Abstract
The preferred type of post-remission therapy (PRT) in patients with acute myeloid leukemia (AML) in first complete remission (CR1) is a subject of continued debate, especially in patients at higher risk of nonrelapse mortality (NRM), including patients >40 years of age. We report results of a time-dependent multivariable analysis of allogenic hematopoietic stem cell transplantation (alloHSCT) (n=337) versus chemotherapy (n=271) or autologous HSCT (autoHSCT) (n=152) in 760 patients aged 40–60 years with AML in CR1. Patients receiving alloHSCT showed improved overall survival (OS) as compared with chemotherapy (respectively, 57±3% vs 40±3% at 5 years, P<0.001). Comparable OS was observed following alloHSCT and autoHSCT in patients with intermediate-risk AML (60±4 vs 54±5%). However, alloHSCT was associated with less relapse (hazard ratio (HR) 0.51, P<0.001) and better relapse-free survival (RFS) (HR 0.74, P=0.029) as compared with autoHSCT in intermediate-risk AMLs. AlloHSCT was applied following myeloablative conditioning (n=157) or reduced intensity conditioning (n=180), resulting in less NRM, but comparable outcome with respect to OS, RFS and relapse. Collectively, these results show that alloHSCT is to be preferred over chemotherapy as PRT in patients with intermediate- and poor-risk AML aged 40–60 years, whereas autoHSCT remains a treatment option to be considered in patients with intermediate-risk AML.
Similar content being viewed by others
Introduction
Although hematological first complete remissions (CR1) may be achieved in ~80% of younger patients with newly diagnosed acute myeloid leukemia (AML), the relapse rate is still unacceptably high and varies according to age and the underlying cytogenetic and molecular profile of the leukemia.1, 2, 3, 4, 5 Post-remission therapy (PRT) is applied for prevention of relapse and may include either consolidation chemotherapy or hematopoietic stem cell transplantation (HSCT) using either allogeneic (alloHSCT) or autologous (autoHSCT) stem cell grafts. Although alloHSCT offers the most effective antileukemic therapy, enhanced nonrelapse mortality (NRM) may compromise that favorable effect. As a result, alloHSCT is no longer indicated for favorable risk AML,6 and currently being discussed in patients with intermediate-risk AML.7, 8, 9
NRM following myeloablative conditioning (MAC) alloHSCT increases with age and/or comorbidities,10, 11, 12 as a result of which a net survival benefit of alloHSCT in AML patients beyond 40 years could not be demonstrated in a meta-analysis of the earlier studies by HOVON, MRC, EORTC and the French BGM group.6, 13, 14, 15, 16 Following that observation, several HOVON centers introduced reduced intensity conditioning (RIC) alloHSCT in patients beyond 40 years of age to reduce NRM, while maintaining graft versus leukemia (GVL) effects.17, 18 Meanwhile, by virtue of the use of peripheral blood stem cells instead of bone marrow, results of autoHSCT gradually improved in AML.19 These developments, as well as results of more recent retrospective and prospective studies,20, 21, 22 urged us to readdress the question of preferred PRT in a more recent cohort of AML patients, aged 40–60 years. Particularly this age cohort allowed us to compare PRT by alloHSCT, using either RIC or MAC, versus chemotherapy or autoHSCT. We evaluated these PRT modalities by time-dependent analysis, a method that has lately increasingly been applied for evaluation of alloHSCT, as the sibling donor versus no-donor methodology can no longer be applied with the increased use of unrelated donors.7, 23, 24, 25, 26 The method allows for comparing patients actually transplanted versus nontransplanted patients without the bias caused by the time to transplant.27
Patients and methods
Patients
A total of 760 patients between 40 and 60 years of age with newly diagnosed AML receiving PRT in CR1, who participated in two consecutive, prospective HOVON–SAKK phase III trials (AML42/42A and AML92), were included (Figure 1).19, 28, 29 Patients were classified for leukemia risk, based on the cytogenetic and molecular profile of the underlying AML, according to the latest European LeukemiaNET (ELN) AML risk classification.1 In the present analysis, the intermediate-I and intermediate-II risk groups of the ELN risk classification were combined because of similar outcome of these subgroups (Supplementary Figure 1). Both AML42/42A and AML92 had been approved by ethics committees of participating institutions and were conducted in accordance with the Declaration of Helsinki. All participants had given written informed consent. Detailed description of the inclusion and exclusion criteria of these studies can be found in the Supplementary Appendix.
Treatment protocols
Treatment in the AML42/42A and AML92 studies involved a maximum of two remission-induction cycles, including anthracyclin with cytarabine chemotherapy, as previously described.28, 29 Three different types of PRT were applied in patients in CR1 according to a predefined strategy as outlined in the AML42 and AML 92 protocol, including a third cycle of chemotherapy with mitoxantrone and etoposide, high-dose chemotherapy with busulfan and cyclophosphamide followed by autoHSCT or alloHSCT following either RIC or MAC. These different therapeutic modalities were applied according a risk-adapted strategy:19, 28, 29 (1) Patients with AML classified as favorable risk, according to cytogenetic and available molecular analysis, were planned for a third cycle of chemotherapy; (2) intermediate-risk patients were preferentially treated by alloHSCT using a human leukocyte antigen (HLA) matched sibling donor or a fully HLA-matched unrelated donor if available; and (3) patients with poor-risk AML proceeded to alloHSCT using either a sibling or unrelated donor, using 7/8 or 8/8 matched donors. Patients alternatively received an autoHSCT or a third cycle of chemotherapy if no suitable donor was available.19
Transplantation protocols
Patients received either a MAC or RIC regimen followed by the infusion of donor cells. RIC-alloHSCT was introduced in patients below 60 years as from 2001, whereby the indication for RIC or MAC was selectively determined by age and consistently adhered to by the individual center throughout the AML42/42A and AML92 studies. Whereas some centers maintained their policy of MAC-alloHSCT for all patients up to the age of 60, a number of centers changed their policy by setting the age limit for MAC at <40 years and RIC for patients of 40 years and beyond. The degree of HLA-matching for unrelated donors was 8/8 allele match for HLA-A, B, C and DRB1 for intermediate-risk patients and ⩾7/8 allele match for poor-risk patients. The MAC regimen contained high-dose cyclophosphamide with total body irradiation (TBI) in 110 (70%) patients, whereas the remainder received busulfan with cyclophosphamide. T-cell depletion was only performed in recipients of MAC-alloHSCT, whereby partial T-cell depletion was performed by CD34-selection and add-back of T cells to the graft to ensure 1 × 105 T cells/kg bodyweight of the recipient, as described earlier.30 Although RIC regimens varied, the majority contained 2.0 Gy TBI preceded by fludarabine (n=126, 70%), as described earlier.17 A calcineurin inhibitor (either ciclosporin or tacrolimus) plus mycophenolate mofetil or methotrexate was given as prophylaxis for graft-versus-host disease (GVHD). Recipients of a T-cell depleted MAC-alloHSCT received a calcineurin inhibitor (either ciclosporin or tacrolimus) plus methotrexate as GVHD prophylaxis.
Endpoints
The primary endpoint of the study was overall survival (OS), according to the type of PRT received. OS and relapse-free survival (RFS) were measured from the date of start of PRT. The event for OS was death, whatever the cause, and patients were censored at the date of last contact, if alive. The events for RFS were death in CR1, designated as NRM or hematological relapse. The cumulative risks of relapse and NRM over time were calculated as competing risks with actuarial methods where patients alive in continuing CR1 were censored at the date of last contact.
Statistical methods
A time-dependent analysis of PRT was performed, as described previously,24 by applying multivariable Cox regression with time-dependent covariates autoHSCT and alloHSCT. The multivariable analysis is conceptually similar to a Mantel–Byar analysis,31 but more general as it allows for adjustment for other factors. Some patients received PRT with chemotherapy (n=39) or autoHSCT (n=3) first before they proceeded to alloHSCT. In both the multivariable analysis and the estimation of the survival curves, these patients were counted as at risk in the chemotherapy or autoHSCT group from the start of PRT until alloHSCT and after that as at risk in the alloHSCT group. Multivariable Cox regression analysis for OS, RFS, relapse and NRM was applied with stratification for leukemia risk and adjustment for late CR (after cycle II instead of I), time from CR to PRT, age, sex and year of treatment before or after 2006. Year of treatment before or after 2006 was included to adjust for a possible overall difference in outcomes between these two periods. Moreover, time from start induction to start post-remission treatment and T-cell depletion were added as factors to the model, but showed no significant effects on OS, RFS, relapse or NRM. A similar analysis restricted to alloHSCT patients was done for a direct comparison of RIC-alloHSCT and MAC-alloHSCT, with stratification by leukemia risk and adjustment for late CR, time from CR to transplantation, age, sex, donor type and year of transplantation before or after 2006. In addition, time from start induction to transplantation, number of induction cycles, stem cell source, TBI, patient/donor gender mismatch and cytomegalovirus mismatch were not included in the model because of no significant effect on outcome. All P-values were based on log likelihood ratio tests, except when explicitly stated otherwise. Log likelihood ratio tests were also used to test for interactions. The proportional hazard assumption was tested on the basis of Schoenfeld residuals.32 P-values have not been adjusted for multiple testing. All analyses were done with Stata Statistical Software: Release 13 (2013, College Station, TX, USA: Stata Corporation).
Results
Characteristics of the patients
Between January 2001 and February 2010, induction chemotherapy was started in 1196 patients aged 40–60 years (Figure 1). CR after induction (2 cycles) was obtained in 937 (78%) patients, of whom 760 proceeded to PRT with either chemotherapy (n=271), autoHSCT (n=152), MAC-alloHSCT (n=157) or RIC-alloHSCT (n=180). One hundred and seventy seven patients in CR1 did not receive PRT, because of toxicity (n=33), early death (n=51), AML progression (n=35) or other reasons (Figure 1). Patient characteristics are presented in Table 1. Owing to the preferred application in poor-risk patients, more patients proceeding to alloHSCT exhibited adverse-risk features. A higher percentage of alloHSCT recipients needed two cycles of chemotherapy instead of one cycle to obtain CR. AutoHSCT and alloHSCT were more frequently applied in recent years. The median follow-up of patients still alive was 79 months and differed between patients receiving chemotherapy (83 months), autoHSCT (71 months), MAC-alloHSCT (88 months) and RIC-alloHSCT (75 months). Table 2 presents transplantation characteristics of alloHSCT recipients, comparing the groups of RIC and MAC. Recipients of RIC-alloHSCT were significantly older and were transplanted more frequently in the recent years. Grafts were not manipulated in all RIC-alloHSCT patients, whereas 24% of MAC-alloHSCT patients received grafts, partially depleted of T cells. Patients receiving RIC-alloHSCT and MAC-alloHSCT had similar donor source, stem cell source, CMV-serology status, female donors/male recipient’s ratio and similar distributions of their leukemia risk profile1 and EBMT-risk scores10 (Tables 1 and 2).
Treatment outcome
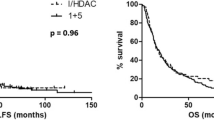
OS appeared to be clearly different in the favorable, intermediate-I/intermediate-II, adverse leukemia risk groups as categorized by the ELN AML risk classification,1 with OS at 5 years of 74±4% in favorable risk, 51±3% in intermediate-I risk, 47±6% in intermediate-II risk and 33±4% in adverse-risk AMLs (Supplementary Figure 1). Because of similar survival in the ELN intermediate I and II risk subcategories, these patients were analyzed as one single intermediate-risk group. Outcome estimates at 5 years for each type of PRT by ELN risk group can be found in Supplementary Table 1. Figures 2a and b show OS and RFS of all patients by type of PRT, stratified for leukemia risk. Improved OS was found for alloHSCT recipients as compared with patients receiving chemotherapy as PRT (57±3% versus 40±3% at 5 years, P<0.001, Figure 2a). In addition, OS was significantly improved in recipients of autoHSCT as compared with recipients of chemotherapy (54±3% versus 40±3% at 5 years, P=0.02, Figure 2a).
OS and RFS in all patients and intermediate-risk patients by post-remission treatment. Kaplan–Meier estimates of OS (a) and RFS (b) of patients with AML in first complete remission from start of post-remission treatment, according to post-remission treatment and with direct adjustment for differences in leukemia risk category among the treatment groups by the method of Gail and Byar.55 Kaplan–Meier estimates of OS (c) and RFS (d) in intermediate-risk patients. Of note, numbers of patients at risk (indicated below the x axis) differ from the patient numbers (indicated in Table 1 and within the figure) because of the time-dependent nature of this analysis, which allows for time to transplantation by switching patients at the time of allograft in CR1 to the transplantation curve. Abbreviations: CT, chemotherapy; Auto, autologous hematopoietic stem cell transplantation; Allo, allogeneic hematopoietic stem cell transplantation; F, number of failures (that is, death whatever the cause for OS, and death or relapse for RFS); N, number of patients; and Cox LR, cox likelihood ratio.
Intermediate-risk AMLs
In intermediate-risk patients, alloHSCT and autoHSCT significantly improved OS as compared with chemotherapy (60±4% and 54±5%, respectively, versus 36±4% at 5 years, P<0.001, Figure 2c), while OS after alloHSCT versus autoHSCT was not significantly different. In contrast, improved RFS was found in patients with intermediate-risk AML receiving PRT with alloHSCT as compared with autoHSCT (56±4% versus 39±5% at 5 years, respectively, P=0.04, Figure 2d). Trends toward improved OS and RFS were found for alloHSCT and autoHSCT as compared with chemotherapy in the relatively small subgroups of favorable and unfavorable risk leukemia’s (Supplementary Figure 2).
Second complete remission
A total of 358 patients developed a relapse after having received PRT. Two hundred and five patients proceeded to salvage chemotherapy and 125 (35%) entered a second CR. Ultimately, only 75 out of 358 relapsing patients proceeded to alloHSCT in second CR and 6 patients received an autoHSCT in second CR. Overall outcome of all relapsing patients was 12±2% at 5 years from relapse.
Multivariable analysis
Table 3 shows the results of the multivariable analysis with stratification for leukemia risk and with adjustment for sex, age, late CR, time from CR to PRT, year of start of PRT before or after 2006, and PRT type. Both OS and RFS were significantly better after alloHSCT as compared to chemotherapy with HRs of 0.64 (P<0.001), and 0.51 (P<0.001), respectively (Supplementary Figure 2). Relapse was significantly reduced following alloHSCT as compared to chemotherapy (HR 0.33, P<0.001). AutoHSCT was also associated with significantly improved RFS (HR 0.69, P=0.005) and a reduced risk of relapse (HR 0.66, P=0.003) as compared to chemotherapy. OS was not significantly different comparing alloHSCT with autoHSCT (HR 0.83, P=0.19), while RFS was significantly improved after alloHSCT as compared to autoHSCT (HR 0.74, P=0.029).
Intermediate-risk AMLs
With respect to OS and RFS in intermediate-risk patients, the HRs comparing alloHSCT with chemotherapy were 0.54 (95% CI: 0.40–0.72, P<0.001) and 0.47 (95% CI: 0.35–0.63, P<0.001), respectively. The HRs comparing autoHSCT with chemotherapy in intermediate-risk patients were 0.72 (95% CI: 0.51–1.02, P=0.058) for OS and 0.73 (95% CI: 0.52–1.00, P=0.048) for RFS. A trend was found toward improved OS comparing alloHSCT with autoHSCT in intermediate-risk patients (HR 0.74, 95% CI: 0.52–1.06, P=0.10), whereas RFS was significantly improved better for alloHSCT in intermediate-risk patients (HR 0.65, 95% CI: 0.46–0.90, P=0.011).
Tests for interaction
We tested for interactions between the type of PRT with age, time from CR to post-remission treatment, year of treatment (<or⩾2006), sex, late CR1 and leukemia risk. Only between age and PRT significant interactions were found, indicating that autoHSCT recipients experienced an increased event rate with age for all endpoints (details not shown). We have also tested for the interaction between the type of PRT and center size (above or below the median number of patients per center). No interaction was found between type of PRT and center size for all outcome parameters.
AlloHSCT conditioning: RIC versus MAC
We additionally performed a direct comparison of patients receiving RIC-alloHSCT with MAC-alloHSCT. Figures 3a and b show OS and RFS, respectively, of alloHSCT recipients by conditioning type stratified for leukemia risk. NRM was significantly increased for recipients of MAC-alloHSCT as compared with recipients of RIC-alloHSCT (23±3% versus 11±2% at 5 years, P=0.009, Figure 3d), whereas the cumulative incidence of relapse comparing RIC-alloHSCT with MAC-alloHSCT was not significantly different (37±4% versus 29±4% at 5 years, Figure 3c), resulting in no significant different OS (57±4% versus 51±4% at 5 years) and RFS (52±4% versus 48±4% at 5 years) for RIC-alloHSCT recipients compared with MAC-alloHSCT patients. Multivariable analysis with stratification for leukemia risk and adjustment for covariates including donor type (Table 4), showed decreased NRM following RIC-alloHSCT (HR 0.44, P=0.004). Of note, within the group of patients receiving MAC-alloHSCT, NRM was increased in recipients of partially T-cell depleted MAC-alloHSCT (HR 4.00, 95% CI: 2.04–7.84, P<0.001), but relapse was not increased. No patients receiving RIC-alloHSCT received grafts that were depleted of T cells. Relapse did not differ between MAC-alloHSCT and RIC-alloHSCT with an HR of 1.24 (P=0.34). It resulted in similar OS (HR 0.78, P=0.16) and RFS (HR 0.85, P=0.34) between RIC-alloHSCT and MAC-alloHSCT. The similar outcome following either RIC or MAC was also observed in subgroups of patients, according to underlying leukemia risk. Specifically, the advantage of RIC or MAC-alloHSCT versus chemotherapy in terms of RFS was observed in both intermediate- and poor-risk subgroups to a similar degree (Supplementary Figure 3).
Outcome of allogeneic transplantation by conditioning type. Kaplan–Meier estimates of OS (a) and RFS (b), and cumulative incidence of relapse (c) and NRM (d) of patients with AML in first CR form start of transplantation, according to conditioning type and with direct adjustment for differences in leukemia risk category among the treatment groups by the method of Gail and Byar.55 The cumulative incidences of relapse and NRM over time were calculated as competing risks with actuarial methods, where patients alive in continuing CR1 were censored at the date of last contact. F, number of failures (that is, death whatever the cause); N, number of patients; and Cox LR, Cox likelihood ratio.
GVHD
Incidences of grade II–IV acute GVHD after RIC-alloHSCT and MAC-alloHSCT were 9% and 26%, respectively. Incidences of chronic limited and chronic extensive GVHD were, respectively, 19% and 36% in RIC-alloHSCT and 32 and 29% in MAC-alloHSCT patients.
Discussion
The preferred type of PRT in younger patients with AML in CR1 is a subject of continued debate. While the GVL effect exerted by alloHSCT strongly reduces relapse irrespective of cytogenetic subcategory,24 counterbalancing NRM may attenuate a favorable effect on OS, which is especially evident in good-risk patients.6, 13, 14, 15, 16, 26 More recently, alloHSCT is also being discussed in intermediate-risk patients,7, 8, 9 especially in patients at higher risk of NRM. We and others previously observed increased NRM in alloHSCT recipients over the age of 40 years, which resulted in similar outcome for AML CR1 patients receiving alloHSCT as compared with conventional PRT using chemotherapy or autoHSCT.6, 13, 14, 15, 16 Following the latter observations, several HOVON centers introduced RIC-alloHSCT for patients as from the age of 40 years, but adhered to alloHSCT as preferred PRT in intermediate-risk patients. The latter approach signified the basis for the current study, addressing the value of alloHSCT versus conventional PRT and comparing recipients of a MAC-alloHSCT versus RIC-alloHSCT. Here, by time-dependent analysis, we observed improved OS by alloHSCT as compared with chemotherapeutic PRT in patients aged 40–60 years with AML in CR1. Of note, alloHSCT and autoHSCT did not significantly differ with respect to OS in intermediate-risk patients, although RFS was better following alloHSCT. In addition, the intensity of the conditioning regimen did not significantly affect the rate of relapse after alloHSCT, thereby questioning the necessity of MAC in this category of patients.
Currently, it is generally accepted that patients with favorable-risk AML do not qualify for alloHSCT as preferred PRT, because of a high probability of obtaining a second CR and subsequent favorable outcome upon proceeding to alloHSCT in second CR.7, 26, 33 More recently, that policy was also advocated for intermediate-risk patients,7 although remission rates in relapsing intermediate-risk patients are generally lower and also the percentage of patients actually proceeding to alloHSCT in second CR is compromised.9, 34 Younger patients with adverse-risk AML are currently recommended for an alloHSCT in CR1 using sibling or alternative donors, provided the risk of NRM is not excessively high.12 However, these recommendations are continuously evolving because of a number of developments, including better results following autoHSCT,19, 20, 21, 22 less NRM following RIC-alloHSCT17, 18 and improved possibilities for risk-adapted therapy,12 using at one hand better prognostic scores for leukemia risk,1 better scores to predict NRM10, 11, 35 and incorporation of quantified minimal residual disease in decision making.36, 37, 38, 39 These developments urged us to readdress PRT, especially focusing on the place of alloHSCT in intermediate-risk patients aged 40–60 years, for whom RIC-alloHSCT had been introduced in recent years. In contrast to our earlier observations,13 the present study clearly showed an overall advantage for alloHSCT recipients as compared with patients proceeding to chemotherapeutic PRT. NRM following alloHSCT in the present study estimated 17±2% at 5 years as compared with 25±4% at 4 years in the earlier HOVON–SAKK study,13 which thereby largely accounted for the observed improvement. Improved outcome following alloHSCT as compared with chemotherapy in the present study was apparent in both MAC-alloHSCT and RIC-alloHSCT recipients, with fairly similar outcome. These results are in accordance with a recent German study, exploring PRT by prospective matched pair analysis.40 That study showed significantly better OS for alloHSCT in nonfavorable-risk patients and especially in patients 45–59 years of age, which compares well with the present report. Also in their study, an increasing number of patients received an alloHSCT preceded by non-MAC or RIC, but recipients of RIC and MAC were not compared. In addition, the option of autoHSCT was not included as a PRT option in the AMLCG99 study.40 Stelljes et al.40 performed a prospective matched pair analysis, whereas alloHSCT was evaluated by time-dependent methodology in the present study. In the past, we and others evaluated the effect of transplantation by ‘biological randomization’ through so-called (sibling) donor versus no-donor studies.6, 13 These studies allow for an intention to treat analysis and thereby to approximate real randomized studies, although variable numbers of patients with a donor in those studies actually proceeded to transplantation. With the advent of MUDs and their increasing application in AML, sibling donor versus no-donor studies have become obsolete. Therefore, other statistical methods were introduced, including landmark analysis, matched pair analysis and multivariable models that include a particular type of PRT as a time-dependent covariate.41 Although only a real randomization would more rigorously rule out selection, the methodology does allow for approximating a prospective comparison without the bias caused by the time to transplant, and by including a multivariate analysis corrects for the most important, but not all, characteristics affecting relapse and NRM.23, 27
Results with autografting have improved following the introduction of peripheral blood stem cells,19, 20, 21, 22 and a recent retrospective study by the Center for International Blood and Marrow Transplant Research suggested similar outcome for younger AML CR1 patients receiving either alloHSCT from an HLA-identical sibling or an autograft using peripheral blood stem cells.21 Although recipients of alloHSCT exhibited more high-risk features, had longer follow-up and experienced a lower risk of treatment failure, no significant difference in OS was noted. Better possibilities for salvage may have accounted for improved survival after autoHSCT. Salvage by MAC-alloHSCT after autologous bone marrow transplantation appeared associated with considerable NRM in the past,42, 43 but currently RIC-alloHSCT using either sibling or alternative donors may provide for better possibilities for PRT in second CR, as was suggested in the present study by a better RFS following alloHSCT, but similar OS for autoHSCT and alloHSCT recipients.
RIC-alloHSCT is generally associated with reduced NRM as compared with MAC regimens, but concern has been raised that a reduction of NRM by RIC-alloHSCT is achieved at the expense of its antileukemic activity.17, 18 Although our study is not a prospective randomized study by design and individual conditioning choice is poorly controllable, the methodology applied allowed to limit the bias associated with time to transplant, while including a multivariable analysis.23, 24, 31, 44 With a mature follow-up of >6 years (median) in recipients of RIC-alloHSCT, we found that the reduction of relapse by RIC-alloHSCT did not significantly differ from what was observed in recipients of MAC-alloHSCT, suggesting overall equivalent antileukemic efficacy. These results are in contrast with earlier observations of a possible higher relapse rate after RIC-alloHSCT.45, 46, 47, 48, 49, 50, 51 A recent, prematurely closed prospective randomized study between RIC-alloHSCT and MAC-alloHSCT did not find major differences in outcome.52 The RIC regimen in the latter study, however, involved a more intensive, near-ablative conditioning with 8 Gy TBI.52 The potent antileukemic effect of RIC-alloHSCT that we observed may be explained by a strong GVL effect given the relatively high incidence of chronic extensive GVHD, which correlates with ongoing GVL.53, 54 Also, the strong antileukemic effects of dose-intensive remission-induction chemotherapy28, 29 may have obviated the need for further intensified chemoradiotherapy as part of the conditioning regimen. Therefore, our results suggest that reducing the intensity of the conditioning regimen before alloHSCT may result in less NRM without a significant increase of relapse in this group of intensively treated AML patients in CR1.
Collectively, our results suggest that alloHSCT is to be preferred over chemotherapy as PRT in patients with intermediate- and poor-risk AML aged 40–60 years, whereas autoHSCT remains a treatment option to be considered in patients with intermediate-risk AML. Further refinement of decision making might result from taking into account at one hand evolving leukemia risk factors and at the other hand risk factors that predict for NRM.12 In addition, risk factors that evolve during treatment, such as MRD currently gain importance.36, 37, 38, 39 A number of recent studies have suggested that especially intermediate-risk patients may be further subclassified on the basis of MRD, which might thereby allow for further optimization of personalized PRT in AML. Last, given the potent GVL activity and limited toxicity profile of RIC-alloHSCT, further evaluation of RIC-alloHSCT in younger AML patients below the age of 40 years appears warranted.
References
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Burnett A, Wetzler M, Lowenberg B . Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29: 487–494.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012; 366: 1079–1089.
Ferrara F, Schiffer CA . Acute myeloid leukaemia in adults. Lancet 2013; 381: 484–495.
Sanders MA, Valk PJ . The evolving molecular genetic landscape in acute myeloid leukaemia. Curr Opin Hematol 2013; 20: 79–85.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009; 301: 2349–2361.
Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 2013; 31: 1293–1301.
Pfirrmann M, Ehninger G, Thiede C, Bornhauser M, Kramer M, Rollig C et al. Prediction of post-remission survival in acute myeloid leukaemia: a post-hoc analysis of the AML96 trial. Lancet Oncol 2012; 13: 207–214.
Forman SJ, Rowe JM . The myth of the second remission of acute leukemia in the adult. Blood 2013; 121: 1077–1082.
Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998; 352: 1087–1092.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 2012; 9: 579–590.
Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 2007; 109: 3658–3666.
Burnett AK, Wheatley K, Goldstone AH, Stevens R, Hann I, Hills RK . Long-term results of the MRC AML10 trial. Clin Adv Hematol Oncol 2006; 4: 445–451.
Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood 2003; 102: 1232–1240.
Jourdan E, Boiron JM, Dastugue N, Vey N, Marit G, Rigal-Huguet F et al. Early allogeneic stem-cell transplantation for young adults with acute myeloblastic leukemia in first complete remission: an intent-to-treat long-term analysis of the BGMT experience. J Clin Oncol 2005; 23: 7676–7684.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol 2006; 24: 444–453.
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 2011; 118: 6037–6042.
Herr AL, Labopin M, Blaise D, Milpied N, Potter M, Michallet M et al. HLA-identical sibling allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning compared to autologous peripheral blood stem cell transplantation for elderly patients with de novo acute myeloid leukemia. Leukemia 2007; 21: 129–135.
Keating A, DaSilva G, Perez WS, Gupta V, Cutler CS, Ballen KK et al. Autologous blood cell transplantation versus HLA-identical sibling transplantation for acute myeloid leukemia in first complete remission: a registry study from the Center for International Blood and Marrow Transplantation Research. Haematologica 2013; 98: 185–192.
Ferrara F . Renaissance of autologous stem cell transplantation for AML? Lancet Oncol 2012; 13: 121–123.
Hospital MA, Thomas X, Castaigne S, Raffoux E, Pautas C, Gardin C et al. Evaluation of allogeneic hematopoietic SCT in younger adults with adverse karyotype AML. Bone Marrow Transplant 2012; 47: 1436–1441.
Cornelissen JJ, Breems D, van Putten WL, Gratwohl AA, Passweg JR, Pabst T et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol 2012; 30: 2140–2146.
Schlenk RF, Dohner K, Mack S, Stoppel M, Kiraly F, Gotze K et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol 2010; 28: 4642–4648.
Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood 2013; 122: 1576–1582.
Anderson JR, Cain KC, Gelber RD . Analysis of survival by tumor reponse. J Clin Oncol 1983; 1: 710–719.
Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med 2011; 364: 1027–1036.
Randomized study to assess the added value of Laromustine in combination with standard remission-induction chemotherapy in patients aged 18-65 years with previously untreated acute myeloid leukemia (AML) or myelodysplasia (MDS) (RAEB with IPSS >= 1.5); Main ID: NTR1446. 2013; Available at: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1446 (accessed on 22 August 2013).
Cornelissen JJ, van der Holt B, Petersen EJ, Vindelov L, Russel CA, Hoglund M et al. A randomized multicenter comparison of CD34(+)-selected progenitor cells from blood vs from bone marrow in recipients of HLA-identical allogeneic transplants for hematological malignancies. Exp Hematol 2003; 31: 855–864.
Mantel N, Byar D . Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc 1974; 69: 81–86.
Grambsch PM, Therneau TM . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526.
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013; 121: 2213–2223.
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 2005; 23: 1969–1978.
Versluis J, Labopin M, Niederwieser D, Socie G, Schlenk RF, Milpied N et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia 2014; e-pub ahead of print 20 May 2014; doi: 10.1038/leu.2014.16.
Maurillo L, Buccisano F, Del Principe MI, Del Poeta G, Spagnoli A, Panetta P et al. Toward optimization of postremission therapy for residual disease-positive patients with acute myeloid leukemia. J Clin Oncol 2008; 26: 4944–4951.
Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42 A study. J Clin Oncol 2013; 31: 3889–3897.
Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 2011; 29: 1190–1197.
Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122: 1813–1821.
Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol 2014; 32: 288–296.
Simon R, Makuch RW . A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med 1984; 3: 35–44.
Breems DA, Boogaerts MA, Dekker AW, Van Putten WL, Sonneveld P, Huijgens PC et al. Autologous bone marrow transplantation as consolidation therapy in the treatment of adult patients under 60 years with acute myeloid leukaemia in first complete remission: a prospective randomized Dutch-Belgian Haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) trial. Br J Haematol 2005; 128: 59–65.
Burnett AK, Goldstone AH, Stevens RM, Hann IM, Rees JK, Gray RG et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: results of MRC AML 10 trial. UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet 1998; 351: 700–708.
Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol 2010; 28: 586–595.
Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol 2009; 27: 4570–4577.
Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312.
Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia 2006; 20: 322–328.
Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol 2007; 82: 867–872.
Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 2006; 12: 1047–1055.
Martino R, de Wreede L, Fiocco M, van Biezen A, von dem Borne PA, Hamladji RM et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant 2013; 48: 761–770.
Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant 2012; 47: 203–211.
Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol 2012; 13: 1035–1044.
Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 1979; 300: 1068–1073.
Rowe JM . Graft-versus-disease effect following allogeneic transplantation for acute leukaemia. Best Pract Res Clin Haematol 2008; 21: 485–502.
Gail MH, Byar DP . Variance calculations for direct adjusted survival curves, with applications to testing for no treatment effect. Biom J 1986; 28: 587–599.
Acknowledgements
We thank the Leukemia Working Group of the HOVON/SAKK Cooperative Groups for conception and design; Ine Meulendijks, Jan van Tuijn, Martine Testroote, Christel van Hooije (HOVON) and Christina Biaggi (SAKK) for collection and assembly of the data.
Author Contributions
JJC, JV, WLJvP and BL contributed to the study design; all authors provided the study materials or patients; all authors were involved in the collection and assembly of clinical data; JJC, JV, WLJvP and BL were involved in analyzing and interpreting the data and writing this report; and all authors reviewed and approved the final version of the manuscript.
Members of the HOVON and SAKK Leukemia Groups
LFV, currently Isala Hospital, Zwolle, The Netherlands; and GH, currently Radboud University Medical Centre, Nijmegen, The Netherlands. All participating HOVON-SAKK institutes and investigators can be found in the supplementary appendix.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Cornelissen, J., Versluis, J., Passweg, J. et al. Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia 29, 1041–1050 (2015). https://doi.org/10.1038/leu.2014.332
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.332
- Springer Nature Limited
This article is cited by
-
CACA guidelines for holistic integrative management of adult acute myeloid leukemia
Holistic Integrative Oncology (2024)
-
Autologous stem cell transplantation in adult patients with intermediate-risk acute myeloid leukemia in first complete remission and no detectable minimal residual disease. A comparative retrospective study with haploidentical transplants of the global committee and the ALWP of the EBMT
Bone Marrow Transplantation (2023)
-
Autologous hematopoietic stem cell transplantation followed by interleukin-2 for adult acute myeloid leukemia patients with favorable or intermediate risk after complete remission
Annals of Hematology (2022)
-
Post-remission measurable residual disease directs treatment choice and improves outcomes for patients with intermediate-risk acute myeloid leukemia in CR1
International Journal of Hematology (2022)
-
Syngeneic hematopoietic stem cell transplantation for acute myeloid leukemia: a propensity score-matched analysis
Blood Cancer Journal (2021)