Abstract
The mechanisms of action of the rapid antidepressant effects of ketamine, an N-methyl-D-aspartate glutamate receptor antagonist, have not been fully elucidated. This study examined the effects of ketamine on ligand binding to a metabotropic glutamatergic receptor (mGluR5) in individuals with major depressive disorder (MDD) and healthy controls. Thirteen healthy and 13 MDD nonsmokers participated in two [11C]ABP688 positron emission tomography (PET) scans on the same day—before and during intravenous ketamine administration—and a third scan 1 day later. At baseline, significantly lower [11C]ABP688 binding was detected in the MDD as compared with the control group. We observed a significant ketamine-induced reduction in mGluR5 availability (that is, [11C]ABP688 binding) in both MDD and control subjects (average of 14±9% and 19±22%, respectively; P<0.01 for both), which persisted 24 h later. There were no differences in ketamine-induced changes between MDD and control groups at either time point (P=0.8). A significant reduction in depressive symptoms was observed following ketamine administration in the MDD group (P<0.001), which was associated with the change in binding (P<0.04) immediately after ketamine. We hypothesize that glutamate released after ketamine administration moderates mGluR5 availability; this change appears to be related to antidepressant efficacy. The sustained decrease in binding may reflect prolonged mGluR5 internalization in response to the glutamate surge.
Similar content being viewed by others
Introduction
Depression is the psychiatric disorder with the greatest impact on the global disability burden.1 This is because it is common2 and because many individuals fail to respond to available treatments.3 Moreover, currently available medications typically require weeks to months to exert their beneficial effects, which are commonly lost within the first year of treatment.4 Identifying novel treatment targets and developing new tools that can rapidly ease the burden of depression to individuals and society is thus of high priority.
Glutamate signaling has an important role in the regulation of affective processes.5, 6, 7 A postmortem study reported elevated tissue glutamate levels in depression in the anterior cingulate cortex8 and magnetic resonance spectroscopy study in the anterior cingulate cortex9 and occipital cortex detected elevated glutamate levels in individuals with major depressive disorder (MDD).10 Elevations in glutamate may produce neuroadaptations in neural structure and function, including alterations in the regulation of glutamate receptors.11, 12 There is, however, also magnetic resonance spectroscopy literature showing lower glutamate and glutamate/glutamine levels in individuals with depression.13, 14
The metabotropic glutamatergic receptor 5 (mGluR5) is a receptor target for glutamate that may show depression-related changes in regulation. MGluR5 is a G-protein coupled receptor located presynaptically and postsynaptically15, 16, 17, 18 and is present on neurons both at the cell surface and intracellularly.19, 20, 21 Preclinical literature suggests that changes in mood states are associated with changes in mGluR5 levels. Knock out of mGluR5 in mice is associated with depressive-like behavior,22 and reductions in mGluR5 protein occur in animal models of depression.23, 24 In human postmortem brain tissue, reductions in mGluR5 protein levels have been reported in depressed individuals in the cerebellum25 and prefrontal cortex (PFC; BA9).26 A different postmortem study, however, failed to demonstrate significant alterations in the anterior cingulate mGluR5 density in MDD.27 In vivo, a positron emission tomography (PET) imaging study with [11C]ABP688 reported lower mGluR5 density in MDD in several regions.26 Chronic, but not acute, antidepressant treatment appears to increase mGluR5 expression,28 raising the possibility that therapeutic effects might be mediated by mGluR5 regulation.
In the present study, we compared ketamine effects upon mGluR5 availability in depressed patients and healthy comparison subjects. Ketamine is an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist that produces clinically meaningful improvement in depression symptoms within 24 h of administration in a majority of patients.29, 30 Previous work in rodent models have shown evidence suggesting that rapid changes in glutamate cycling are related to the rapid antidepressant effects of ketamine and other rapidly acting agents.31, 32
Using [11C]ABP688, a PET tracer specific for the negative allosteric modulator (NAM) site on the mGluR5, our prior study demonstrated a rapid and large (20%) reduction in [11C]ABP688 binding after ketamine infusion in healthy humans.11 As glutamate does not compete with radioligands that target the NAM site on mGluR5 (Novartis, personal communication; Lin, in preparation), the ketamine-induced decrease in ligand binding could be an indicator of reduction in mGluR5 availability (that is, internalization) in response to glutamate released following ketamine administration11 or a glutamate-induced conformational change in the receptor that reduces the likelihood of radioligand binding at the NAM site. Presently, we examined ketamine effects on mGluR5 availability in depressed and healthy subjects. We hypothesized that the administration of ketamine would lead to an immediate reduction in mGluR5 availability and that the magnitude of the reduction of mGluR5 in the PFC or hippocampus would predict improvement in depressive symptoms in the depressed cohort. Secondary exploratory analyses investigated whether the reduction in mGluR5 availability during infusion is maintained at 24 h post-ketamine, the time point with highest antidepressant effect.
Materials and methods
Subjects
The Yale University Institutional Review Board, the Yale Radiation Safety Committee and the Yale-New Haven Hospital Radiation Safety Committee approved this study. After providing informed consent, inclusion criteria were assessed by: a physical examination, routine blood tests, and psychiatric and neurological examination. A drug screen (urine), electrocardiogram, and a pregnancy test (for female subjects) were performed as part of subject screening, before radiotracer administration. Inclusion criteria for both cohorts were: (1) Subjects are between 18 and 60 years and (2) English speaking. Inclusion criteria for healthy controls (HCs) were: (1) No current, or history of, any Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis as assessed by structured clinical interview for DSM (SCID); (2) No first-degree relative with history of psychotic, mood or anxiety disorder; (3) No regular medication use within the past 2 months and no history of psychiatric medication use; (4) No lifetime drug abuse or dependence disorder (including for alcohol and nicotine). Inclusion criteria for depressed subjects were: (1) Currently in a depressed mood state as defined by Montgomery–Åsberg Depression Rating Scale (MADRS) severity scores, (2) Primary diagnosis of MDD as assessed by DSM-IV SCID, (3) No other current DSM-IV diagnosis, besides anxiety disorder, (4) No use of psychiatric medication in the past month preceding the study. (5) No significant suicidal ideation. (6) No lifetime drug abuse or dependence disorder (including for alcohol and nicotine).
Thirty-three subjects met eligibility criteria for the study (16 control and 17 depressed). Of those, one HC subject was withdrawn from the study after the baseline PET scan owing to high blood pressure (prior to ketamine administration); two HCs could not tolerate the full ketamine dose owing to psychotomimetic effects and were unable to continue with the scanning procedures, thus their data were discarded; two subjects with depression were excluded for abnormally rapid plasma radiotracer metabolism, which prevented an accurate fit of the plasma data; and one subject exhibited too much motion during the ketamine scan for accurate analysis. In total, 13 contemporaneous healthy non-smoking volunteers (6 males, 7 females) and 14 unmedicated mild to moderately depressed subjects (4 males, 10 females) completed this study (mean age: 33.5±13.2 years) (Table 1). Data from the first 10 HCs (same day assessment only) were published previously.11
Psychiatric assessments
A structured clinical interview (SCID-Non-Patient) and psychiatric history were conducted at screening. MADRS33 and Beck Depression Inventory (BDI-II)34 were used to assess subjects’ depressive symptoms both during intake and on PET scan day (before and 30 min and 24 h after ketamine administration). The effects of ketamine on the subject’s mental state were assessed using the Clinician Administered Dissociative State Scale35 and Profile of Mood States.36
Positron emission tomography
[11C]ABP688 of high E/Z ratio (70:1; n=20) is produced from reacting [11C]methyl iodide with desmethyl-ABP688 in the presence of tetrabutylammonium hydroxide using the loop method developed by Nabulsi and colleagues.37 The average radiochemical and chemical purities is 97% and 93% average, respectively. The E/Z ratio of [11C]ABP688 conformers in the final PET drug product (range: 42/1–98/1) was determined for each produced batch by analytical radio high-performance liquid chromatography area percent. The E-isomer exhibits a higher KD in vivo.38 PET imaging was performed and analyzed as described previously.11
Subjects participated in three scans: two on same day—baseline [11C]ABP688 scan and [11C]ABP688 scan with ketamine infusion—and [11C]ABP688 scan 24 h after ketamine infusion. The radiotracer [11C]ABP688 was administered as a bolus in all scans. After the baseline [11C]ABP688 scan, subjects were given a short break (~1 h). A second [11C]ABP688 dose was administered (as a bolus over 1 min), and following successful radioligand administration, ketamine was immediately administered. The study was designed as such to decrease subject burden in case of equipment failure. In addition, the design ensures the immediate effects of ketamine (rapid glutamate release) are captured. Blood pressure, pulse and peripheral capillary oxygen saturation (SPO2) were acquired before and after ketamine administration and during the ketamine infusion (at 5–10 min intervals). Twelve (of the 14) depressed and 10 (of the 13) HC subjects returned for the 24-h scan. Of these scans, five (two from the depressed cohort, three from the HC cohort) were removed from analysis because arterial lines could not be placed.
The Yale New Haven Hospital Pharmacy provided racemic ketamine, which was administered intravenously11, 39, 40 (initial bolus: 0.23 mg kg−1 over 1 min, followed by constant infusion: 0.58 mg kg−1 over 1 h). The dose administered here is considered a psychotomimetic dose and not an antidepressant dose.
T1-weighted magnetic resonance images (MRIs) were acquired on a 3-T Trio imaging system (Siemens Medical Systems, Erlangen, Germany) and used to delineate anatomical regions on the PET (coregistered to the MRI). The MRI voxel size was 1 × 1 × 1 mm3.
Input function measurement
Prior to PET imaging, catheters were inserted in the forearm veins and radial artery for radioisotope injection and arterial blood sampling, respectively, as previously described.11 Radioactivity was analyzed as previously described.41 After correction for dispersion and delay,42 the automated and manual plasma concentration values were combined and convolved with a Gaussian function (full-width half-maximum=24 s) for smoothing.
A high-performance liquid chromatography assay of arterial blood samples (five samples at 0, 4, 12, 30, and 60 min) provided unmetabolized parent compound levels.43 These levels were fit with a Hill function.44 To calculate the input function, the fit parent fraction curve and merged plasma counts were multiplied. A combination of a straight line (before the peak) and the sum of three exponentials (after the peak) was used to fit the combined data in order to generate the metabolite-corrected arterial input function. Free fraction (fP) measurements were considered unreliable.45
Image analysis
Image analysis routines were implemented in MATLAB (The MathWorks, Natick, MA, USA) as previously described.11 Regions of interest were probabilistic, as determined by nonlinear registration of previously drawn regional atlases to the subject’s MRI as previously described.46 Cortical regions of interest were gray matter masked by voxel-wise multiplication (regional probability multiplied by the probability of that the voxel is in gray matter, as assessed by Statistical Parametric Mapping, SPM5; Institute of Neurology, University College of London, London, England). In this work, the striatum was defined as a weighted (by volume) average binding in the dorsal caudate, dorsal putamen and ventral striatum. A semiautomated technique was used to coregister the subject’s mean PET image to the MRI.47 The mean activity of a region, which was weighted by regional label probabilities, within each PET frame was then calculated to generate time activity curves.
Outcome measure calculation
An unconstrained two-tissue compartment mode was used to calculate regional outcome measures.11, 48 Given the unreliable fP values and a non-ideal reference region,49 VT (volume of distribution: ratio, at equilibrium, of the ligand concentration in the region of interest to that in the plasma50) was used as the main outcome measure. Percentage of change in binding was then calculated as ((VT,baseline−VT,ketamine or 24_hour_scan)/VT,baseline) × 100.
Statistical analysis
To determine the significance of measured VT differences due to ketamine administration or between groups or sexes at baseline, a linear mixed-effects model with region as a fixed effect was used. The dependence structure among regions and images from the same subject was modeled using the Kronecker product between unrestricted symmetry (to model the correlation among all regions) and compound symmetry (to model the correlation between two scans). The interaction term between region and scan was examined and, if appropriate, included in the model. To model change in subjects’ vital signs following ketamine administration, linear mixed models for longitudinal data were also used. The dependence structure used for these models was compound symmetry. The paired comparisons of scores from subjective reports from two time points were performed using Wilcoxon’s signed-rank test. Both unadjusted P-values and false discovery rate corrected values, based on Benjamini and Hochberg method, are provided for these paired comparisons. Tests were all two-sided. All analyses were performed using R 3.0.2 (http://www.r-project.org/) and SAS 9.2 (SAS, Cary, NC, USA).
Results
All mean results are presented as an average±s.d.
Vital signs/subjective report
Significant increases in blood pressure and heart rate were observed during the ketamine scan. Systolic and diastolic blood pressure and heart rate were significantly (P<0.05) higher than baseline until ~30 min after ketamine, similar to our previous report.11 SPO2 values differed from baseline only at the 4-min time point.
There were statistically significant, large reductions in depression scores following ketamine administration (45±70% on MADRS, 52±29% on BDI-II; all Ps<0.001) and 24 h after ketamine (53±69% on MADRS, 49±35% on BDI-II; all Ps<0.001) in the MDD group, which is similar to previously reported results29, 30 (Table 1).
There was a significant increase in Clinician Administered Dissociative State Scale scores during ketamine administration in both the HC and MDD groups (amnesia: F(2,22)=15.08, P<0.001; depersonalization: F(2,22)=24.48, P<0.001; derealization: F(2,22)=25.85, P<0.001), which returned to baseline at 24 h. No between-group differences were observed, however, suggesting that the immediate psychotomimetic effects of ketamine were similar between control and depressed groups (all Fs<1.97, all Ps>0.16).
Tracer metabolism/clearance
No significant differences were observed between scans in injected dose (568±143 MBq), specific activity (329.8±208.6 MBq nmol−1), injected mass (0.7±0.7 μg) or delivery rate of tracer from arterial plasma to brain tissue (K1),50 in either cohort (listed values represent averages over all scans). Clearance rates (injected dose divided by the calculated area under the metabolite-corrected arterial input function51) were not significantly different across time points in the depressed cohort (baseline: 93.0±12.5 l h−1, ketamine: 87.1±12.9 l h−1, 24 h: 91.7±14.4 l h−1, P=0.37). However, there was a significant decrease in clearance between the baseline and ketamine scans (−11.3±21.6% difference, on average) in the HC cohort. The average clearance values for the HC cohort were: baseline: 104.1±36.6 l h−1, ketamine: 89.5±29.2 l h−1, 24 h: 83.2±17.7 l h−1, P=0.03 (linear mixed-effects model across times).
Baseline analysis
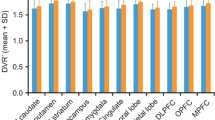
Baseline mGluR5 availability (VT) was significantly lower in the depressed group as compared with the healthy cohort in all regions assessed (Figure 1, P=0.01, linear mixed-effects model across all regions).
Average volume of distribution (VT) across healthy control (n=13, black) and depressed (n=14, white) subjects. All individual regional differences were significant in post hoc analysis. Error bars represent s.d. across subjects.
Ketamine-induced change in [11C]ABP688 binding
There were no significant between-group differences in the amount of ketamine injected (P=0.16). As indicated in Figure 2, significant binding (VT) reductions from baseline were observed on average during the ketamine scan and the 24-h scan both within the depressed group and the HCs (P<0.01, linear mixed-effects model, in both cases). No significant differences were observed between the ketamine scan and the 24-h scan in either group (P>0.8, in both cases). The linear mixed-effects model did not indicate a diagnosis by scan interaction (P=0.46), showing that the binding reductions (VT; from baseline to ketamine scan and from baseline to 24-h scan) were not significantly different between the depressed and control groups. Figures 2c and d show the average percentage of difference in VT from baseline. Across all subjects and regions, the percentage of reductions from baseline in the control and depressed cohort, respectively, were: 19±22% and 14±9% in the ketamine scan and 31±30% and 14±13% in the 24-h after ketamine scan. As indicated by this and Figures 2c and d, variability was particularly high for the 24-h after ketamine scan in controls (gray bars).
Top: Average volume of distribution (VT) across (a) healthy control (n=13 total, 7 at 24 h) and (b) depressed (n=14 total, 10 at 24 h) subjects. Average values from the baseline (black bars), ketamine challenge (white bars) and 24 h after ketamine (gray bars) scans are shown. Regions are organized from left to right in order of the highest to the lowest mean baseline binding. Differences in all regions shown are statistically significant (P<0.05, uncorrected) in post hoc testing. Error bars represent s.d. across subjects. For comparison, the depressed and control cohort data are displayed with the same scale. Bottom: Average percentage of reduction in VT from baseline. Percentage of differences were measured across all subjects within a region and averaged. All average percentage of differences are negative. Absolute values are shown for easier visualization. Error bars represent s.d. across subjects. For comparison, the control (c) and depressed (d) cohort data are displayed with the same scale.
To examine this variability further, Figure 3 shows the changes in VT for each individual subject. On average, there was a significant decrease in binding during the ketamine scan, and this pattern was similar for individual brain regions. However, two HC subjects (Figure 3 left) showed increases in binding. Further, there was high variability in the 24-h scan in the HCs (subject binding differences from baseline ranged from −75±13% to 14±3%).
Average volume of distribution (VT) within a subject. Each subject is represented by a single line. Plotted values are the average VT values across all regions.
Associations with mood during ketamine PET scan
We examined whether there was an association between ketamine-induced changes in mGluR5 availability and mood in the PFC and hippocampus. Given the sample size and exploratory nature of the analyses, we did not correct for multiple comparisons. Exploratory analyses (four in total: MADRS and BDI-II as measures and PFC and hippocampus as regions) revealed that reduction in mGluR5 availability in the hippocampus was associated with a reduction in total depressive symptoms assessed by the MADRS (r=0.52, P=0.035, Figure 4). The questions that MADRS and BDI-II include have several latent factors, including psychic anxiety and dysphoric apathy.52, 53 In our data, the change in mGluR5 availability in the hippocampus was strongly correlated with the psychic anxiety symptoms on both the MADRS (r=0.6, P=0.031, Figure 4) and the BDI-II (r=0.68, P=0.010). We did not observe similar associations between change in mGluR5 availability and the dysphoric symptoms.
Relationship between ketamine-induced change in Montgomery–Åsberg Depression Rating Scale (MADRS) Score (total (blue) and psychic anxiety (red)) and percentage of change in volume of distribution (VT) in the hippocampus. Each symbol represents a different subject.
Discussion
The current study highlights the acute reduction in mGluR5 availability in vivo in humans following administration of ketamine, consistent with the capacity of this drug to enhance glutamate release.54 Reductions in mGluR5 availability persisted for 24 h following ketamine administration, suggesting that glutamate release triggers persisting adaptations in mGluR5s, perhaps through internalization. Interestingly, hippocampal mGluR5 changes during infusion correlated with ketamine’s rapid antidepressant effects, in particular the latent factor of psychic anxiety symptoms.
At baseline, mGluR5 availability (measured as VT) in the depressed cohort was significantly lower than in the controls. Lower mGluR5 availability in the depressed cohort was consistent with a previous report of lower mGluR5 levels in individuals with MDD in several brain regions and reduced PFC mGluR5 protein expression in the postmortem brains of depressed subjects.26
In this work, ketamine-induced reductions in mGluR5 availability were observed in both the depressed and control cohorts, and these reductions persisted 24 h after the ketamine infusion. The immediate decreases in mGluR5 availability are likely due to the rapid surge in extracellular glutamate leading to mGluR5 downregulation or internalization. The glutamate surge hypothesis is consistent with rodent data showing that a single administration of ketamine rapidly, but only transiently, induces increases in glutamate efflux54 and cycling.31, 32 The hypothesis is also in line with other recent studies demonstrating that activation of postsynaptic AMPA receptors is a critical component related to the rapid antidepressant-like effects associated with several additional compounds.55, 56 Evidence exists from other studies suggesting that activation of the AMPA receptors leads to changes in the mGluR5 signaling and facilitation of downstream effects,57 which could have a role in the current work.
Given the apparently similar ketamine-induced decrease in mGluR5 availability and similarity in the psychotomimetic response, it appears that the ketamine-induced surge in glutamate does not differ between these groups. Previous studies have found both elevated8, 9, 10 and reduced13, 14 levels of total tissue glutamate levels to be associated with MDD. These findings may suggest that some difference in ketamine-induced release would be expected between the MDD and healthy comparison subjects. However, it is critical to note that these studies are providing measures of total tissue glutamate levels and do not truly inform us about glutamate transmission to any extent, thus making it very difficult to predict the effect on stimulated glutamate release.
As noted above, the allosteric relationship between the radioligand used and the endogenous neurotransmitter glutamate is different in our study as compared with many published PET studies where the radiotracer interacts with the orthosteric site and thus is subject to direct competition by endogenous neurotransmitter. In our study, however, the radioligand is a NAM that binds to a site in the transmembrane domain of the receptor and not to the N-terminal orthosteric site where glutamate binds. Consistent with its different sites of binding, glutamate does not directly impact the binding of [11C]ABP688 in a membrane preparation but it does lead to receptor internalization (Lin et al., in preparation). Thus the measured reduction in receptor availability is potentially due to rapid receptor internalization. It is also possible that the rapid surge in glutamate leads to a conformational change of the receptor, reducing the ability of the radioligand to bind to the receptor. The overall result would be an observation of lower receptor availability. Overall, the decrease in ligand binding observed here immediately following treatment likely represents an indirect in vivo measure of the ketamine-induced glutamate surge. However, the exact mechanism leading to mGluR5 downregulation in the current study cannot be assessed with PET imaging; nonetheless, our data suggest that long-term reduced mGluR5 availability may have an important role in relieving symptoms of anxiety specifically.
The glutamate surge has been shown to be transient in nature.32, 54, 58 Thus the persistent mGluR5 downregulation at 24 h is unlikely to reflect continued exposure of these receptors to elevated glutamate levels. We hypothesize that the persistent reduction in mGluR5 availability reflects an imbalance between mGluR5 internalization and the rate of recycling of internalized receptor to the plasma membrane within the 24-h period between assessments. Preclinical studies are in line with this interpretation. mGluR5 receptors are anchored to the cytoskeleton via the proteins Homer and Shank. mRNA levels for Homer decline as early as 90 min after ketamine infusion, with downregulation of Homer 1B (although upregulation of Homer 1A has been reported).59 Persistent downregulation of mGluR5 may be among the persisting neuroadaptations to acute ketamine administration that are associated with the persistence of antidepressant effects beyond the brief occupancy of NMDA receptors by ketamine.56
It is possible that we are underestimating the ketamine-induced decrease in mGluR5 density during infusion. In studies of test–retest reliability of mGluR5 radioligand binding, when test and retest studies are conducted on the same day, we have consistently found an increase in binding in the retest (afternoon) scan60, 61 (average percentage of increase in VT: 27±32%). In prior work, we also reported a significant correlation between percentage of change in VT from baseline and percentage of change in K1/clearance from baseline using two tracers that bind to mGluR5—[11C]ABP688 and [18F]FPEB.61 We have hypothesized that the significant correlation between the percentage of change in VT and the percentage of change in clearance/K1 was due to the diurnal variation in glutamate underlying all three parameters: K1 (as there are multiple pathways by which glutamate influences blood flow in the brain62), clearance, and VT changes. This may be the case in this study as well: namely, the post-ketamine surge in glutamate may similarly affect tracer binding, K1 and clearance, as a similar correlation was observed (ρ=0.3/0.6, P=0.04/<0.01 for correlation of VT with K1/clearance across all subjects and all time points). Given that diurnal glutamate changes appear to be in the opposite direction of ketamine-induced changes, it is likely that we are underestimating the ketamine-induced decreases in mGluR5 binding by ~25%, on average. Further, the ultimate goal of this study was to examine the change in specific binding (that is, mGluR5 binding) due to ketamine. However, in this study, change in total binding (VT=specific plus nonspecific binding) was evaluated. The ratio of nonspecific to specific binding in the cerebellum was estimated by Kågedal et al.63 to be 1.33. As this ratio has only been measured in controls, it is unknown whether the ratio is different in a depressed cohort. Assuming that it is not, based on cerebellar VT, the non-specific binding of the depressed subjects is estimated to be ~1.3 and ~1.1 ml cc−1 for the controls, on average. Therefore, in the high binding regions of Figure 1 (all regions shown excluding the cerebellum), the non-specific binding is approximately one-third of the total binding (~27–38%). Ketamine is not expected to change the non-specific binding. Therefore, following ketamine treatment, when specific binding is decreased, the non-specific binding is expected to become a greater portion of the total binding (~34–44%, in this case). Taken together, this means that examining percentage of change in VT underestimates the total effect on specific binding. Given the values above, this underestimation is ~7% for the depressed cohort and ~10% for the HCs. As such, given these assumptions, using specific binding rather than total binding (VT) should not greatly affect overall conclusions.
We observed that individuals who exhibited the greatest reduction in mGluR5 density during ketamine infusion also exhibited the greatest response, primarily on symptoms related to psychic anxiety (Figure 4), immediately after treatment. Although still preliminary, the results are consistent with prior evidence suggesting a model in which the ketamine-induced glutamate surge, and presumably mGluR5 internalization, is a critical step relating to the rapid acting antidepressant effects of the drug.64 Briefly, blockade of NMDA receptors on tonically firing GABAergic interneurons is thought to lead to glutamatergic disinhibition and thus a surge of glutamate in the PFC. This surge increases AMPA receptor activation. which, coupled with ketamine’s inhibition of extrasynaptic NMDA receptors, initiates and enables postsynaptic activation of signaling pathways related to neuroplasticity. These pathways include those involving mammalian target of rapamycin (mTOR) and brain-derived neutrotrophic factor (BDNF).30, 65 These neuroplasticity pathways are hypothesized to underlie ketamine’s antidepressant response. mGluR5 antagonism and internalization have also been associated with rapid, although not long lasting, antidepressant effects in preclinical studies.66, 67 Furthermore, the functional interplay between mGluR5 and NMDA was previously related to antidepressant activity,68 potentially through facilitation of BDNF signaling pathways. A recent, phase 2b study (n=333), examining the efficacy of a selective mGluR5 NAM (basimglurant) as adjunctive therapy for previously treatment non-responsive MDD, failed to show the drug to produce a statistically significant difference in the primary end point (mean change in clinician-rated MADRS) compared with a placebo group.69 However, the study was complicated by very high placebo response rates and secondary analyses (at 1.5 mg) showed statistically significant differences in basimglurant adjuvant therapy versus placebo in multiple other scales, including the patient-rated MADRS and Clinical Global Impression-Improvement mean score. Thus the findings suggest that future studies are required to more fully understand the clinical utility of this psychopharmacological approach.
mGluR5 antagonism has also been shown to be effective in reducing anxiety symptoms.70, 71, 72, 73 The rapid anxiolytic effects of mGluR5 antagonists such as 2-methyl-6-(phenylethynyl)-pyridine do not appear to rely on new protein synthesis but may partially involve facilitation of mTOR signaling pathways and regulation of glutamate neurotransmission.70, 74 Sustained effects of mGluR5 antagonism, however, as seen at the 24 h time point, may rely on new protein synthesis and mTOR signaling pathways that lead to normalization of synaptic plasticity,75, 76 as well as other factors that have yet to be elucidated.
Recently, it has been suggested that a metabolite of ketamine, (2S,6S;2R,6R)-hydroxynorketamine (HNK) may be responsible for the antidepressant response as in preclinical studies HNK was necessary and sufficient for this response.55 The metabolite pathway is independent of NMDA receptors, acting through AMPA receptors to activate BDNF and mTOR pathways. However, even this independent pathway would affect mGluR5 downstream. Evidence from other studies suggests that activation of the AMPA receptors leads to changes in the mGluR5 signaling and facilitation of downstream effects,57 which could have a role in the current work. The exact mechanism leading to mGluR5 downregulation in the current study cannot be assessed with PET imaging; nonetheless, our data suggest that mGluR5 may have an important role in relieving symptoms of anxiety specifically.
Limitations
There are several limitations of this study. First, our study lacks a placebo control group. Ketamine is an increasingly well-known therapeutic agent, and individuals with MDD may have the expectation of reduction in symptoms after ketamine administration; some of the antidepressant response might be due to these expectations. However, the reduction in depressive symptoms was associated with a change in receptor availability, suggesting that the change in symptoms was not solely due to expectation but potentially due to the therapeutic effects of the glutamate surge and mGluR5 downregulation. Second, hemodynamic ketamine effects may alter tracer delivery and washout. The kinetic models used assume equilibrium conditions;50 however, heart rate and blood pressure were elevated (transiently) during ketamine administration. Furthermore, ketamine has been associated with increased blood flow in frontal regions,77, 78, 79 measured with functional MRI, although the effect reported was not great and isolated to a few regions. In this study, however, the delivery of tracer (K1) did not significantly change post-ketamine, and clearance only differed post-ketamine in the control group. Thus, given the magnitude of the previously reported effects and the lack of difference in K1 over the whole scan, even if ketamine were to cause transient changes in K1, these are unlikely to affect outcome. Further, the outcome VT, by incorporating the metabolite-corrected arterial input function, accounts for other potential changes induced by ketamine (for example, metabolism). Third, our study lacks long-term follow up. Repeat scanning about 2 weeks after ketamine administration, at the time when the antidepressant response wanes, would help determine the time course of mGluR5 re-insertion into the membrane and whether this corresponds to the increases in depressive symptoms. Fourth, only nonsmokers were studied in the present experiment. The extent of ketamine-induced changes on mGluR5 availability (or [11C]ABP688 binding) in smokers has yet to be determined. Although previous work showed that there is downregulation in mGluR5 with chronic tobacco exposure80 and both mGluR5 NAMs and NMDA antagonists appear to reduce smoking-related behaviors, whether there are smoking-specific differences in the glutamate surge (and mGluR5 downregulation) after ketamine administration is not known. Thus the current results might only apply to the nonsmoking MDD population. Finally, the ketamine dose administered here is higher than the typical dose used for clinical purposes. Thus the extent of change in receptor availability might be greater in this study as compared with the general practice.
Conclusion
We conducted a novel examination to evaluate ketamine-induced changes in mGluR5 availability using PET in healthy and depressed cohorts. Significant decreases in [11C]ABP688 binding, a tracer specific for mGluR5, were observed in both cohorts. It is likely that this decreased binding reflects mGluR5 downregulation/internalization. Exploratory analyses suggest that mGluR5 downregulation (and/or the likely surge in glutamate) has a role in the reduction of depressive symptomatology. These data support the development and implementation of mGluR5 therapies for the relief of depressive symptomatology.
References
Global Burden of Disease Study Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800.
World Health Organization The Global Burden of Disease: 2004 Update, Table A2: Burden of Disease in DALYs by Cause, Sex and Income Group in WHO Regions, Estimates for 2004. WHO: Geneva, Switzerland, 2008; http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_AnnexA.pdf.
McClintock S, Husain M, Wisniewski S, Nierenberg A, Stewart J, Trivedi M et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol 2011; 31: 180–186.
Zisook S, Ganadjian K, Moutier C, Prather R, Rao S . Sequenced Treatment Alternatives to Relieve Depression (STAR*D): lessons learned. J Clin Psychiatry 2008; 69: 1184–1185.
Krystal J, Mathew S, D'Souza D, Garakani A, Gunduz-Bruce H, Charney D . Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 2010; 24: 669–693.
Krystal J, Sanacora G, Duman R . Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73: 1133–1141.
Sanacora G, Zarate CAJ, Krystal JH, Manji HK . Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008; 7: 426–437.
Hashimoto K, Sawa A, Iyo M . Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007; 62: 1310–1316.
Abdallah C, Mason G, DellaGioia N, Sanacora G, Jiang L, Matuskey D et al. mGluR5 and glutamate involvement in MDD: a multimodal imaging study. Biol Psychiatry, (under review).
Sanacora G, Gueorguieva R, Epperson C, Wu Y-T, Appel M, Rothman D et al. Subtype-specific alterations of GABA and glutamate in major depression. Arch Gen Psychiatry 2004; 61: 705–713.
DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C et al. In vivo ketamine-induced changes in [11C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol Psychiatry 2015; 77: 266–275.
Miyake N, Skinbjerg M, Easwaramoorthy B, Kumar D, Girgis R, Xu X et al. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry 2011; 69: 822–824.
Luykx J, Laban K, Heuvel Mvd, Boks M, Mandl R, Kahn R et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev 2012; 36: 198–205.
Hasler G, Veen Jvd, Tumonis T, Meyers N, Shen J, Drevets W . Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64: 193–200.
Sistiaga A, Herrero I, Conquet F, Sánchez-Prieto J . The metabotropic glutamate receptor 1 is not involved in the facilitation of glutamate release in cerebrocortical nerve terminals. Neuropharmacology 1998; 37: 1485–1492.
Manahan-Vaughan D, Braunewell K . Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA 1999; 96: 8739–8744.
Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 1997; 17: 7503–7522.
Takumi Y, Matsubara A, Rinvik E, Ottersen O . The arrangement of glutamate receptors in excitatory synapses. Ann NY Acad Sci 1999; 868: 474–482.
Hubert G, Paquet M, Smith Y . Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. J Neurosci 2001; 21: 1838–1847.
López-Bendito G, Shigemoto R, Fairén A, Luján R . Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex 2002; 12: 625–638.
O'Malley K, Jong Y, Gonchar Y, Burkhalter A, Romano C . Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem 2003; 278: 28210–28219.
Shin S, Kwon O, Kang JI, Kwon S, Oh S, Choi J et al. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat Neurosci 2015; 18: 1017–1024.
Kovacevic T, Skelin I, Minuzzi L, Rosa-Neto P, Diksic M . Reduced metabotropic glutamate receptor 5 in the Flinders Sensitive Line of rats, an animal model of depression: an autoradiographic study. Brain Res Bull 2012; 87: 406–412.
Wieronska JM, Branski P, Szewczyk B, Palucha A, Papp M, Gruca P et al. Changes in the expression of metabotropic glutamate receptor 5 (mGluR5) in the rat hippocampus in an animal model of depression. Pol J Pharmacol 2001; 53: 659–662.
Fatemi SH, Folsom TD, Rooney RJ, Thuras PD . mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Transl Psychiatry 2013; 3: e271.
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 2011; 168: 727–734.
Matosin N, Fernandez-Enright F, Frank E, Deng C, Wong J, Huang XF et al. Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: implications for novel mGluR-based therapeutics. J Psychiatry Neurosci 2014; 39: 407–416.
Smialowska M, Szewczyk B, Branski P, Wieronska JM, Palucha A, Bajkowska M et al. Effect of chronic imipramine or electroconvulsive shock on the expression of mGluR1a and mGluR5a immunoreactivity in rat brain hippocampus. Neuropharmacology 2002; 42: 1016–1023.
Berman R, Cappiello A, Anand A, Oren D, Heninger G, Charney D et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354.
Abdallah C, Adams T, Kelmendi B, Esterlis I, Sanacora G, Krystal J . Ketamine's mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 2016; 33: 689–697.
Chowdhury G, Behar K, Cho W, Thomas M, Rothman D, Sanacora G . ¹H-[¹3C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry 2012; 71: 1022–1025.
Chowdhury G, Zhang J, Thomas M, Banasr M, Ma X, Pittman B et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 2016; 22: 120–126.
Montgomery SA, Asberg M . A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389.
Beck S, Ward C, Mendelsohn M, Erbaugh J . An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571.
Bremner J, Krystal J, Putnam F, Southwick S, Marmar C, Charney D et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1999; 11: 125–136.
Norcross J, Guadagnoli E, Prochaska J . Factor structure of the profile of mood states (POMs), two partial replications. J Clin Psychol 1984; 40: 1270–1277.
Sandiego CM, Nabulsi N, Lin SF, Labaree D, Najafzadeh S, Huang Y et al. Studies of the metabotropic glutamate receptor 5 radioligand [(1)(1)C]ABP688 with N-acetylcysteine challenge in rhesus monkeys. Synapse 2013; 67: 489–501.
Kawamura K, Yamasaki T, Kumata K, Furutsuka K, Takei M, Wakizaka H et al. Binding potential of (E)-[(11)C]ABP688 to metabotropic glutamate receptor subtype 5 is decreased by the inclusion of its (11)C-labelled Z-isomer. Nucl Med Biol 2014; 41: 17–23.
Anticevic A, Gancsos M, Murray J, Repovs G, Driesen N, Ennis D et al. NMDA receptor function in large-scale anti-correlated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci 2012; 109: 16720–16725.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D'Souza DC et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 2013; 18: 1199–1204.
Ametamey S, Kessler L, Honer M, Wyss M, Buck A, Hintermann S et al. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med 2006; 47: 698–705.
Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S et al. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 2011; 31: 1807–1816.
Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T et al. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab 2010; 30: 196–210.
Wu S, Ogden RT, Mann JJ, Parsey RV . Optimal metabolite curve fitting for kinetic modeling of 11C-WAY-100635. J Nucl Med 2007; 48: 926–931.
DeLorenzo C, Milak MS, Brennan KG, Kumar JS, Mann JJ, Parsey RV . In vivo positron emission tomography imaging with [(11)C]ABP688: binding variability and specificity for the metabotropic glutamate receptor subtype 5 in baboons. Eur J Nucl Med Mol Imaging 2011; 38: 1083–1094.
DeLorenzo C, Kumar JS, Mann JJ, Parsey RV . In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab 2011; 31: 2169–2180.
DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ et al A New Method for Assessing PET-MRI Coregistration. Proceedings of SPIE; 7 February 2009; Lake Buena Vista, Florida, USA.
Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U et al. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med 2007; 48: 1207–1215.
Patel S, Hamill T, Connolly B, Jagoda E, Li W, Gibson R . Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol 2007; 34: 1009–1017.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539.
Hirvonen J, Johansson J, Teras M, Oikonen V, Lumme V, Virsu P et al. Measurement of striatal and extrastriatal dopamine transporter binding with high-resolution PET and [11C]PE2I: quantitative modeling and test-retest reproducibility. J Cereb Blood Flow Metab 2008; 28: 1059–1069.
Parker R, Flint E, Bosworth H, Pieper C, Steffens D . A three-factor analytic model of the MADRS in geriatric depression. Int J Geriatr Psychiatry 2003; 18: 73–77.
Huang C, Chen J . Meta-analysis of the factor structures of the Beck Depression Inventory-II. Assessment 2015; 22: 459–472.
Moghaddam B, Adams B, Verma A, Daly D . Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486.
Duman R, Aghajanian G, Sanacora G, Krystal J . Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–249.
Kim H, Lee K, Lee D, Han Y, Lee S, Sohn J et al. Costimulation of AMPA and metabotropic glutamate receptors underlies phospholipase C activation by glutamate in hippocampus. J Neurosci 2015; 35: 6401–6412.
Lorrain D, Baccei C, Bristow L, Anderson J, Varney M . Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 2003; 117: 697–706.
Bartolomeis Ad, Sarappa C, Buonaguro E, Marmo F, Eramo A, Tomasetti C et al. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry 2013; 46: 1–12.
DeLorenzo C, Kumar JD, Mann J, Parsey R . In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. J Cereb Blood Flow Metab 2011; 31: 2169–2180.
DeLorenzo C, Gallezot J, Gardus J, Yang J, Planeta B, Nabulsi N et al. In vivo variation in same-day estimates of metabotropic glutamate receptor subtype 5 binding using [11C]ABP688 and [18F]FPEB JCBFM. J Cereb Blood Flow Metab 2016 (e-pub ahead of print).
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA . Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243.
Kågedal M, Cselényi Z, Nyberg S, Raboisson P, Ståhle L, Stenkrona P et al. A positron emission tomography study in healthy volunteers to estimate mGluR5 receptor occupancy of AZD2066 - estimating occupancy in the absence of a reference region. Neuroimage 2013; 82: 160–169.
Abdallah C, Sanacora G, Duman R, Krystal J . Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523.
Li N, Lee B, Liu R, Banasr M, Dwyer J, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Pałucha A, Brański P, Szewczyk B, Wierońska J, Kłak K, Pilc A . Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 2005; 81: 901–906.
Tatarczyńska E, Klodzińska A, Chojnacka-Wójcik E, Palucha A, Gasparini F, Kuhn R et al. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol 2001; 132: 1423–1430.
Pałucha-Poniewiera A, Wierońska J, Brański P, Burnat G, Chruścicka B, Pilc A . Is the mGlu5 receptor a possible target for new antidepressant drugs? Pharmacol Rep 2013; 65: 1506–1511.
Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M et al. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry 2016; 73: 675–684.
Iijima M, Fukumoto K, Chaki S . Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res 2012; 235: 287–292.
Fontanez-Nuin D, Santini E, Quirk G, Porter J . Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 2011; 21: 727–735.
Chen L, Liu J, Ali U, Gui Z, Hou C, Fan L et al. Chronic, systemic treatment with a metabotropic glutamate receptor 5 antagonist produces anxiolytic-like effects and reverses abnormal firing activity of projection neurons in the basolateral nucleus of the amygdala in rats with bilateral 6-OHDA lesions. Brain Res Bull 2011; 84: 215–223.
Molina-Hernández M, Tellez-Alcántara N, Pérez-García J, Olivera-Lopez J, Jaramillo M . Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1129–1135.
Mora MPdl, Lara-García D, Jacobsen K, Vázquez-García M, Crespo-Ramírez M, Flores-Gracia C et al. Anxiolytic-like effects of the selective metabotropic glutamate receptor 5 antagonist MPEP after its intra-amygdaloid microinjection in three different non-conditioned rat models of anxiety. Eur J Neurosci 2006; 23: 2749–2759.
Page G, Khidir F, Pain S, Barrier L, Fauconneau B, Guillard O et al. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int 2006; 49: 413–421.
Hou L, Klann E . Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 2004; 24: 6352–6361.
Breier A, Malhotra A, Pinals D, Weisenfeld N, Pickar D . Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 1997; 154: 805–811.
Vollenweider F, Leenders K, Scharfetter C, Antonini A, Maguire P, Missimer J et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose(FDG). Eur Neuropsychopharmacol 1997; 7: 9–24.
Rowland L, Beason-Held L, Tamminga C, Holcomb H . The interactive effects of ketamine and nicotine on human cerebral blood flow. Psychopharmacology 2010; 208: 575–584.
Akkus F, Ametamey S, Treyer V, Burger C, Johayem A, Umbricht D et al. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci USA 2013; 110: 334.
Acknowledgements
We thank Ms Samantha Rossano for her contributions to the data analysis and manuscript development. We also thank the Yale University Positron Emission Tomography Center staff for their aid with radiotracer syntheses, related analyses and subject imaging. We acknowledge the biostatistical consultation and support from the Biostatistical Consulting Core at the School of Medicine, Stony Brook University. Support was provided by K01MH092681 (to IE), VA National Center for PTSD (to IE, JHK, RHP, CGA), K01MH091354 (to CD) and R01MH104512 (to CD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JHK: (1) Vladimir, Coric, Krystal, John H, Sanacora, Gerard—Glutamate Modulating Agents in the Treatment of Mental Disorders US Patent No. 8,778,979 B2 Patent Issue Date: 15 July 2014. (2) Charney D, Krystal JH, Manji H, Matthew S, Zarate C—Intranasal Administration of Ketamine to Treat Depression United States Application No. 14/197,767 filed on 5 March 2014; United States application or PCT International application No. 14/306,382 filed on 17 June 2014. He was also on consultant/advisory board for: AMGEN, AstraZeneca Pharmaceuticals, Biogen, Idec, MA, Biomedisyn Corporation, Forum Pharmaceuticals, Janssen Research & Development, Otsuka America Pharmaceutical, Inc., Sunovion Pharmaceuticals, Inc., Takeda Industries, Taisho Pharmaceutical Co., Ltd, Biohaven Pharmaceuticals, Blackthorn Therapeutics, Inc., Lohocla Research Corporation, Luc Therapeutics, Inc., Pfizer Pharmaceuticals, and TRImaran Pharma. GS has received consulting fees form Allergan, Alkermes, AstraZeneca, BioHaven Pharmaceuticals, Hoffman La-Roche, Janssen, Merck, Naurex, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valenant pharmaceutical North America and Vistagen therapeutics over the past 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex and Servier over the past 24 months. Free medication was provided to GS for an NIH sponsored study by Sanofi-Aventis. In addition, he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a patent ‘Glutamate agents in the treatment of mental disorders’ Patent number: 8778979. RHP: Scientific Consultant to Cogstate, Ltd. CGA served on advisory boards for Genentech. REC received research support from Astra-Zeneca, Astellas, BMS, Pfizer, Siemens, Taisho and UCB. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Esterlis, I., DellaGioia, N., Pietrzak, R. et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol Psychiatry 23, 824–832 (2018). https://doi.org/10.1038/mp.2017.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.58
- Springer Nature Limited
This article is cited by
-
Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms
Neuropsychopharmacology (2024)
-
Challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression
Neuropsychopharmacology (2024)
-
Loss of mGlu5 receptors in somatostatin-expressing neurons alters negative emotional states
Molecular Psychiatry (2024)
-
The role of mGluR5 on the therapeutic effects of ketamine in Wistar rats
Psychopharmacology (2024)
-
Imaging synaptic density in depression
Neuropsychopharmacology (2023)