXCR4 also increased MFE preferentially in malignant breast cancer cell lines compared with normal breast cell lines.26 It is interesting to speculate that as CCR7 is less important for homeostasis than CXCR4, as has been inferred from animal models,33 CCR7 may represent a more attractive target for future CSC-targeting therapies.
As stimulation of CCR7 had no effect on proliferation of the bulk population of cells when seeded into adherent culture, compared with a highly significant effect in non-adherent culture, it is likely that CCR7 predominantly mediates specific cellular properties of stemness. Moreover, we have previously reported that CCR7 activation on breast cancer cells inhibits anoikis,13 a characteristic of breast and other CSCs.25, 34 Therefore, it is plausible that CCR7 supports CSC survival without attachment to the extracellular matrix, a hypothesis that may form the basis for future studies.
CCR7 appeared to have a quantitative rather than a qualitative role in normal mammary stem cells compared with CSCs. When CCR7 was deleted we saw a mild effect on the normal mammary gland. In contrast, a major effect was seen in mammary tumorigenesis. Interestingly, whereas the morphological effect on normal mammary gland development was not extensive, CCR7 deletion discreetly affected normal mammary gland stem-like cells. Therefore, it is possible that CCR7 has a role in regulating the properties of stemness within the mammary epithelial cell population, an effect that appears more prominent during cancer progression. As highlighted in a recent study by Cheresh and colleagues,35 dysregulation of normal stem cells may contribute to breast cancer progression and stemness, and CCR7 may emerge as a novel mediator of this transition.
Translation of our findings from the mouse model to human disease is of particular significance, considering that there is currently no clear consensus on the markers that define functional mammary stem cells in both mice and humans. Thus, we show here that CCR7 not only has a role in mouse mammary tissue but is also expressed, is functional and is highly responsive in the stem-like populations within human breast cancer tissue. Intriguingly, circulating tumor cells, an indicator of metastatic spread and poor outcome in breast and other cancers, have been recently equated to CSCs.36 In the last decade numerous studies also suggested a role for CCR7 in malignant dissemination of mammary tumors to distant sites.13, 15, 17 Taken together, these results suggest a novel causative link between CCR7 activity on stem-like populations and metastatic breast cancer.
To seek proof-of-principle on the utility of pharmacologically targeting CCR7 we tested the receptor antagonist CCL19(8–83).30 We found that pharmacological inhibition of CCR7 through direct mammary fat pad injection of CCL19(8–83) afforded a significant reduction in early-stage primary mammary tumorigenesis. As the relative contribution of the malignant lesions to the weight of the whole mammary fat pad was very small at this early stage the reduction in total weight between antagonist and vehicle-treated glands was not statistically significant. However, the cellularity, a characteristic that directly reflects the extent of epithelial malignant outgrowth and is used in clinical pathology to evaluate the response to chemotherapy in breast cancer, was strongly impacted by treatment with the CCR7 antagonist.
More importantly, directly targeting CCR7 using the antagonist significantly depleted the stem-like cell pools in both early and late-stage mammary neoplasia as was shown using the transplantation approach. These findings strongly suggest that the CCR7 receptor axis is a potential point of intervention in stem cell-targeting therapies. Furthermore, the results of this study provide a rationale for the use of antagonists of the CCR7 pathway as adjuvants to conventional cytotoxic drugs unable to eliminate quiescent CSCs.2
In conclusion, the characterization of CCR7 in primary breast tumorigenesis in vitro and in vivo, and in mouse and human tissue, strongly suggests a role for this molecule in breast cancer development and progression. These insights raise the possibility of pharmacologically targeting CCR7 for the development of new therapies in breast cancer.
Materials and methods
Mice
Mice were maintained in pathogen-free conditions in the University of Adelaide’s Laboratory Animal Services facility. Ccr7−/− mice were purchased from Jackson Laboratory. FVB MMTV-PyMT (+) mice were backcrossed for 14 generations to C57Bl/6 background, and C57Bl/6 background was confirmed by microsatellite analysis. PyMT-carrying males were then crossed with Ccr7−/− females, and the offspring were interbred to produce MMTV-PyMT Ccr7WT and MMTV-PyMT Ccr7−/− mice. The University of Adelaide institutional animal ethics committee approved all experimentation. For the assessment of CCR7 expression and CCL19(8–83) function both C57Bl/6 and FVB backgrounds were tested to eliminate any strain bias. For experiments involving knockout mice, only C57Bl/6 mice were tested. Nomenclature used for genotypes is as follows: Ccr7WT=WT, Ccr7−/−=CCR7 KO, MMTV-PyMT Ccr7WT=WT+, MMTV-PyMT Ccr7−/−=CCR7 KO+.
Human mammary tissue
Ethical approval was granted by the Royal Adelaide Hospital Ethics Committee and all patients gave written, informed consent prior to surgery. Pathology reports for tumors used are available on request. Normal breast tissue samples were obtained from the Queen Elizabeth Hospital, Adelaide.
Whole mount staining
Mammary glands were fixed in Carnoy’s and were stained overnight in Carmine Alum before dehydration and mounting. Image 'stitching' and analysis was performed using Image J.
Histology
Lungs of MMTV-PyMT WT+ or CCR7 KO+ mice were perfused and dissected, then cryoembedded in optimal cutting temperature (OCT) reagent and serially sectioned at 9μm. Formalin-fixed paraffin-embedded mammary glands/tumors were sectioned at 5μm. All slides were stained using haemotoxylin and eosin, dehydrated and mounted. Slides were scanned using the NanoZoomer Digital Pathology System (Hamamatsu Photonics, Shizuoka, Japan) and lung sections were manually quantitated using the NanoZoomer Digital Pathology Virtual Slide Viewer software for number of metastases and area at the largest point.
Immunofluorescent microscopy
Antigen retrieval of formalin-fixed paraffin-embedded mouse mammary sections was performed by boiling slides in 0.1 m sodium citrate buffer (pH 6.0). Slides were stained with rabbit anti-CCR7 (Epitomics, Burlingame, CA, USA) overnight at 4 °C, and primary antibody was detected with Alexa Fluor 488-conjugated goat anti-rabbit (Invitrogen, Carlsbad, CA, USA) for 30 min. Slides were counterstained with DAPI (4',6-diamidino-2-phenylindole), mounted and analyzed using the Leica TCS SP5 Confocal Microscope System.
Processing mouse mammary tissue to single cell suspension
Mouse mammary glands/tumors were dissected, with removal of the lymph node if possible. Tissue was manually dissociated and then digested in 10% collagenase/hyaluronidase (Stem Cell Technologies, Vancouver, BC, Canada) in Dulbecco's Modified Eagle's medium (DMEM) for 3-4 h with gentle tilting. Single cells were extracted as previously described37 and filtered through a 70 μm nylon mesh. To remove contaminating infiltrating cells of hematopoietic origin, Biotin Binder Dynabeads (Invitrogen) in combination with a biotinylated anti-mouse lineage antibody panel (BioLegend, San Diego, CA, USA) were used as suggested by the manufacturer.
Processing human mammary tissue to single cell suspension
Surgical specimens were minced and digested in 10% collagenase/hyaluronidase (Stem Cell Technologies) in DMEM supplemented with 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), penicillin–streptomycin and 0.25 μg/ml fungazone. Organoids were then extensively washed with DMEM, and red blood cells were lysed by isotonic lysis buffer (150 mM NH4Cl in 17 mM Tris-HCl, pH 7.2). Single cell suspensions were obtained by digesting organoids with trypsin for 10 min at room temperature, with subsequent filtration through a 70 μm nylon mesh.
Flow cytometry
Cells were fixed in 4% formaldehyde and immunostained for 45 min on ice in phosphate-buffered saline/0.5% bovine serum albumin (PBS/0.5% BSA). Antibodies used were: Alexa Fluor 647-conjugated anti-mouse CCR7, phycoerythrin (PE)-conjugated anti-mouse/anti-human CD24, fluorescein isothiocyanate (FITC)/PECy5-conjugated anti-human CD49f, FITC-conjugated anti-human CD44 (all from BD, North Ryde, NSW, Australia), FITC-conjugated anti-mouse CD29, PE-conjugated anti-mouse/anti-human DLL1 (all from BioLegend), and biotinylated anti-mouse/anti-human DNER (R&D Systems, Minneapolis, MN, USA). Samples containing biotinylated antibodies were resuspended in PerCP/Cy5.5 or Alexa Fluor 488-conjugated streptavidin in PBS/0.5% BSA for 30 min on ice. Fluorescence-minus-one samples or conjugated isotypes were used as negative controls. Flow cytometry was carried out using FACSCanto equipment (BD). Data analysis was performed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Mammosphere assay
Cells were seeded in mammosphere medium (1:1 mixture of DMEM and Ham’s F12 medium (Gibco, Life Technologies, Mulgrave, VIC, Australia) supplemented with 1 × B27 (Invitrogen), 20ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor, 4 μg/ml heparin (Sigma-Aldrich, St Louis, MO, USA), penicillin–streptomycin, and 0.25 μg/ml fungazone) at 4 × 104 cells/ml to an ultra-low attachment tray (Corning Inc., Corning, NY, USA). Where indicated, CCL21, CCL19 and CCL19(8–83) were added at concentrations of 10 ng/ml, 200 ng/ml and 100 ng/ml, respectively. Media was replenished every second day. After 7–10 days, mammospheres were counted and passaged.
Limiting dilution assay
Mammosphere colonies derived from 8-week-old pre-neoplastic MMTV-PyMT WT+ and CCR7 KO+ mice were dissociated using trypsin and triturated through a 19G needle. Following filtration, cells were injected in 20% Matrigel (BD):80% DMEM into the fourth inguinal mammary glands of anaesthetized WT-recipient mice (8-weeks-old) at limiting dilutions as previously described.24 Mice were killed after 6 weeks and glands whole-mounted. Tumor-initiating cell-frequency and statistical calculations were performed using L-Calc software (Stem Cell Technologies).
Tissue transplants
Mammary gland fragments of 1mm3 size from donor MMTV-PyMT mice were transplanted into contralateral sides of anaesthetized congenic non-PyMT WT-recipient mice (8-weeks-old) within the fourth inguinal mammary glands. Mice were monitored for adverse reactions to surgery and subsequent tumor growth.
In vivo treatment with CCR7 antagonist
Mice were injected under anesthetic into an inguinal mammary fat pad, with 1 μg CCL19(8–83) truncated ligand in 50 μl saline vehicle, as indicated. As a control, mice were injected in the contralateral inguinal mammary fat pad with vehicle alone as previously reported.38, 39
Statistical analysis
Unless otherwise indicated, analyses were carried out using GraphPad Prism and data is shown as mean±s.e.m. Significant statistical difference was estimated using student’s t-tests, or χ2-tests for distribution analysis. Tumor-free survival curves were graphed using the Kaplan–Meier method and distributions were compared by the log-rank statistic (Mantel–Cox test). All measurements were done in triplicate. P-values were used to denote statistical significance. Levels of significance were *P⩽0.05, **P⩽0.01 and ***P⩽0.001.
References
Maxmen A . The hard facts. Nature 2012; 485: S50–S51.
Visvader JE, Lindeman GJ . Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012; 10: 717–728.
Medema JP . Cancer stem cells: the challenges ahead. Nat Cell Biol 2013; 15: 338–344.
Visvader JE . Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev 2009; 23: 2563–2577.
Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 2008; 3: 429–441.
Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006; 66: 6063–6071.
van Amerongen R, Bowman AN, Nusse R . Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012; 11: 387–400.
Iwanaga R, Wang CA, Micalizzi DS, Harrell JC, Jedlicka P, Sartorius CA et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res 2012; 14: R100.
Chin AR, Wang SE . Cytokines driving breast cancer stemness. Mol Cell Endocrinol 2014; 382: 598–602.
Balkwill FR . The chemokine system and cancer. J Pathol 2012; 226: 148–157.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–56.
Li J, Sun R, Tao K, Wang G . The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig Liver Dis 2011; 43: 40–47.
Kochetkova M, Kumar S, McColl SR . Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ 2009; 16: 664–673.
Cunningham HD, Shannon LA, Calloway PA, Fassold BC, Dunwiddie I, Vielhauer G et al. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl Oncol 2010; 3: 354–361.
Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol 2006; 17: 945–951.
Cassier PA, Treilleux I, Bachelot T, Ray-Coquard I, Bendriss-Vermare N, Menetrier-Caux C et al. Prognostic value of the expression of C-Chemokine Receptor 6 and 7 and their ligands in non-metastatic breast cancer. BMC Cancer 2011; 11: 213.
Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res 2005; 11: 5686–5693.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 2003; 163: 2113–2126.
Guy CT, Cardiff RD, Muller WJ . Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12: 954–961.
Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res 2001; 61: 8298–8305.
Schwab LP, Peacock DL, Majumdar D, Ingels JF, Jensen LC, Smith KD et al. Hypoxia-inducible factor 1alpha promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res 2012; 14: R6.
Ma J, Lanza DG, Guest I, Uk-Lim C, Glinskii A, Glinsky G et al. Characterization of mammary cancer stem cells in the MMTV-PyMT mouse model. Tumour Biol 2012; 33: 1983–1996.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010; 140: 62–73.
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–1270.
Ablett MP, O'Brien CS, Sims AH, Farnie G, Clarke RB . A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget 2014; 5: 599–612.
Pastrana E, Silva-Vargas V, Doetsch F . Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011; 8: 486–498.
Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther 2009; 8: 490–498.
Biswas S, Sengupta S, Roy Chowdhury S, Jana S, Mandal G, Mandal PK et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat 2014; 143: 265–276.
Pilkington KR, Clark-Lewis I, McColl SR . Inhibition of generation of cytotoxic T lymphocyte activity by a CCL19/macrophage inflammatory protein (MIP)-3beta antagonist. J Biol Chem 2004; 279: 40276–40282.
Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33.
Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX et al. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol 2000; 164: 4558–4563.
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR . Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393: 595–599.
Luo M, Guan JL . Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 2010; 289: 127–139.
Desgrosellier JS, Lesperance J, Seguin L, Gozo M, Kato S, Franovic A et al. Integrin alphavbeta3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev Cell 2014; 30: 295–308.
Giordano A, Gao H, Cohen EN, Anfossi S, Khoury J, Hess K et al. Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann Oncol 2013; 24: 2515–2521.
Smalley MJ, Kendrick H, Sheridan JM, Regan JL, Prater MD, Lindeman GJ et al. Isolation of mouse mammary epithelial subpopulations: a comparison of leading methods. J Mammary Gland Biol Neoplasia 2012; 17: 91–97.
Lavergne E, Combadiere C, Iga M, Boissonnas A, Bonduelle O, Maho M et al. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J Immunol 2004; 173: 3755–3762.
Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol 2003; 170: 4638–4648.
Acknowledgements
We are grateful to Dr Timothy Proudman and Dr Pallave Dasari from the Queen Elizabeth Hospital for supply of normal human breast tissue and Dr Deepak Dhatrak of the Department of Anatomical Pathology at SA Pathology for procurement of human breast cancer samples. We would also like to sincerely thank Professor Angel Lopez for critical reading of the manuscript, and Dr Michael Samuel and Ms Natasha Pyne for assistance with immunohistochemistry. This work was supported by an NHMRC project grant. WVI is a National Breast Cancer Foundation/The QEH Research Foundation Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Boyle, S., Ingman, W., Poltavets, V. et al. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene 35, 105–115 (2016). https://doi.org/10.1038/onc.2015.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.66
- Springer Nature Limited
This article is cited by
-
ROCK-mediated selective activation of PERK signalling causes fibroblast reprogramming and tumour progression through a CRELD2-dependent mechanism
Nature Cell Biology (2020)
-
Chemokines in homeostasis and diseases
Cellular & Molecular Immunology (2018)
-
Promotion of tumor progression and cancer stemness by MUC15 in thyroid cancer via the GPCR/ERK and integrin-FAK signaling pathways
Oncogenesis (2018)
-
Interplay between CCR7 and Notch1 axes promotes stemness in MMTV-PyMT mammary cancer cells
Molecular Cancer (2017)
-
Regulation of CCR7-dependent cell migration through CCR7 homodimer formation
Scientific Reports (2017)