Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is most frequently used to treat acute myeloid leukemia (AML). Whether patients should routinely receive consolidation chemotherapy before proceeding to transplant after achieving first complete remission (CR1) has been a subject of debate. We performed a systematic review and meta-analysis of studies examining the impact of post-remission chemotherapy before allo-HSCT in patients with AML in CR1. Six studies including 1659 patients were included in the meta-analysis. The pooled hazard ratio (HR) for overall survival was 0.9 (95% confidence interval [CI] 0.77–1.05, P = 0.182), and the pooled HR for leukemia-free survival was 0.87 (95% CI 0.75–1.0, P = 0.07). No survival advantage was observed for post-remission chemotherapy before reduced-intensity conditioning or myeloablative conditioning (MAC) allo-HSCT for AML in CR1. The pooled relative risk for relapse incidence (RI) was 1.02 (95% CI 0.82–1.28, P = 0.834). Post-remission chemotherapy before allo-HSCT did not significantly affect the RI in patients with AML in CR1. The analyses revealed no significant benefit of post-remission consolidation chemotherapy in patients who received allo-HSCT. We recommend proceeding to allo-HSCT as soon as CR1 is attained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is most frequently used to treat acute myeloid leukemia (AML) because it is potentially curative [1]. Most patients who receive allo-HSCT have achieved first complete remission (CR1) [2]. In non-transplant settings, consolidation chemotherapy plays a critical role in achieving a durable complete response in patients with AML and is considered the standard of care for defined risk groups [3, 4]. Consolidation chemotherapy in CR1 is commonly used in the allogeneic setting as a bridge to transplantation with the goal of preventing early relapse prior to allo-HSCT, allowing time to identify a fully matched donor, insurance clearance, or scheduling an appointment at a transplant center. However, after achieving CR, the option of routinely receiving consolidation chemotherapy before proceeding to transplant is a particularly important and frequently debated issue. A study by the European Cooperative Group for Bone Marrow Transplantation (EBMT) included 826 patients who underwent allo-HSCT for AML in CR1 following a myeloablative conditioning regimen (MAC). The authors found no significant difference in relapse incidence (RI), leukemia-free survival (LFS), and overall survival (OS) among patients who received no consolidation or consolidation with standard-dose, intermediate-dose, or high-dose cytarabine [5]. These findings are consistent with those reported by the Center for International Blood and Marrow Transplant Research, which also found no significant difference in non-relapse mortality, RI, LFS, and OS among the three groups [6]. It appears that post-remission consolidation chemotherapy should no longer be considered before allo-HSCT for patients with AML in CR1. Moreover, potential toxicities resulting from consolidation chemotherapy could be severe enough to preclude subsequent transplantation or might produce complications that could increase the risk of transplant-related death. Although narrative reviews are available, no systematic review or meta-analysis has addressed this issue.
Here, we report the results of a meta-analysis of available studies that examined the impact of post-remission chemotherapy before allo-HSCT for patients with AML in CR1.
Methods
Search strategy and study selection
We searched the Medline (Pubmed), Embase, and Cochrane Registry of Controlled Trials databases in September 2017. We performed a manual search of abstracts from the annual meetings of the American Society of Hematology, the American Society of Clinical Oncology, and the European Hematology Association from 2000 to 2016. We also screened the reference lists of all identified studies and relevant articles including review papers. We used the following search terms: (Post-remission or Pre-transplant consolidation chemotherapy) AND (Allogeneic SCT or stem cell transplantation) AND (Acute myeloid leukemia and First complete remission). Studies comparing post-remission consolidation chemotherapy with no consolidation chemotherapy before allo-HSCT for patients with AML in CR1 were included. Two reviewers (QYG and JD) independently screened the titles and abstracts of all identified studies to assess their eligibility for inclusion.
Data extraction and quality assessment
Two reviewers (JH and YMZ) independently extracted the data from each study including first author, publication year, study region, patient number, median age, period of enrollment, transplantation type, and study outcome. Disagreements between the two reviewers were resolved via discussion. Two researchers (JD and XL) accessed the quality of the included studies using the Newcastle-Ottawa Scale (NOS) [7]. The NOS consists of nine items classified into three dimensions including selection (four items), comparability (two items), exposure, and outcome (three items). Based on the NOS, the quality of the studies was classified into high-quality (scores 7–9), intermediate-quality (scores 4–6), and low-quality (scores 1–3) studies.
Definition of outcomes
The main outcomes of this study were OS and LFS. Secondary outcomes were RI and treatment-related mortality (TRM). OS was defined as the time to death from any cause or at the last follow-up (censored). LFS was defined as survival without leukemia relapse or censor. Relapse was defined as recurrence of leukemia confirmed by cytology. TRM was defined as mortality after allo-HSCT not related to relapse.
Statistical analyses
All statistical analyses were performed using Stata ver.14.0 software (Stata Corp., College Station, TX, USA). We measured the hazard ratios (HRs) for OS and LFS and relative risk (RR) for RI and TRM. The HR or RR and their 95% confidence intervals (CIs) were directly extracted from the studies. When the HR was not explicitly provided, we estimated it according to Tierney et al. [8]. The statistical heterogeneity of the studies was assessed using the chi-square-based Q-test and quantified with the I2 statistic (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity) [9]. Estimates of the pooled HR and RR and their respective 95% CIs were used as a fixed-effect model with the inverse variance approach unless moderate heterogeneity (I2 > 50% or P value < 0.1) was found, in which a random effect model using the DerSimonian and Laird method was chosen.
Results
Included studies
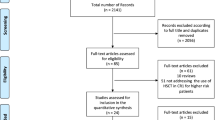
We acquired 85 citations in the electronic database and manual searches, and 16 potentially relevant citations were retrieved as full-text or were identified for more detailed information (Fig. 1). Of these, six reviews were excluded and four studies were excluded for insufficient data. Ultimately, six studies with 1659 patients met the predefined selection criteria, which included four original articles and two abstracts (Table 1).
Characteristics of the included studies
All studies reported the outcomes of patients with AML in CR1 receiving consolidation chemotherapy compared to no consolidation chemotherapy before allo-HSCT. The studies were published between 2000 [6] and 2016 [10], and all were retrospective studies. Four studies evaluated the impact of post-remission consolidation chemotherapy on outcome after reduced-intensity conditioning (RIC) allo-HSCT for patients with AML in CR1 [11,12,13,14]. Two studies examined the effects of post-remission chemotherapy before MAC allo-HSCT for patients with AML in CR1 [6, 10]. Of the six trials, the case collection period ranged from 1989 [6] to 2011 [11]. Sample sizes ranged from 52 [14] to 604 [12]. The median age was 28–32 [6] to 59–60 years [12]. All patients received one or more cycles of chemotherapy in the consolidation groups of all studies. One study reported the outcomes of patients with AML in CR1 receiving no consolidation chemotherapy, standard-dose cytarabine consolidation therapy, and high-dose cytarabine consolidation therapy before allo-HSCT [6]. Primary outcomes were reported by six trials, and secondary outcomes were reported by four trials [6, 10,11,12,13]. The quality of the studies in the analyses was high, with a mean overall NOS assessment score of 8.7 (range, 8–9).
Primary outcomes
Six studies including 1659 patients reported OS in the meta-analysis. The pooled HR for OS was 0.9 (95% CI 0.77–1.05, P = 0.182, I2 = 0%) (Fig. 2). These data indicate that post-remission chemotherapy before allo-HSCT did not significantly affect OS in patients with AML in CR1. We also performed different subgroup analyses. We found no difference between the use of post-remission chemotherapy and no consolidation chemotherapy before MAC allo-HSCT in patients with AML in CR1 (HR = 0.92, 95% CI 0.68–1.24, P = 0.57, I2 = 0%). In addition, no difference was observed between the two cohorts of patients with AML in CR1 who received RIC allo-HSCT (HR = 0.89, 95% CI 0.74–1.07, P = 0.223, I2 = 0%). When OS was analyzed according to patient age, there were no differences between the use of post-remission chemotherapy and no consolidation chemotherapy before allo-HSCT in patients with median age < 50 years (HR = 0.85, 95% CI 0.53–1.38, P = 0.182, I2 = 44.8%) or > 50 years (HR = 0.89, 95% CI 0.75–1.07, P = 0.226, I2 = 0%). We also analyzed OS according to the publication year (> 2013 and ≤ 2013) and number of patients (> 100 and < 100), but no difference was observed between the two cohorts of patients with AML in CR1 who received allo-HSCT (data not shown).
Five studies including 1599 patients reported LFS in the meta-analysis. The pooled HR for LFS was 0.87 (95% CI 0.75–1.01, P = 0.07, I2 = 0%) (Fig. 3), which indicates that post-remission chemotherapy before allo-HSCT did not significantly affect LFS in patients with AML in CR1. Subgroup analyses showed no difference between the use of post-remission chemotherapy and no consolidation chemotherapy before RIC allo-HSCT in patients with AML in CR1 (HR = 0.89, 95% CI 0.74–1.06, P = 0.19, I2 = 18.8%). No difference was observed between the two cohorts of patients with AML in CR1 who received MAC allo-HSCT (HR = 0.82, 95% CI 0.61–1.10, P = 0.18, I2 = 0%). When LFS was analyzed according to patient age, there were no differences between the use of post-remission chemotherapy and no consolidation chemotherapy before allo-HSCT in patients with a median age < 50 years (HR = 0.81, 95% CI 0.60–1.09, P = 0.166, I2 = 0%) or > 50 years (HR = 0.89, 95% CI 0.75–1.06, P = 0.201, I2 = 0%). We analyzed LFS according to the publication year (> 2013 and ≤ 2013) and patient number (> 100 and < 100), but no difference was detected between the two cohorts of patients with AML in CR1 who received allo-HSCT (data not shown).
Secondary outcomes
Three studies including 1095 patients reported RI and TRM in the meta-analysis. The pooled RR for RI was 1.02 (95% CI 0.82–1.28, P = 0.834, I2 = 0%) (Fig. 4). These data indicate that post-remission chemotherapy before allo-HSCT did not significantly affect RI in patients with AML in CR1. The pooled RR for TRM was 0.74 (95% CI 0.58–0.96, P = 0.021, I2 = 0%) (Fig. 5), which indicates that post-remission chemotherapy before allo-HSCT significantly decreased TRM compared to no post-remission chemotherapy for patients with AML in CR1.
Discussion
Allo-HSCT is a potentially curative treatment option for AML due to its effects on chemotherapy and/or radiation in the preparative regimen, and more importantly, by immunologic graft-versus-leukemia (GVL) effects [15]. Some form of post-remission consolidation chemotherapy has been proven to decrease the recurrence of leukemia and improve survival of patients with AML in CR1 who are not undergoing transplantation [3, 16]. In the transplant setting, patients with AML in CR1 received allo-HSCT have been demonstrated superior to those who received conventional post-remission therapy, particularly patients in a defined risk and age group [17]. Despite extensive studies and ongoing research, the use and timing of allo-HSCT in AML vary considerably. Whether to proceed directly to allo-HSCT after achieving CR by induction chemotherapy is a frequently debated issue in clinical practice. A retrospective study from the EBMT showed that high-dose cytarabine may not be needed in patients who have planned MAC allo-HSCT as post-remission therapy [5]. In the RIC allo-HSCT setting, a report from the acute leukemia working party of the EBMT suggested that pre-HSCT consolidation chemotherapy does not significantly alter outcomes and supports prompt transition to transplant as soon as morphological CR1 is attained [13]. Whether to proceed directly to allo-HSCT after achieving CR from induction chemotherapy is an important issue because toxicities resulting from consolidation chemotherapy may preclude subsequent allo-HSCT or increase the risk of transplant-related mortality. Although non-systemic reviews are available, no systematic review and meta-analysis has addressed this issue. This is the first meta-analysis of available studies examining the impact of post-remission chemotherapy before allo-HSCT in patients with AML in CR1.
This systemic review and meta-analysis of data from six studies representing a large population of patients demonstrated no survival advantage from post-remission chemotherapy before allo-HSCT for patients with AML in CR1. We also found that post-remission chemotherapy did not significantly affect RI. However, post-remission chemotherapy before allo-HSCT significantly decreased TRM compared to no post-remission chemotherapy for patients with AML in CR1. Notably, no significant decrease in TRM was found in three studies [6, 11, 12]. In fact, we observed a decrease in TRM for patients receiving consolidation chemotherapy in each of the three studies, likely reflecting their superior hematopoietic cell transplantation-specific comorbidity index scores [12]. This result suggests that a patient comorbidity index may affect the decision-making of the referring physician regarding who can tolerate chemotherapy and who will receive pre-transplant consolidation chemotherapy. Moreover, more patients received two consecutive induction cycles in the consolidation chemotherapy group than in the no consolidation chemotherapy group, which would expose them to added toxicity before transplantation [13]. The decision to proceed directly to allo-HSCT without consolidation chemotherapy is probably not the standard of care, but rather, reflects the fewer options for patients with comorbidities who poorly tolerate chemotherapy.
Several studies have demonstrated that minimal residual disease (MRD) detectable before allo-HSCT is associated with adverse outcomes in patients with AML in CR1 [18, 19]. Because most of these studies did not collect data on pre-transplant MRD status, it is unknown whether the lack of benefit of post-remission consolidation chemotherapy extends equally to MRD-negative and MRD-positive patients. The role of MRD in decision-making to proceed with pre-transplant consolidation chemotherapy remains a crucial question, and future studies attempting to address the value of consolidation chemotherapy in AML prior to allo-HSCT should include an in-depth assessment of CR including MRD and its impact on the final results.
This meta-analysis had several limitations that should be considered when interpreting the results. The two patient populations in the studies were somewhat heterogeneous in terms of median age, disease characteristics, cytogenetics, pre-transplant therapy, and donor source. However, multivariate analyses adjusting for potentially confounding effects of other prognostic factors produced the same results as univariate analyses in most of the studies. In all non-randomized studies, the patient population that encountered early relapse prior to planned allo-HSCT was excluded from the analyses. As expected, there was a significantly longer interval between CR1 and allo-HSCT in patients who received post-remission chemotherapy. This delay in transplantation was an inherent survival bias in favor of patients who received consolidation chemotherapy because patients with early relapse would be excluded from the comparison. Indeed, patients who underwent transplant beyond the median time between CR1 and allo-HSCT had a significantly different RI compared to those who underwent a transplant within the median time in one of the included studies [13]. However, comparable outcomes were observed for patients treated with or without pre-transplant consolidation chemotherapy in the subgroup in which the transplant was within or beyond the median time between CR1 and allo-HSCT [13]. Moreover, the median time to allo-HSCT for most of the studies was 2 and 5 months for the two patient populations, reflecting most of the higher risk period. Despite the finding that patients differed in some other potentially confounding prognostic factors, we still observed comparable results from each study. The most likely explanation for this is the strength of the GVL effect provided by RIC or MAC allo-HSCT, which may overcome differences in the pre-transplant patient or disease-related characteristics. We also acknowledge the most important inherent limitation, which was selection bias due to using non-randomized observational studies. There was no information regarding patients who were precluded from transplantation because of a lower risk for early disease recurrence or due to toxicities or mortality from consolidation chemotherapy. We also lacked data regarding the reasons why patients were assigned to their respective treatment. Furthermore, patients in these studies did not receive precisely equivalent doses of high-dose cytarabine, so the intensity of consolidation chemotherapy in these studies varied. Our findings may not be applicable to patients with AML in CR1 who are candidates for allo-SCT but for whom a suitable donor is not immediately available. Those patients may still need to receive consolidation chemotherapy while waiting for a donor to be identified due to the risk of early disease recurrence before transplantation. Hence, we highlight the need for prospective studies to examine the role of post-remission therapy before allo-HSCT, during which intention-to-treat analyses will be extremely important.
In conclusion, providing that a suitable donor is readily available and has confirmed potential transplant eligibility, there is no significant benefit to adding consolidation chemotherapy before RIC or MAC allo-HSCT, and that support a recommendation to proceeding promptly to allo-HSCT as soon as CR1 is attained. However, further prospective studies are warranted to confirm these results.
References
Baldomero H, Gratwohl M, Gratwohl A, Tichelli A, Niederwieser D, Madrigal A, Frauendorfer K (2011) The EBMT activity survey 2009: trends over the past 5 years. Bone Marrow Transplant 46:485–501
Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, Duarte RF, Dufour C, Kuball J, Farge-Bancel D, Gennery A, Kroger N, Lanza F, Nagler A, Sureda A, Mohty M (2016) Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant 51:786–792
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei ER (1994) Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 331:896–903
Gupta V, Tallman MS, Weisdorf DJ (2011) Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood 117:2307–2318
Cahn JY, Labopin M, Sierra J, Blaise D, Reiffers J, Ferrant A, Bergmann L, Visani G, Cornelissen J, De Witte T, Bosi A, Frassoni F, Gorin NC (2000) No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Br J Haematol 110:308–314
Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D, Keating A, Gale RP, Geller RB, Laughlin MJ, Lazarus HM, Luger SM, McCarthy PL, Rowe JM, Saez RA, Vowels MR, Horowitz MM (2000) Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood 96:1254–1258
Wells G, Shea B, Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Assal A, Jakubowski AA, Maloy M, Zheng J, Devlin SM, Avecilla S, Barker JN, Castro-Malaspina H, Dahi P, Gyurkocza B, Meagher R, Perales M, Shaffer B, Tamari R, Young JW, Giralt SA, Papadopoulos EB (2016) The role of post-remission chemotherapy before T-cell depleted allogeneic stem cell transplant for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant 22:S197–S198
McCormack SE, Cao Q, Oran B, Weisdorf DJ, Warlick ED (2011) Pre-transplant consolidation chemotherapy may not improve outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for acute myeloid leukemia in first complete remission. Leuk Res 35:757–761
Warlick ED, Paulson K, Brazauskas R, Zhong X, Miller AM, Camitta BM, George B, Savani BN, Ustun C, Marks DI, Waller EK, Baron F, Freytes CO, Socie G, Akpek G, Schouten HC, Lazarus HM, Horwitz EM, Koreth J, Cahn JY, Bornhauser M, Seftel M, Cairo MS, Laughlin MJ, Sabloff M, Ringdén O, Gale RP, Kamble RT, Vij R, Gergis U, Mathews V, Saber W, Chen YB, Liesveld JL, Cutler CS, Ghobadi A, Uy GL, Eapen M, Weisdorf DJ, Litzow MR (2014) Effect of postremission therapy before reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow 20:202–208
Yeshurun M, Labopin M, Blaise D, Cornelissen JJ, Sengeloev H, Vindelov L, Kuball J, Chevallier P, Craddock C, Socie G, Bilger K, Schouten HC, Fegueux N, Goker H, Maertens J, Bunjes D, Arnold R, Nagler A, Mohty M (2014) Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 120:855–863
Ganapule A, Phillip C, Lakshmi K, George B, Viswabandya A, Abraham A, Ahmed R, Srivastava A, Mathews V (2013) Clinical outcome of allogeneic SCT with a RIC regimen in young adults with AML in CR1: impact of prior consolidation therapy. Biol Blood Marrow Transpl 19:S246
Kolb H, Schmid C, Barrett AJ, Schendel DJ (2004) Graft-versus-leukemia reactions in allogeneic chimeras. Blood 103:767
Cassileth PA, Lynch E, Hines JD, Oken MM, Mazza JJ, Bennett JM, McGlave PB, Edelstein M, Harrington DP, O’Connell MJ (1992) Varying intensity of postremission therapy in acute myeloid leukemia. Blood 79:1924–1930
Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, Ligges S, Sauer T, Tschanter P, Thoennissen GB, Berning B, Kolb HJ, Reichle A, Holler E, Schwerdtfeger R, Arnold R, Scheid C, Müller-Tidow C, Woermann BJ, Hiddemann W, Berdel WE, Büchner T (2014) Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia: a prospective matched pairs analysis. J Clin Oncol 32:288–296
Ustun C, Wiseman AC, Defor TE, Yohe S, Linden MA, Oran B, Burke M, Warlick E, Miller JS, Weisdorf D (2013) Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant 48:1415–1420
Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, Fang M, Gyurkocza B, Delaney C, Radich JP, Estey EH, Appelbaum FR (2013) Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 122:1813–1821
Acknowledgments
We sincerely thank all patients and clinical investigators who were involved in the studies selected for this meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhu, Y., Gao, Q., Du, J. et al. Effects of post-remission chemotherapy before allo-HSCT for acute myeloid leukemia during first complete remission: a meta-analysis. Ann Hematol 97, 1519–1526 (2018). https://doi.org/10.1007/s00277-018-3414-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3414-6