Abstract
Background/Objectives:
Recent findings have highlighted the detrimental influence of maternal overnutrition and obesity on fetal development and early life development. However, there are no evidence-based guidelines regarding the optimal strategy for dietary intervention before pregnancy.
Subjects/Methods:
We used a murine model to study whether switching from a high-fat (HF) diet to a normal-fat (NF) diet (H1N group) 1 week before pregnancy could lead to in utero reprogramming of female offspring obesity; comparator groups were offspring given a consistent maternal HF group or NF group until weaning. After weaning, all female offspring were given the HF diet for either 9 or 12 weeks before being killed humanely.
Results:
H1N treatment did not result in maternal weight loss before pregnancy. NF offsprings were neither obese nor glucose intolerant during the entire experimental period. H1N offsprings were most obese after the 12-week postweaning HF diet and displayed glucose intolerance earlier than HF offsprings. Our mechanistic study showed reduced adipocyte insulin receptor substrate 1 (IRS1) and hepatic IRS2 expression and increased adipocyte p-Ser636/639 and p-Ser612 of H1N or HF offspring compared with that in the NF offspring. Among all groups, the H1N offspring had lowest level of IRS1 and the highest levels of p-Ser636/639 and p-Ser612 in gonadal adipocyte. In addition, the H1N offspring further reduced the expression of Glut4 and Glut2, vs those of the HF offspring, which was lower compared with the NF offspring. There were also enhanced expression of genes inhibiting glycogenesis and decreased hepatic glycogen in H1N vs HF or NF offspring. Furthermore, we showed extremely higher expression of lipogenesis and adipogenesis genes in gonadal adipocytes of H1N offspring compared with all other groups.
Conclusions:
Our results suggest that a transition from an HF diet to an NF diet shortly before pregnancy, without resulting in maternal weight loss, is not necessarily beneficial and may have deleterious effects on offspring.
Similar content being viewed by others
Introduction
The rapid rise in obesity and associated diseases throughout the world is having a major impact on human health and health-care resources. In 2008, 68% of the adult population in the United States was reportedly overweight or obese.1 In 2012, 16.9% of US children and adolescents were obese2 and nearly 50% of women of childbearing age were overweight or obese.3 The demographic shift toward a more obese phenotype in just one or two generations is not likely supported by a major genetic contribution, but rather is primarily attributable to environmental or epigenetic mechanisms. It is now well established that in utero and early life exposure to under- or overnutrition can disrupt normal growth and development and change offspring phenotype to one that might lead to disease in the future.4, 5, 6, 7, 8 This concept is known as the developmental origins of health and disease.9, 10, 11, 12
Unlike initial studies that focused on the effects of maternal undernutrition, such as a diet low in protein or calories, recent studies have more targeted maternal overnutrition such as a high-fat (HF) diet, which reflects the dietary habits of Western society and one of the major causes for obesity. Retrospective studies have indicated that babies exposed to overnutrition during gestation have higher risks of developing obesity, diabetes and other complications in adult life.13, 14, 15, 16 In animal models, offspring of mothers exposed to overnutrition have common phenotypes that include catch-up growth, increased adiposity, impaired glucose tolerance, impaired insulin sensitivity and liver dysfunction.5, 17, 18, 19, 20 Therefore, it is suggested that prevention of obesity may need to begin before pregnancy,21, 22, 23, 24, 25 and thus there is an emerging need to evaluate the impact of maternal diet structure on offspring obesity and the risk of associated disorders.
Women of childbearing age are recommended to adopt a healthy lifestyle, composed of regular physical activity, ideal prenatal diets and avoidance of harmful practices, to optimize pregnancy outcomes.26, 27 It is important that obese women or women who have been on long-term unhealthy Western-style diet select the ideal diets, before and during pregnancy, that limit overconsumption for the mother and prevent undernutrition for the fetus.28 However, according to the 2010 Dietary Guidelines for American, there are no guidelines that doctors and registered dietitian nutritionists could follow to help these women transit to a healthy diet strategy that meet the special needs of the mothers and the fetus. There are also no human study and very few animal studies aiming on evaluating the impact of multiple prenatal diet practices on pregnancy outcomes for obese/overweight mothers. In a recent mouse study, obese mothers were switched from an HF diet to a low-fat diet starting before the second pregnancy and maintained this diet until the third pregnancy.29 Interestingly, unlike the pups from the first and the second pregnancy, offspring from the third pregnancy had normal body weight and did not increase neonatal adiposity; this finding suggests the possibility that a simple diet intervention such as switching from an HF diet to a low-fat diet before pregnancy can reduce the risk of offspring obesity and thus serve as a possible prevention strategy.
In the current study, we used a murine model to study whether switching the maternal HF diet to an NF diet 1 week before pregnancy could lead to in utero reprogramming of offspring and thus prevent or promote offspring obesity.
Research design and methods
Study design
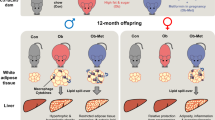
The study design is shown in Figure 1a. Three-month-old female and male mice were fed either the control diet (NF, 10% fat) or the HF diet (60% fat) for 9 weeks before conception. The breeding pairs given the HF diet either continued this diet throughout gestation and lactation (the HF group) or were switched to an NF diet 1 week (the H1N group) before conception and continued this diet throughout gestation and lactation. The offspring were therefore members of one of three groups: the NF group, the HF group or the H1N group. After weaning, the female offspring were given the HF diet for 9 or 12 weeks and then humanely killed. The female offspring of the NF mothers served as a control group in the evaluation of the metabolic response of offspring to maternal diets in later life. Another group of offspring (the CTL group), which were fed the control diet and were born to breeding pairs given the control diet, served as the reference group to show the normal ranges for a given characteristic. Body weight and food consumption of the offspring and the mother were recorded once every week during the experimental period.
H1N female offspring were more obese and had glucose intolerance after 9 weeks on postweaning HF diet. (a) Diet protocol used in the study is presented in this scheme of study design. (b) Body weight of female offspring from wean to week 12. H1N offspring significantly gained body weight from week 9 to week 12, compared with any of the other groups. Result is presented as mean±s.e., n=7 to 11. (c) Tissue (subcutaneous fat, gonadal fat and liver) weight of female offspring at week 12. H1N offspring and HF offspring had significantly larger gonadal fat and subcutaneous fat than either NF offspring or CTL offspring. Weight of liver was not different among all four groups. The result are presented as mean±s.e., n=4–11. Significance (P<0.05) is presented by different characters comparing among treatment groups in each tissue. (d) Weight of gonadal adipose tissue of HF and H1N offspring at weeks 9 and 12. H1N offspring, but not HF offspring, gained a significant amount of gonadal fat from week 9 to 12. The results are presented as mean±s.e., n=4–6. Significance (P<0.05) is presented by different characters comparing among treatment groups in each tissue. (e) At week 9, H1N offspring had impaired insulin response, compared with CTL offspring (P=0.028) or NF offspring (P=0.032). (f) At week 12, HF offspring started to present impaired glucose intolerance. The impaired glucose tolerance of H1N offspring was maintained till week 12 (P=0.039 vs CTL). The result are presented as mean±s.e., n=4–11.
Diet
Diet was purchased from Research Diets, LLC (New Brunswick, NJ, USA). The control diet (cat. no. D12450B) had an energy density of 3.771 kcal g−1 (10% fat energy, 70% carbohydrate energy and 20% protein energy). The HF diet (cat. no. D12492) had an energy density of 5.157 kcal g−1 (60% fat energy, 20% carbohydrate energy and 20% protein energy). The fat source is composed of 92% of lard and 8% of soybean oil. The concentrations of vitamins, minerals and proteins were modified to ensure that these nutrients in the HF diet were equivalent to those in the NF diet on a per kilocalorie basis.
Intraperitoneal injected glucose tolerance test
Offspring mice from each experimental group fasted overnight and were subsequently subjected to a intraperitoneal injected glucose tolerance test (IPGTT) early the next morning. Glucose tolerance tests were conducted with 20% d-glucose in 0.9% saline, so that the final concentration of the administered dose was 2.0 g/kg body weight (for details, please see the Supplementary Methods).
Statistical analysis
Differences among the control, NF, HF and H1N groups at the single time points, were analyzed by Fishers’ least significant difference test such that the multiple comparisons between groups were taken into account. Fisher’s least significant difference test was performed by first carrying out one-way analysis of variance for all four treatment groups. For the longitudinal data such as body weight and food consumption, a linear mixed model was used for the analysis of repeated measures with each individual mouse as a random effect. All analyses were carried out by using SAS JMP software (SAS Institute Inc., Cary, NC, USA) and R statistical programming language.
Results
Consistent with previous reports,30, 31 we noticed gender-specific phenotype of the offspring induced by either a maternal H1N diet or a maternal HF diet (see Supplemental Materials and Supplementary Figure 1S for male and Figures 1b–f for female), suggesting sex-dependent mechanisms involved. Therefore, we investigated the impact of maternal diet switching on male and female offspring separately. In this study, we focused on the phenotypes and molecular mechanisms in the female offspring.
The mean body weight of female offspring in the H1N group was significantly greater than that of female offspring in the HF group after 9 weeks of the postweaning HF diet
Female offspring of the CTL dams weighed significantly less than female offspring in the HF (11.89±0.43 vs 13.22±1.27 g, P=0.018) and H1N groups (11.89±0.43 vs 13.47±0.90 g, P=0.009) at the time of weaning. There was no mean weight difference between CTL offspring and NF offspring at the time of weaning (Figure 1b). The preweaning maternal diet had an obvious effect on the mean weight gain: analysis of repeated measures revealed that the female offspring of the H1N or HF group had significantly higher body weight over the entire 12 weeks compared with that of the CTL or NF group (P<0.0001). For the period of weeks 10–12, the H1N group had significantly higher body weight compared with the HF (P=0.035).
We measured the weight of subcutaneous fat, gonadal adipose tissue fat and the liver of mice in each group. There was no difference in the mean liver weight of the offspring among all four groups at week 12 (Figure 1c). However, the HF and H1N offspring had significantly greater subcutaneous fat than did the NF and control offspring. In addition, the H1N offspring had the largest gonadal fat pads among all groups (Figure 1c). As the H1N offspring experienced rapid catch-up growth from weeks 9 to 12, we hypothesized that a rapidly enlarged adipose depot contributed to the increased body weight. At week 9, female offspring of the HF and H1N groups had similar mean amounts of gonadal adipose tissue (Figure 1d, P=0.055). Between weeks 9 and 12, the amount of gonadal fat pad increased twofold in the H1N offspring (Figure 1d), whereas that of the HF offspring remained the same at weeks 9 and 12 (Figure 1d).
H1N female offspring developed glucose intolerance as early as 9 weeks on a postweaning HF diet
To determine whether H1N treatment would alter the glucose tolerance of female offspring on postweaning HF diets, we conducted IPGTTs at the end of weeks 9 and 12 (Figures 1e and f). As early as week 9, H1N offspring had an impaired glucose tolerance when compared with CTL offspring (P=0.028) or NF offspring (P=0.032). HF offspring first experienced impaired glucose tolerance at week 12 (P=0.003 vs CTL offspring) rather than week 9 (P=0.108 vs CTL offspring). The impaired glucose tolerance of H1N offspring continued until week 12 (P=0.039 vs CTL offspring). At week 12, NF offspring had a trend toward higher blood glucose concentration during IPGTT, but it was not statistically higher compared with that of CTL when measured as the AUC (P=0.104).
Body weight gain of H1N offspring was neither due to increased energy intake nor due to an association with maternal body weight change and glucose intolerance
We considered whether differences in energy consumption caused the differences in body weight and adipose weight; therefore, the energy consumption of each mouse was calculated (number of kilocalories per week; Figure 2a). Consistent with their body weight, CTL offspring consumed significantly fewer calories than the other groups through 12 weeks, as evaluated by repeated measures (P<0.0001): each CTL mouse took in ~50 kcal per week, whereas offspring from other groups took in at least 70 kcal per week. Interestingly, there was a sudden decrease in energy intake by H1N offspring starting at week 9. The energy intake was significantly lower compared with that of the HF offspring during the following 3 weeks (Figure 2d), despite the fact that the H1N offspring gained significant weight during the same period (Figure 1a). Therefore, it is clear that the body weight gain of H1N offspring was not due to increased energy intake.
Body weight gain of the H1N offspring was neither due to increased energy intake nor due to maternal obesity and glucose intolerance. (a). Food consumption of each offspring mouse was calculated as kilocalories from weeks 1 to 12. The offspring mice were separately housed and the food consumption (kcal) of each cage was calculated as follows: food consumption (kcal)=(weight of input−weight of leftover) × 5.157 for H1N, HF and NF offspring (or × 3.771 for CTL offspring). The result are presented as mean±s.e., n=7–14. (b–d) Mean body weight (b), IPGTT result before pregnancy (c) or after weaning (d) of mother mice. The results are presented as mean±s.e., n=3–4. Significance (P<0.05) is presented by different characters comparing among four groups.
Maternal factors such as obesity, significant gestational weight gain and glucose intolerance may independently contribute to the obesity phenotype observed in the offspring.32, 33, 34, 35, 36, 37 We therefore evaluated the body weight change and glucose tolerance of the mothers before and after pregnancy. A 1-week maternal diet transition seemed not to cause body weight loss, as there was no difference in the body weight between HF and H1N mother before pregnancy (Figure 2b). Although HF and H1N mothers were slightly heavier than CTL and NF mothers before pregnancy as a result of the 9-week HF diet (Figure 2b), none of the mothers had gained body weight during pregnancy and the lactation period. The results of the IPGTTs of NF, HF and H1N mothers were similar to those of the CTL mothers before (Figure 2c) or after pregnancy (Figure 2d).
A short-term transition from a maternal HF diet to an NF diet (H1N) exacerbated deleterious effects on insulin signaling
Ser/Thr phosphorylation of insulin receptor substrate 2 (IRS2) proteins interferes with insulin signaling in several ways and is associated with insulin resistance.38 We therefore evaluated the expression of IRS1 and phosphorylation of IRS1 at Ser612 and Ser636/639 in the liver and adipose tissue in offspring given a 9-week, postweaning HF diet. Expression levels of IRS1 in NF offspring were no different than those of CTL offspring, regardless of the tissue type analyzed (Figures 3a and d). However, IRS1 levels of the three types of analyzed tissue in HF and H1N offspring were significantly lower compared with those in NF or CTL offspring (Figures 3a–d). IRS1 expression in the gonadal fat of H1N offspring was remarkably lower compared with that of HF offspring (Figures 3a and d, 0.10±0.01 vs 0.68±0.12, respectively; P=0.014).
H1N offspring were less sensitive to insulin signaling. (a–c) IRS1 and phosphorylation of IRS1 at Ser636/639 and Ser612 on ISR1 of subcutaneous adipose tissue (a), gonadal adipose tissue (b) and liver (c) were detected on western blots. (d–f) Relative amounts of IRS1 and phosphorylation of IRS1 were expressed as the ratio of IRS1/actin (d) and as the ratios of Ser636/639/IRS1 (e) and Ser612/IRS1 (f), respectively. IRS1 levels in the adipose tissues of both HF and H1N offspring were significantly lower compared with those of either NF or CTL offspring. Phosphorylation of IRS1 at Ser636/639 and Ser612 was significantly greater in adipose tissue of HF and H1N offspring than in NF or CTL offspring. Among all four groups, H1N offspring had the highest level of phosphorylation at IRS1-Ser636/639 in both subcutaneous and gonadal adipose tissue and the highest level of IRS1-Ser612 in gonadal adipose tissue. (g) Hepatic IRS2 expression was detected by western blot analysis. (h) Relative amounts of IRS2 were expressed as the ratio of IRS2/actin. Compared with CTL or NF offspring, H1N and HF offspring expressed less hepatic IRS2. The results are presented as mean±s.e., n=3–4. Significance (P<0.05) is presented by different characters comparing among groups in each tissue.
Compared with NF or CTL offspring, HF and H1N offspring had greater phosphorylation levels of Ser612 in the adipose tissue (Figures 3a, b and e). H1N offspring had the highest level of Ser612 phosphorylation in gonadal fat (Figures 3b and e). Phosphorylation of Ser612 was less in the NF offspring, but was not different in the liver among other three groups (Figures 3c and e). Ser636/639 phosphorylation of IRS1 in the adipose tissue of HF and H1N offspring was significantly greater than that in either NF offspring or CTL offspring, with that of H1N offspring being the highest (Figures 3a, b and f). The hepatic level of phosphorylated Ser612 was not different among all four groups (Figures 3c and f).
Insulin receptor substrate 2 (IRS2) is a cytoplasmic signaling molecule that mediates the effects of insulin by acting as a molecular adaptor between diverse receptor tyrosine kinases and downstream effectors;39, 40, 41 therefore, we determined whether the maternal diet affects the expression of hepatic IRS2. Hepatic IRS2 expression in order from the group with the greatest to the one with the least was CTL>NF>HF≈H1N (Figures 3g and h).
H1N female offspring expressed less Glut4 and Glut2 than HF offspring
To elucidate the underlying mechanism(s) for the impaired glucose tolerance of HF and H1N offspring, we assessed the protein levels of Glut4, that is, the insulin-responsive glucose transporter protein, in gonadal adipose tissue, subcutaneous adipose tissue and liver. Compared with CTL offspring, the NF offspring had significantly lower levels of Glut4 expression in the gonadal adipose tissue and liver (Figures 4b, c, e and f) but a significantly greater levels in the subcutaneous fat (Figures 4a and d). Glut4 expression in the adipose tissue, but not in the liver, of the HF offspring was significantly less than that in the NF offspring. Importantly, Glut4 expression in the H1N offspring was the lowest among all four groups regardless of tissue types (Figures 4a–f).
Preweaning H1N diet resulted in impaired glucose homeostasis via decreased expression of Glut4 and Glut2 and increased mRNA expression of glycogenesis inhibitors. (a–c) Glut4 expression in subcutaneous adipose tissue (a), gonadal adipose tissue (b) and liver (c) were detected by western blot analysis. (d–f) Relative amounts of Glut4 were expressed as the ratio Glut4/actin. H1N offspring had the lowest level of Glut4 expression in the subcutaneous adipose tissue (d), gonadal adipose tissue (e) and liver (f). (g) Glut2 expression in the liver was detected by western blot analysis. (h) Relative amounts of Glut2 were expressed as the ratio Glut2/actin. H1N offspring had the lowest level of Glut2 expression in the liver. (i) H1N offspring had highest amount of hepatic glucose content after 9-week exposure of postweaning HF diet. (j) Hepatic expression of genes involved in glycogenesis, gluconeogenesis and glycolysis was measured by real-time PCR. (k) H1N offspring had lowest amount of hepatic glycogen content after 9-week exposure of postweaning HF diet. The results are presented as mean±s.e., n=4–6. Significance (P<0.05) is presented by different characters comparing among groups in each tissue. *P<0.05 vs CTL group.
Glut2 is required for hepatocyte glucose uptake but is dispensable for glucose output.42, 43, 44, 45 We therefore evaluated the expression of hepatic Glut2. Compared with the NF diet, the HF diet did not affect Glut2 expression; however, the H1N offspring had significantly lower levels of hepatic Glut2 than did HF and NF offspring (Figures 4g and h).
H1N offspring had increased expression of the inhibitory genes of hepatic glycogenesis and lower level of glycogen than did HF offspring
We next investigated whether the impact of maternal diet on offspring insulin sensitivity was associated with an altered glucose metabolism. First, we tested the offspring hepatic glucose level 9 weeks after the postweaning HF diet. Hepatic glucose level in order from the group with the greatest to the one with the least was H1N>HF>NF>CTL (Figure 4i). The hepatic glucose level in H1N offspring (9.439 μmol mg protein−1) was ~9-fold (0.999 μmol mg protein−1), 4-fold (2.140 μmol mg protein−1) and 2-fold (5.238 μmol mg protein−1) the value of CTL, NF and HF offspring, respectively (Figure 4i).
Second, we evaluated the impact of maternal diet on insulin-sensitive glucose metabolism by evaluating gene expression. Expression of Gsk3β, Phkα1 and Phkγ1, three genes that encode inhibitors of glycogenesis, was significantly lower in the liver of NF offspring compared with that in the CTL offspring (Figure 4j, NF vs CTL). Exposure to a preweaning HF diet increased expression of Gsk3β, Phkα1 and Pyg to at least a normal level, but further inhibited Phkβ and Phkγ1 expression (Figure 4j, HF vs NF); this result suggested a moderate effect of a preweaning HF diet on glycogenesis inhibitor genes. Simultaneously, exposure to a preweaning HF diet enhanced mRNA level of genes involved in gluconeogenesis, including Fbp, G6pc and Pepck in the liver (Figure 4j, NF vs HF). Interestingly, a preweaning H1N diet increased the expression of Gsk3β, Phkα, Phkγ1 and Pyg than did the NF diet (Figure 4j, H1N vs NF) and increased the expression of Gsk3β, Phkβ and Phkγ1 than did the HF diet (Figure 4j, H1N vs HF). However, expression levels of genes involved in gluconeogenesis of H1N offspring were normal. This result suggested an inhibitory effect of H1N treatment on glycogenesis. Consistent with this result, there was significant reduction in hepatic glycogen level seen in H1N offspring than in other groups (Figure 4k).
H1N offspring exhibited greater expression of genes involved in adipocyte lipogenesis than did HF offspring
Since a very high hepatic glucose level was observed in H1N offspring, we asked if this glucose accumulation would stimulate lipid storage in the liver by measuring the hepatic triglyceride. The result showed that triglyceride content in the liver was not different in all four groups on either a 9-week or a 12-week postnatal HF diet (Figure 5a).
Expression of genes related with lipid metabolism in adipocytes of offspring was significantly affected by H1N diet. (a) The hepatic triglyceride content was not different among all four groups on a 9-week or a 12-week exposure to a postweaning HF diet. (b and c) Adipocyte expression of genes involved in de novo lipogenese was measured by real-time PCR. (d and e) Adipocyte expression of genes involved in adipogenesis was measured by real-time PCR. The results are presented as mean±s.e., n=4–6. Significance (P<0.05) is presented by different characters. *P<0.05 vs CTL group.
We evaluated insulin-responsive lipogenesis in adipose tissue by measuring the expression of Acc1, Fas and Srebp-1 genes. Exposure of offspring to a postweaning HF diet (NF offspring) had an overall moderate impact on adipose lipogenesis: expression of Srebp-1 was increased in subcutaneous fat, but Acc1 expression was reduced in both subcutaneous and gonadal fat (Figures 5b and c, NF vs CTL). A preweaning HF diet resulted in normal levels of Acc1 expression in subcutaneous adipose tissue but decreased in gonadal adipose tissue, whereas the Srebp-1 expression level was higher than normal in both subcutaneous and gonadal adipose tissue (Figures 5b and c, HF vs CTL). The preweaning H1N diet had a remarkable effect on lipogenesis: Acc1, Fas and Srebp-1 were overexpressed in subcutaneous and gonadal adipose tissue (Figures 5b and c). This effect in gonadal adipose tissue was quite distinct: expression of Acc1 and Fas in H1N offspring was very high in relation to that in either NF offspring or HF offspring.
Ppar-γ, Ppargc1a and C/Ebp, each of which encodes a transcription element important for lipid uptake and adipogenesis by fat cells, were evaluated by real-time PCR. The postweaning HF diet resulted in slightly lower levels of Ppargc1a and C/Ebp expression in subcutaneous adipocyte (Figure 5d, NF vs CTL), while the preweaning HF diet led to significantly overexpressed Ppar-γ and C/Ebp in subcutaneous and gonadal adipocyte (Figures 5d and e, HF vs NF). This effect was exacerbated in gonadal adipocytes of H1N offspring, which expressed even greater levels of Ppar-γ and C/Ebp than did the HF offspring (Figure 5e, Ppar-γ: 94.46±20.66 vs 3.18±1.31, respectively; P=0.010; and C/Ebp: 238.03±59.85 vs 11.04±4.61, respectively; P=0.010).
Discussion
Overconsumption of energy throughout the reproductive cycle is responsible for short- and long-term maternal health risks, including obesity, diabetes and cardiovascular disease. It is believed that maternal obesity and excessive gestational weight gain alter the intrauterine environment and contribute to an increased risk of obesity in offspring.8, 46, 47 Current evidence to advocate preconception weight loss in humans to decrease the risk of childhood obesity is mostly from association studies of surgically induced interpregnancy weight loss in mother and incidence of obesity in children.48, 49, 50, 51, 52, 53, 54 These studies reported that children delivered after surgery have lower risks of being overweight and obese than do their siblings born before surgery. Of note, these surgeries are also likely to alter patients' eating habits, and this effect may contribute to the improvement in the child’s overall health status in addition to maternal weight loss. Other studies of interpregnancy weight loss achieved by adopting a healthy lifestyle before pregnancy that consisted of a balanced diet and regular physical activity have shown to reduce the risk of gestational diabetes and preeclampsia,55, 56, 57 but the improvement of pregnancy outcome on children is unknown.
Current recommendations from the National Institute for Health and Care Excellence, the American Congress of Obstetricians and Gynecologists and the Royal Australian and New Zealand College of Obstetricians and Gynecologists are that women of childbearing age should modulate their body mass index to a value within the normal range before conception by engaging in lifestyle changes such as moderate increases in exercise activity or improvements in quality of diets.58, 59, 60, 61 To obese/overweight women who have high risks of gestational diabetes mellitus, intervention is urgently needed to reduce the risk for poor maternal and child health outcomes. According to the Academy's gestational diabetes mellitus Evidence-Based Nutrition Practice Guideline (http://andevidencelibrary.com/topic.cfm?cat=3733), registered dietitian nutritionists can provide valuable guidance to these women about healthy food selection and optimal weight before, during and postpregnancy. However, there is no evidence-based strategy regarding the optimal duration of the dietary intervention or the most beneficial rate of maternal weight loss. The lack of data support about this critical issue may be due, in part, to the difficulty of carrying out large-scale cohort human studies.
Using mouse models, we tested a strategy of a short-term transition from an HF diet to an NF diet 1 week before pregnancy and maintaining this diet until weaning (H1N diet). This diet was evaluated for its impact on the metabolic response of the offspring during adult life. This model describes and reflects the situation of a rapid diet switch from an unhealthy toward a healthier one, without body weight loss, before pregnancy, and thus provides evidence regarding whether the maternal diet change independent of preconception weight loss would reduce the risk of offspring obesity. Surprisingly, the H1N diet did not ameliorate but exacerbated offspring obesity and obesity-related complications via increased mean body weight and increased gonadal adipose tissue, earlier onset of glucose intolerance, desensitized insulin signaling, decreased Glut4 and Glut2 expression, inhibited hepatic glycogen synthesis and increased expression of lipogenesis and adipogenesis genes in gonadal adipose tissue in our mouse model of diet-induced obesity vs NF or HF offspring (Figure 6). Notably, NF female offspring is neither obese nor glucose intolerant, consistent with previous reports that female mice are protected against an HF diet-induced metabolic syndrome,30, 62 whereas a preweaning H1N diet seemed to block the natural protection of female offspring upon postnatal HF diet. This detrimental effect of the H1N diet on offspring obesity was independent of maternal weight change as well as maternal glucose intolerance. Our results suggest that a short-term switch in maternal diet without weight loss before pregnancy is not necessarily beneficial and may even be harmful to offspring health.
Working model of how preweaning H1N diet exacerbated obesity of female mouse offspring given a postweaning HF diet, compared with HF offspring. H1N diet before pregnancy reduced IRS1 expression and induced overphosphorylation of Ser636/639 and Ser612-IRS in gonadal adipocytes, compared with HF diet. Hepatic IRS2 expression was also disrupted by the H1N diet. In addition, H1N diet decreased the expression of adipocyte and hepatic Glut4 and hepatic Glut2, and reduced hepatic glycogen storage associated with increased expressions of genes inhibiting glycogenesis. These all contributed to the imbalanced glucose homeostasis. Furthermore, there were increased expressions of Srebp-1c, Acc1, Fas, Ppar-γ and C/ebp in gonadal adipocytes that contributed to enhanced lipogenesis, as well as enhanced adipogenesis associated with enlarged gonadal adipose tissue when compared by HF.
We sought to investigate at the molecular level how the H1N diet caused severe obesity and early onset of glucose intolerance in offspring. Phosphorylation at Ser636/639 and Ser612 negatively regulates insulin signaling and is involved in insulin resistance.63 Mice lacking IRS2 develop lethal diabetic ketoacidosis as a result of combined insulin resistance and insulin deficiency.39, 64 Compared with the HF diet, the preweaning H1N diet caused increased phosphorylation of Ser636/639 and Ser612 of IRS1 in adipocytes. In addition, hepatocytes in H1N offspring had significantly lower levels of IRS2 compared with NF offspring. These results suggested repressed insulin signaling as a potential mechanism of reprogramming induced by the H1N diet. In addition, decreased Glut4 in adipocytes and hepatocytes in the H1N offspring compared with the HF offspring might contribute to less insulin-responsive glucose transport as well. Our results suggested that the maternal H1N diet predisposed and reprogrammed the insulin response in offspring adipocytes and hepatocytes.
A previous study of non-human primates demonstrated that a maternal HF diet led to elevated hepatic expression of gluconeogenic enzymes and that switching the maternal HF diet to a low-fat diet during a subsequent pregnancy normalized gluconeogenic enzyme expression.65 Our results are consistent with this report that increased expressions of key genes of gluconeogenesis in hepatocytes were observed in the HF offspring but not in the H1N offspring. We reported decreased levels of hepatic glycogen associated with greatly enhanced levels of glycogenesis inhibitors in H1N offspring, which might contribute to its extremely high level of hepatic glucose. These results suggested that the preweaning HF diet and H1N diet both altered glucose metabolism but went through different metabolic events.
That impaired glucose metabolism via insulin resistance coexists with preserved lipid metabolism is known as ‘selective insulin resistance’.66, 67 Exposure to a preweaning H1N diet raised expression of the de novo lipogenesis genes Acc1, Fas and Srebp-1 on a 9-week postweaning HF diet. A preweaning H1N diet significantly raised Ppar-γ and C/Ebp expression in adipose tissue vs preweaning NF diet, whereas the preweaning HF diet resulted in moderate overexpression of those genes. As we observed, severe obesity, adipocyte hypertrophy and rapid growth of gonadal adipose tissue were more prominent in the H1N offspring than in the HF offspring at postweaning week 12. We noticed significant growth of the H1N offspring starting at postweaning week 9, when glucose intolerance was reported but adipocyte hypertrophy or inflammation as shown in Supplementary Figures 2S and 3S was not observed. This evidence supports the hypothesis that early catch-up fat of the H1N offspring is not associated with overt adipocyte hypertrophy or inflammation.68 These results suggested that a preweaning H1N diet programmed the pathophysiological changes of adipose tissue at least partially through the upregulation of genes involved in adipogenesis and lipogenesis.
Do the exacerbation of offspring obesity and glucose intolerance associated with the maternal H1N diet suggest that switching from an HF diet to an NF diet before pregnancy is harmful for offspring health and thus should be avoided? One previously reported hypothesis is that the postweaning diet needs to match the perinatal diet to minimize the detrimental effect on offspring health.69 However, the detrimental effect of an HF diet on pregnant mothers and their offspring has been revealed previously, and our results clearly showed that a maternal HF diet matched with the offspring HF diet (HF group) neither reduced the gain in offspring body weight nor ameliorated glucose intolerance, but actually promoted the female offspring obesity. Therefore, an HF diet during pregnancy and childhood is not recommended. Another hypothesis is that such a rapid switch results in a relative state of ‘dietary restriction’ during pregnancy because the maternal body had adapted to the long-term HF diet and the 1-week transition was not long enough for readjustment. Thus, a thrifty phenotype hypothesis might partially explain the earlier onset of glucose intolerance in the H1N offspring than in the HF offspring.70, 71 It is possible that a longer transition period from an HF diet to an NF diet would allow maternal readaptation for an optimized environment in utero. This hypothesis is partially supported by results of a recent study: compared with the offspring of dams consistently given an HF diet until weaning, offspring of dams switched from a long-term HF diet to an NF diet 8 weeks before pregnancy showed decreased neonatal adiposity and body weight gain.29 Future studies are needed to evaluate the impact of different durations of diet transition before pregnancy on the in utero reprogramming of offspring.
In summary, our study has suggested that a transition from an HF diet shortly before pregnancy, without resulting in weight loss, is not necessarily beneficial and may even be harmful to offspring. Thus, it is important to ensure that any recommended prenatal dietary interventions are evidence based to have potential metabolic benefits for the offspring. Our findings are of great importance for designing clinical trials to evaluate the urgently required intervention strategies aiming to minimize the intergenerational cycle of obesity.
References
Flegal KM, Carroll MD, Ogden CL, Curtin LR . Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241.
Ogden CL, Carroll MD, Kit BK, Flegal KM . Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 2012; 82: 1–8.
Vahratian A . Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J 2009; 13: 268–273.
Yang Z, Huffman SL . Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern Child Nutr 2013; 9: 105–119.
Williams L, Seki Y, Vuguin PM, Charron MJ . Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta 2014; 1842: 507–519.
Simar D, Chen H, Lambert K, Mercier J, Morris MJ . Interaction between maternal obesity and post-natal over-nutrition on skeletal muscle metabolism. Nutr Metab Cardiovasc Dis 2012; 22: 269–276.
Rooney K, Ozanne SE . Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obes (Lond) 2011; 35: 883–890.
Muhlhausler BS, Ong ZY . The fetal origins of obesity: early origins of altered food intake. Endocr Metab Immune Disord Drug Targets 2011; 11: 189–197.
Vickers MH . Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes 2007; 14: 17–22.
Taylor PD, Poston L . Developmental programming of obesity in mammals. Exp Physiol 2007; 92: 287–298.
Barker DJ . The developmental origins of adult disease. J Am Coll Nutr 2004; 23: 588S–595S.
Barker DJ . Developmental origins of adult health and disease. J Epidemiol Commun Health 2004; 58: 114–115.
Tenenbaum-Gavish K, Hod M . Impact of maternal obesity on fetal health. Fetal Diagn Ther 2013; 34: 1–7.
Leddy MA, Power ML, Schulkin J . The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008; 1: 170–178.
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH . Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997; 337: 869–873.
van den Broek M, Leermakers ET, Jaddoe VW, Steegers EA, Rivadeneira F, Raat H et al. Maternal dietary patterns during pregnancy and body composition of the child at age 6 y: the Generation R Study. Am J Clin Nutr 2015; 102: 873–880.
Ainge H, Thompson C, Ozanne SE, Rooney KB . A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011; 35: 325–335.
Alfaradhi MZ, Ozanne SE . Developmental programming in response to maternal overnutrition. Front Genet 2011; 2: 27.
Masuyama H, Hiramatsu Y . Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012; 153: 2823–2830.
Ornellas F, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB . Programming of obesity and comorbidities in the progeny: lessons from a model of diet-induced obese parents. PLoS One 2015; 10: e0124737.
Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA . Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr 2003; 77: 1374–1378.
Ravelli GP, Stein ZA, Susser MW . Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976; 295: 349–353.
Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP . Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 1999; 70: 811–816.
Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ . Early growth and abdominal fatness in adult life. J Epidemiol Commun Health 1992; 46: 184–186.
Danielzik S, Czerwinski-Mast M, Langnase K, Dilba B, Muller MJ . Parental overweight, socioeconomic status and high birth weight are the major determinants of overweight and obesity in 5-7 y-old children: baseline data of the Kiel Obesity Prevention Study (KOPS). Int J Obes Relat Metab Disord 2004; 28: 1494–1502.
Procter SB, Campbell CG . Position of the Academy of Nutrition and Dietetics: nutrition and lifestyle for a healthy pregnancy outcome. J Acad Nutr Diet 2014; 114: 1099–1103.
Kaiser LL, Campbell CG . Practice paper of the Academy of Nutrition and Dietetics abstract: nutrition and lifestyle for a healthy pregnancy outcome. J Acad Nutr Diet 2014; 114: 1447.
Shapira N . Prenatal nutrition: a critical window of opportunity for mother and child. Womens Health (Lond Engl) 2008; 4: 639–656.
Krasnow SM, Nguyen ML, Marks DL . Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am J Physiol Endocrinol Metab 2011; 301: E1243–E1253.
Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity 2010; 18: 463–469.
Mischke M, Pruis MG, Boekschoten MV, Groen AK, Fitri AR, van de Heijning BJ et al. Maternal Western-style high fat diet induces sex-specific physiological and molecular changes in two-week-old mouse offspring. PLoS One 2013; 8: e78623.
Yan X, Huang Y, Zhao JX, Rogers CJ, Zhu MJ, Ford SP et al. Maternal obesity downregulates microRNA let-7 g expression, a possible mechanism for enhanced adipogenesis during ovine fetal skeletal muscle development. Int J Obes (Lond) 2013; 37: 568–575.
Wu T, Deng S, Li WG, Yu Y, Li F, Mao M . Maternal obesity caused by overnutrition exposure leads to reversal learning deficits and striatal disturbance in rats. PLoS One 2013; 8: e78876.
Nicholas LM, Morrison JL, Rattanatray L, Ozanne SE, Kleemann DO, Walker SK et al. Differential effects of exposure to maternal obesity or maternal weight loss during the periconceptional period in the sheep on insulin signalling molecules in skeletal muscle of the offspring at 4 months of age. PLoS One 2013; 8: e84594.
Martin-Gronert MS, Fernandez-Twinn DS, Poston L, Ozanne SE . Altered hepatic insulin signalling in male offspring of obese mice. J Dev Origins Health Dis 2010; 1: 184–191.
Kahraman S, Dirice E, De Jesus DF, Hu J, Kulkarni RN . Maternal insulin resistance and transient hyperglycemia impacts the metabolic and endocrine phenotypes of offspring. Am J Physiol Endocrinol Metab 2014; 307: E906–E918.
Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG . Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol 2014; 211: 237 e231–237 e213.
Boura-Halfon S, Zick Y . Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 2009; 296: E581–E591.
Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF et al. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes 2003; 52: 2239–2248.
Rother KI, Imai Y, Caruso M, Beguinot F, Formisano P, Accili D . Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J Biol Chem 1998; 273: 17491–17497.
Dong X, Park S, Lin X, Copps K, Yi X, White MF . Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest 2006; 116: 101–114.
Wood IS, Trayhurn P . Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 2003; 89: 3–9.
Burcelin R, Dolci W, Thorens B . Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes 2000; 49: 1643–1648.
Guillam MT, Burcelin R, Thorens B . Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci USA 1998; 95: 12317–12321.
Hosokawa M, Thorens B . Glucose release from GLUT2-null hepatocytes: characterization of a major and a minor pathway. Am J Physiol Endocrinol Metab 2002; 282: E794–E801.
Rkhzay-Jaf J, O'Dowd JF, Stocker CJ . Maternal obesity and the fetal origins of the metabolic syndrome. Curr Cardiovasc Risk Rep 2012; 6: 487–495.
Oken E, Gillman MW . Fetal origins of obesity. Obes Res 2003; 11: 496–506.
Willmer M, Berglind D, Sørensen TI, Näslund E, Tynelius P, Rasmussen F . Surgically induced interpregnancy weight loss and prevalence of overweight and obesity in offspring. PLoS One 2013; 8: e82247.
Dixon JB, Dixon ME, O'Brien PE . Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol 2005; 106: 965–972.
Bennett WL, Gilson MM, Jamshidi R, Burke AE, Segal JB, Steele KE et al. Impact of bariatric surgery on hypertensive disorders in pregnancy: retrospective analysis of insurance claims data. BMJ 2010; 340: c1662.
Hezelgrave NL, Oteng-Ntim E . Pregnancy after bariatric surgery: a review. J Obes 2011; 2011: 501939.
Karmon A, Sheiner E . Pregnancy after bariatric surgery: a comprehensive review. Arch Gynecol Obstet 2008; 277: 381–388.
Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 2009; 94: 4275–4283.
Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006; 118: e1644–e1649.
Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara et al. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011; 117: 1323–1330.
Villamor E, Cnattingius S . Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006; 368: 1164–1170.
Bogaerts A, Van den Bergh BR, Ameye L, Witters I, Martens E, Timmerman D et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol 2013; 122: 999–1009.
KM Rasmussen, AL Yaktinen (eds). Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Collection, National Institutes of Health: Bethesda, MD, USA, 2009.
Gynecologists, ACoOa. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013; 121: 5.
National Institute for Health and Care Excellence. Weight Management Before, During and after Pregnancy (PH27). National Institute for Health and Care Excellence: London, UK, 2010.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Management of Obesity in Pregnancy (C-Obs 49), 2013.
Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M . Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 2012; 7: e46057.
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004; 431: 200–205.
Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998; 391: 900–904.
McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009; 119: 323–335.
Matsumoto M, Han S, Kitamura T, Accili D . Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 2006; 116: 2464–2472.
Brown MS, Goldstein JL . Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 2008; 7: 95–96.
Marcelino H, Veyrat-Durebex C, Summermatter S, Sarafian D, Miles-Chan J, Arsenijevic D et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes 2013; 62: 362–372.
Sasaki A, Nakagawa I, Kajimoto M . Effect of protein nutrition throughout gestation and lactation on growth, morbidity and life span of rat progeny. J Nutr Sci Vitaminol 1982; 28: 543–555.
Hales CN, Barker DJ . Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35: 595–601.
Hales CN, Barker DJ . The thrifty phenotype hypothesis. Br Med Bull 2001; 60: 5–20.
Acknowledgements
This project was supported by grants from the National Institutes of Health (NIH-1R15HL117238 to LX and National Center for Research Resources, 5P20RR016471-12/8 P20 GM103442-12 to LX and KZ) and the American Heart Association (Scientist Development Grant13SDG14650009 to LX).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Fu, Q., Olson, P., Rasmussen, D. et al. A short-term transition from a high-fat diet to a normal-fat diet before pregnancy exacerbates female mouse offspring obesity. Int J Obes 40, 564–572 (2016). https://doi.org/10.1038/ijo.2015.236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.236
- Springer Nature Limited
This article is cited by
-
Maternal diet intervention before pregnancy primes offspring lipid metabolism in liver
Laboratory Investigation (2020)
-
Adiponectin homolog osmotin, a potential anti-obesity compound, suppresses abdominal fat accumulation in C57BL/6 mice on high-fat diet and in 3T3-L1 adipocytes
International Journal of Obesity (2019)
-
Sex-associated preventive effects of low-dose aspirin on obesity and non-alcoholic fatty liver disease in mouse offspring with over-nutrition in utero
Laboratory Investigation (2019)