Abstract
Previous studies have suggested that tacrolimus (TAC) is more potent than cyclosporine (CSA) for prophylaxis against acute GVHD after allogeneic hematopoietic stem cell transplantation (HSCT). However, the target blood concentrations of these drugs in these studies were not consistent with the current recommendations. Therefore, we performed a randomized controlled trial to compare CSA and TAC with target blood concentrations of 500 and 15 ng/ml, respectively, to prevent acute GVHD after unrelated HSCT. A total of 107 patients were randomized into a CSA group (n=53) or a TAC group (n=54). During the first 4 weeks after HSCT, more than 90% of the patients achieved a mean blood concentration of between 80 and 120% of the target concentration. The incidences of grade II–IV and grade III–IV acute GVHD were 39.6 and 7.5% for the CSA group and 33.3 and 9.4% for the TAC group, respectively (P=0.41 and P=0.76). Other clinical outcomes, including overall survival, disease-free survival and the incidences of relapse, non-relapse mortality, and organ toxicities, were also equivalent. We concluded that the combinations of CSA and TAC with strict dose adjustment showed similar efficacies and toxicities as prophylaxis against acute GVHD after unrelated HSCT.
Similar content being viewed by others
Introduction
Cyclosporine (CSA), which inhibits calcineurin and prevents T-cell activation, has a major role in the pharmacological prevention of GVHD after allogeneic hematopoietic stem cell transplantation (HSCT).1, 2 Tacrolimus (TAC) is another calcineurin inhibitor, and three randomized controlled trials as well as a meta-analysis of these trials showed that the combination of TAC and methotrexate (TAC-MTX) was associated with a significantly lower incidence of acute GVHD than the combination of CSA-MTX, although there was no survival benefit in the TAC-MTX group.3, 4, 5, 6 A Japanese nation-wide retrospective study confirmed that TAC was superior to CSA for the prevention of acute GVHD.7 Therefore, it has been believed that TAC is more effective than CSA in terms of preventing acute GVHD, although this benefit is not translated into better survival, probably due to increased toxicity.
However, the effects of these agents may be strongly affected by their blood concentrations.8 In two of the three randomized controlled trials mentioned above, CSA was continuously infused with a target blood concentration between 150 and 450 ng/ml.4, 6 This target concentration is much lower than that recommended in the recent guidelines. For example, in the recent EBMT (European Group for Blood and Marrow Transplantation) and the ELN (European LeukemiaNet) recommendation, a target trough concentration of 200–300 ng/ml is recommended when CSA is given in two divided doses.1 This target trough concentration in bolus infusion corresponds to a target concentration of 400–600 ng/ml when CSA is administered continuously, since the target steady-state concentration in the continuous infusion of CSA should be 2–2.5 times higher than the trough concentration in twice-daily administration to provide an equal area under the concentration-time curve.9, 10 In fact, the incidence of acute GVHD was significantly higher in patients who received a continuous infusion of CSA with a target concentration of 250–400 ng/ml than in those who received a twice-daily infusion with a target trough concentration of 150–300 ng/ml.11 On the other hand, the target concentration of TAC during continuous infusion was much higher than that in current transplantation practice; it was 10–30 ng/ml in the two randomized trials in the United States and 20–25 ng/ml in the Japanese trial, whereas a blood concentration of 8–12 or 10–20 ng/ml has been recommended based on retrospective and prospective studies.8, 12, 13
Wingard et al.8 analyzed the relationship between the blood concentrations of CSA and TAC and their efficacies and toxicities.8 For both CSA and TAC, higher blood concentrations were associated with an increased incidence of renal dysfunction. In addition, a lower blood concentration of CSA (<300 ng/ml) was associated with an increased incidence of acute GVHD, although this relationship was not statistically significant.
Therefore, in previous randomized controlled trials, the target blood concentrations were not optimal in either the CSA or TAC groups. The higher incidence of acute GVHD in the CSA group may be improved by elevating the target concentration of CSA, while the higher incidence of renal toxicity in the TAC group could be improved by lowering the target concentration of TAC. In fact, in a Japanese study, the incidence of acute GVHD was significantly decreased by increasing the target blood concentration from 300 to 500 ng/ml.14
These considerations prompted us to perform a randomized controlled trial to compare CSA-MTX and TAC-MTX for the pharmacological prevention of acute GVHD, with target blood concentrations of 500 and 15 ng/ml, respectively.
Patients and methods
Trial design
This open-label multicenter randomized controlled trial was performed by the Kanto Study Group for Cell Therapy to compare CSA-MTX and TAC-MTX with a 1:1 allocation. Patient entry was started in February 2008 and closed in December 2012. During the entry period, inclusion criteria were expanded in October 2009 as described in the next paragraph. This study was approved by the institutional review boards of all participating centers and was registered in the UMIN Clinical Trial Registry (UMIN000001151).
Patients
The inclusion criteria were age between 16 and 55 years, underlying disease of AML, ALL or myelodysplastic syndrome, transplantation from an HLA-A, -B and -DRB1 allele matched donor or an HLA-DRB1 1 allele mismatched donor, ECOG performance status less than 2, the absence of severe organ dysfunction (for example, SaO2 less than 94% or ejection fraction less than 55%) and the absence of active infection. HLA-C typing was not routinely performed until 2009.
In October 2009, patients aged between 56 and 65 years became eligible to accelerate patient inclusion. In addition, lymphoma and chronic myeloid leukemia were added to the inclusion criteria. Low-risk diseases were defined as acute leukemia in first or second remission, chronic myeloid leukemia in accelerated phase or first or second chronic phase, myelodysplastic syndrome of refractory anemia or refractory anemia with ringed sideroblast. More advanced diseases were considered as high-risk diseases. All patients provided their written informed consent before being enrolled in these studies.
Conditioning regimen and stem cell source
While the conditioning regimen was decided at the discretion of the participating centers, regimens that were considered to be frequently associated with mixed chimera, such as low-dose TBI alone, were not allowed. Bone marrow was exclusively used as the stem cell source, since unrelated peripheral blood stem cell transplantation was not available in Japan during the study period.
GVHD prophylaxis
Patients were randomly allocated to either CSA-MTX or TAC-MTX as prophylaxis against GVHD, stratified by patient age, conditioning regimen, disease status and institutions. CSA was started on day −1 at a dose of 3 mg/kg/day by continuous infusion and the dose was adjusted to maintain a blood concentration of around 500 ng/ml. For example, when the blood concentration of CSA was 600 ng/ml under the administration of CSA at 180 mg/day, the dose of CSA was decreased to 150 mg/day (5/6th of the original dose). TAC was started on day −1 at a dose of 0.03 mg/kg/day by continuous infusion and the dose was adjusted to maintain a blood concentration of around 15 ng/ml. CSA and TAC were changed to an oral form when it could be tolerated by the patient at doubled and tripled doses, respectively. For patients without GVHD, we started to taper CSA or TAC from day 50 by 5% per week and discontinued the drugs at around day 180.15 Methotrexate was administered at 10 mg/m2 on day 1 and at 7 mg/m2 on days 3, 6 and 11.16
Blood concentrations of CSA and TAC were measured at each center by various methods including AxSYM (Abbott, North Chicago, IL, USA), TDx (Abbott), EMIT (Siemens, Munich, Germany), ACMIA (Siemens) and CEDIA PLUS (Thermo Scientific, Waltham, MA, USA). Therefore, before this study was started, pilot samples were sent to each center and the measurement results were checked. The mean values and standard deviations of the measured results were 147.7±23.5, 224.9±24.5 and 520.9±69.8, respectively, for samples prepared at 150, 250 and 500 of CSA. For TAC, samples prepared at 3.0, 8.0, 15.6 and 21.8 ng/ml resulted in values of 3.6±0.76, 7.3±0.77, 14.0±1.10 and 21.9±3.19 ng/ml, respectively.
Other transplantation procedures
Prophylaxis against bacterial, fungal and Pneumocystis carinii infection was performed according to the policy of each center, but mainly consisted of fluoroquinolones, azoles and sulfamethoxazole/trimethoprim. As prophylaxis against HSV infection and varicella zoster infection, acyclovir was given from days −7 to 35, followed by long-term low-dose (200 mg/day) administration.17, 18 CMV antigenemia assay using C10/C11 antibody was performed at least once a week after engraftment and preemptive therapy with ganciclovir was started for high-risk patients.19
Acute GVHD was graded as previously described.20 Patients who developed grade II–IV acute GVHD were treated with 1–2 mg/kg of (methyl-)prednisolone, while grade II acute GVHD limited to the skin was treated with topical steroid with or without hydrocortisone infusion. Chronic GVHD was diagnosed and graded according to the National Institutes of Health Consensus Criteria.21
Statistical considerations
GVHD prophylaxis was considered successful if none of the following four criteria were present; death within 100 days after HSCT, development of grade II–IV acute GVHD before day 100, discontinuation of assigned calcineurin inhibitor before day 100, and the use of immunosuppressive agents other than the assigned calcineurin inhibitor and MTX. The use of hydrocortisone infusion and topical steroid was allowed.
Non-inferiority was predefined as a difference in the success rates between the two groups of no more than 15%. On the basis of the assumption of a success rate of 60% in the CSA-MTX group and 50% in the TAC-MTX group,7, 14, 22 49 patients in each treatment group were required to show non-inferiority with an alpha error of 5% and a power of 80%. Based on the assumption of a 10% loss of patients between the enrollment and randomization, a total of 110 patients needed to be enrolled in this study.
Fisher’s exact test was used to compare categorical variables and the Mann–Whitney U-test was used to compare continuous variables. Overall survival was estimated according to the Kaplan–Meier method, and compared among groups with the log-rank test. The incidence of GVHD was calculated while treating death without GVHD as a competing event, and compared using Gray’s test.23 All P-values were two-sided and P-values of 0.05 or less were considered as statistically significant. All statistical analyses were performed with EZR (version 1.27, Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (version 3.1.2, The R Foundation for Statistical Computing).24 More precisely, it is a modified version of R commander (version 2.1-5) that was designed to add statistical functions frequently used in biostatistics.
Results
Patients
A total of 108 patients were enrolled in the study between February 2008 and December 2012. Among these patients, one patient was excluded because of the presence of HLA mismatch at a serological level. Therefore, a total of 107 patients were randomized into the CSA-MTX group (n=53) or the TAC-MTX group (n=54) (Figure 1). There were no differences in age, sex, underlying disease, disease status or conditioning regimen between the two groups (Table 1). The proportion of patients who received HLA-mismatched graft (based on HLA-A, -B and -DRB1 information) was higher in the CSA-MTX group (22.6 vs 11.1%), but this difference was not statistically significant (P=0.13). For patients for whom we had complete information regarding HLA-A, -B, -C and -DRB1 alleles, 20 of the 48 patients (41.7%) and 13 of the 47 patients (27.7%) in the CSA-MTX and TAC-MTX groups, respectively, had at least one HLA mismatch (P=0.20).
Success rate, survival, relapse and non-relapse mortality
Predefined success rates were 39.6% in the CSA-MTX group and 38.9% in the TAC-MTX group (Figure 2). The difference in the success rates was 0.7% (95% confidence interval (CI) −17.8–19.2%).
Figures 3a and b show overall and disease-free survival curves stratified by the disease status before HSCT. Overall survival rates at 3 years were 66.3% (95% CI 48.9–79.0%) and 33.3% (95% CI 10.3–58.8%) for low- and high-risk patients in the CSA-MTX group and 70.4% (95% CI 53.8–82.0%) and 30.0% (95% CI 7.7–56.9%) in the TAC-MTX group, respectively (P=0.79). Disease-free survival rates at 3 years were 62.1% (95% CI 45.1–75.2%) and 33.3% (95% CI 10.3–58.8%) for low- and high-risk patients in the CSA-MTX group and 59.0% (95% CI 42.4–72.2%) and 25.0% (95% CI 6.0–50.5%) in the TAC-MTX group, respectively (P=0.80).
The incidences of relapse and non-relapse mortality at 3 years were 27.1% (95% CI 15.7–39.7%) and 17.5% (95% CI 8.5–29.2%) in the CSA-MTX group and 35.6% (95% CI 22.9–48.5%) and 13.0% (5.6–23.4%) in the TAC-MTX group, respectively (P=0.49 and P=0.71, Figure 2c).
Engraftment and GVHD
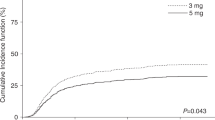
Neutrophil engraftment was achieved in all but three patients (97.2%). The incidences of grade II–IV acute GVHD were 39.6% (95% CI 26.4–52.5%) and 33.3% (95% CI 21.1–46.0%) in the CSA-MTX and TAC-MTX groups, respectively (P=0.41, Figure 4a). We performed a post hoc subgroup analysis, since there was a non-significant trend toward a higher number of patients with HLA mismatch in the CSA-MTX group. The incidences of grade II–IV acute GVHD were 28.6% (95% CI 13.3–46.0%) and 35.3% (95% CI 19.7–51.3%) in the CSA-MTX and TAC-MTX groups, respectively, among patients who received graft from an HLA-A, -B, -C and -DRB1 allele matched donor (P=0.62, Figure 4b). There was no significant difference in the incidence of acute GVHD among the participating centers (data not shown).
The incidences of grade III–IV acute GVHD were also similar; 7.5% (95% CI 2.4–16.7%) and 9.4% (95% CI 3.4–19.2%) for the CSA-MTX and TAC-MTX groups, respectively, in the whole population (P=0.76) and 3.6% (95% CI 0.2–15.7%) and 12.1% (95% CI 3.7–25.8%) among patients who received graft from an HLA-A, -B, -C and -DRB1 allele matched donor (P=0.23).
The incidences of chronic GVHD at 3 years were 38.1% (95% CI 23.5–52.6%) and 46.9% (95% CI 3235-60.3%) in the CSA-MTX and TAC-MTX groups, respectively (P=0.60), among patients who survived disease free for at least 100 days after HSCT.
Adverse events
With regard to the adverse events during the first 4 weeks, the maximum values of serum creatinine and total bilirubin levels were not different between the two groups (P=0.26 and P=0.46), but the maximum value of serum alanine aminotransferase level was significantly higher in the TAC-MTX group (P=0.0056, Figure 5). Thrombotic microangiopathy was observed in four and three patients in the CSA-MTX and TAC-MTX groups, respectively (P=0.72).
Pharmacokinetics
During the continuous infusion of CSA or TAC, a significantly higher percentage of blood samples in the CSA-MTX group than in the TAC-MTX group was within the range of good blood concentration control (60.8 vs 52.4%, P=0.00013, Figure 6a), which was defined as a blood concentration between 80 and 120% of the target concentration. Especially, within the first week after HSCT, more than half of the blood samples in the TAC-MTX group were higher than the good control range (Figure 6b). In the second week after HSCT, the proportion of samples with good control increased in both groups, but thereafter, the number of samples with a higher concentration tended to increase in the TAC-MTX group. However, when we analyzed the mean blood concentration during the first 4 weeks in each patient, the value was between 80 and 120% of the target concentration in 100 of the 107 patients (93%).
The numbers of samples within the range of good blood concentration control, which was defined as a blood concentration between 80 and 120% of the target concentration, and those with higher or lower concentrations during the intravenous administration of calcineurin inhibitors (a), and grouped according to the weeks after transplantation (b).
The actual daily doses of CSA were 2.89±0.64, 3.26±0.91, 3.13±1.05 and 2.98±1.08 mg/kg and those of TAC were 0.025±0.015, 0.021±0.0068, 0.024±0.020 and 0.020±0.0068 mg/kg, respectively, during the first, second, third and fourth weeks after HSCT.
There were no significant associations between blood concentration and clinical outcomes including overall survival, disease-free survival and the incidences of GVHD and adverse events (data not shown).
Discussion
In this randomized controlled trial, we evaluated the efficacy of CSA and TAC with a strict dose adjustment to target 500 ng/ml for CSA and 15 ng/ml for TAC. Dose adjustment was successful, as 93% of the patients achieved a mean blood concentration of between 80 and 120% of the target concentration during the first 4 weeks after HSCT. The proportions of patients in the CSA-MTX and TAC-MTX groups with prophylaxis success were similar, although the lower limit of the 95% CI of their difference was just outside the predefined non-inferiority margin. Other clinical outcomes including overall survival, disease-free survival and the incidences of acute GVHD, relapse, non-relapse mortality and organ toxicities were also equivalent, except that the incidence of liver toxicity was higher in the TAC-MTX group, although this study was not sufficiently powered for these secondary end points.
The lack of a difference in the incidence of acute GVHD between the CSA-MTX and TAC-MTX groups in the current study highlights the importance of the target blood concentration of calcineurin inhibitors. In the previous randomized controlled trials, insufficient blood concentrations of CSA might have resulted in higher incidences of acute GVHD and excessive concentrations of TAC might have resulted in the higher incidences of renal toxicity. In the current study, the target blood concentrations of both drugs were determined at the clinically recommended levels, which resulted in a similar efficacy and toxicity for CSA and TAC. This situation is very similar to the comparison of idarubicin (IDR) and daunorubicin (DNR) in remission induction therapy for AML. A meta-analysis of randomized controlled trials revealed that the CR rate was higher and fewer patients relapsed, albeit slightly more died in remission, in the IDR group than in the DNR group.25 However, these differences disappeared when the total dose of DNR was increased up to 250 mg/m2.26 The previously reported difference between CSA and TAC, like that between IDR and DNR, might simply result from the dose setting.
In contrast to previous studies,8, 11, 14, 27, 28 there were no significant correlations between blood concentration and various clinical outcomes. This may be due to the successful control of blood concentration with small variances in this study. Some of the blood samples showed blood concentrations that were too low or too high, but the subsequent doses were adjusted to obtain appropriate concentrations, and finally, the mean blood concentration of CSA or TAC in each patient was considered to be achieved within an acceptable range.
Dose adjustment of TAC appeared to be more difficult than that of CSA, since the proportion of blood samples with a good concentration was lower in the TAC-MTX group. Especially, in more than half of the blood samples in the TAC-MTX group within the first week after HSCT, the blood concentration of TAC exceeded 120% of the target concentration. If we consider that the actual daily doses of TAC were within the range of 0.020–0.025 mg/kg thereafter, the starting dose of TAC at 0.03 mg/kg may have been too high and should be reconsidered. Another possible reason for the difficulty in the dose adjustment of TAC may be the preparation of intravenous CSA and TAC. Intravenous TAC is provided as a 5-mg/ml solution, and therefore, for a patient with a body weight of 60 kg, 0.03 mg/kg of TAC corresponds to only 0.36 ml of intravenous TAC preparation, whereas 3.0 mg/kg of CSA corresponds to 3.6 ml of intravenous CSA preparation, which contains 50 mg/ml of CSA.
This study has several limitations. First, HLA-C was not typed in 12 of the 107 patients. However, the conclusions of this study did not change when we re-analyzed the data among patients who received graft from an HLA-A, -B, -C and -DRB1 allele matched donor. Second, the measurement of blood concentrations of CSA and TAC was not centralized and was performed at each center according to various methods. Nevertheless, the measurement of pilot samples of CSA and TAC before the study began resulted in acceptable variances. Finally, single-nucleotide polymorphisms of cytochrome P 450 family genes may have affected the blood concentrations of CSA and TAC, but we did not have access to DNA samples of the patients.
In conclusion, the combinations of CSA-MTX and TAC-MTX with strict dose adjustment showed similar efficacies and toxicities as prophylaxis against acute GVHD after unrelated HSCT. Both calcineurin inhibitors appear to be acceptable agents for preventing acute GVHD, provided that their doses are adjusted to maintain appropriate blood concentrations.
References
Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant 2014; 49: 168–173.
Choi SW, Reddy P . Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol 2014; 11: 536–547.
Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 181–185.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–2068.
Ram R, Gafter-Gvili A, Yeshurun M, Paul M, Raanani P, Shpilberg O . Prophylaxis regimens for GVHD: systematic review and meta-analysis. Bone Marrow Transplant 2009; 43: 643–653.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303–2314.
Yanada M, Emi N, Naoe T, Sakamaki H, Takahashi S, Hirabayashi N et al. Tacrolimus instead of cyclosporine used for prophylaxis against graft-versus-host disease improves outcome after hematopoietic stem cell transplantation from unrelated donors, but not from HLA-identical sibling donors: a nationwide survey conducted in Japan. Bone Marrow Transplant 2004; 34: 331–337.
Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant 1998; 4: 157–163.
Nakamura Y, Takeuchi H, Okuyama K, Akashi T, Jojima Y, Konno O et al. Evaluation of appropriate blood level in continuous intravenous infusion from trough concentrations after oral administration based on area under trough level in tacrolimus and cyclosporine therapy. Transplant Proc 2005; 37: 1725–1727.
Kimura S, Oshima K, Okuda S, Sato K, Sato M, Terasako K et al. Pharmacokinetics of CsA during the switch from continuous intravenous infusion to oral administration after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2010; 45: 1088–1094.
Ogawa N, Kanda Y, Matsubara M, Asano Y, Nakagawa M, Sakata-Yanagimoto M et al. Increased incidence of acute graft-versus-host disease with the continuous infusion of cyclosporine A compared to twice-daily infusion. Bone Marrow Transplant 2004; 33: 549–552.
Couriel DR, Thall P, Mickler K, De Lima M, Giralt S, Qazilbash MH et al. Phase II/III Randomized Study Comparing Two Different Tacrolimus Blood Levels for the Prevention of Graft-Versus-Host Disease (GVHD). Blood 2005; 106: 45a (abstract 142).
Mori T, Kato J, Shimizu T, Aisa Y, Nakazato T, Yamane A et al. Effect of early posttransplantation tacrolimus concentration on the development of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation from unrelated donors. Biol Blood Marrow Transplant 2012; 18: 229–234.
Oshima K, Kanda Y, Nakasone H, Arai S, Nishimoto N, Sato H et al. Decreased incidence of acute graft-versus-host disease by continuous infusion of cyclosporine with a higher target blood level. Am J Hematol 2008; 83: 226–232.
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–735.
Morishima Y, Morishita Y, Tanimoto M, Ohno R, Saito H, Horibe K et al. Low incidence of acute graft-versus-host disease by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone marrow transplantation from human leukocyte antigen compatible siblings; possible role of genetic homogeneity. The Nagoya Bone Marrow Transplantation Group. Blood 1989; 74: 2252–2256.
Kawamura K, Wada H, Yamasaki R, Ishihara Y, Sakamoto K, Ashizawa M et al. Prophylactic role of long-term ultra-low-dose acyclovir for varicella zoster virus disease after allogeneic hematopoietic stem cell transplantation. Int J Infect Dis 2014; 19: 26–32.
Kawamura K, Wada H, Yamasaki R, Ishihara Y, Sakamoto K, Ashizawa M et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus disease after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2013; 15: 457–465.
Kanda Y, Yamashita T, Mori T, Ito T, Tajika K, Mori S et al. A randomized controlled trial of plasma real-time PCR and antigenemia assay for monitoring CMV infection after unrelated BMT. Bone Marrow Transplant 2009; 45: 1325–1332.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Nishida T, Murayama T, Hirai H, Okamoto S, Sao H, Hara M et al. Phase II study of tacrolimus and methotrexate for prophylaxis of acute graft-versus-host disease after HLA-A, B, and DRB1 genotypically mismatched unrelated bone marrow transplantation among Japanese patients. Int J Hematol 2009; 89: 98–105.
Gray RJ . A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Kanda Y . Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458.
A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. AML Collaborative Group. Br J Haematol 1998; 103: 100–109.
Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N et al. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood 2011; 117: 2358–2365.
Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 414–422.
Kanda Y, Hyo R, Yamashita T, Fujimaki K, Oshima K, Onoda M et al. Effect of blood cyclosporine concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor. Am J Hematol 2006; 81: 838–844.
Acknowledgements
We thank the staffs at the participating centers, including Tokyo Medical University Hospital, Yokohama City University Medical Center, Jikei University Hospital and Yokohama City University Hospital. We also thank the staffs at the Japan Clinical Research Support Unit and the data center of the Kanto Study Group for Cell Therapy.
Author contributions
YK designed the study and analyzed the data. TK, TM, MT, CN, AY, RW, SK, KK, JK, ND, MA, S-IK, MK, HK and SO participated in the study and gathered the data. AT contributed as a data manager. YK wrote the first draft of the paper and all other authors contributed to the final version.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
YK and CN received an honorarium and a research grant from Astellas Pharma Inc. and Novartis Pharma KK. SO received research a grant from Astellas Pharma Inc. Division of Hematology, Department of Medicine, Keio University School of Medicine had courses endowed by Novartis Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Kanda, Y., Kobayashi, T., Mori, T. et al. A randomized controlled trial of cyclosporine and tacrolimus with strict control of blood concentrations after unrelated bone marrow transplantation. Bone Marrow Transplant 51, 103–109 (2016). https://doi.org/10.1038/bmt.2015.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.222
- Springer Nature Limited
This article is cited by
-
Substitution of calcineurin inhibitors with corticosteroids after allogeneic hematopoietic cell transplantation
International Journal of Hematology (2023)
-
Clinical trials: design, endpoints and interpretation of outcomes
Bone Marrow Transplantation (2022)
-
Risk factors for adverse outcomes following haploidentical hematopoietic cell transplantation with posttransplant cyclophosphamide: a two-center analysis
Annals of Hematology (2022)
-
Negative impact of chronic graft-versus-host disease and glucocorticoid on the recovery of physical function after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2019)
-
Pharmacokinetic comparison of cyclosporin A and tacrolimus in graft-versus-host disease prophylaxis
Annals of Hematology (2017)