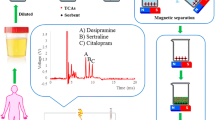
Abstract
In this study, an off-line coupling of dispersive solid-phase extraction (DSPE) and ion mobility spectrometry (IMS) was introduced to extract and determine bupropion (BUP). A magnetic nanocomposite adsorbent (Fe3O4@CuO&GO) was fabricated by combining graphene oxide (GO) sheets with Fe3O4 and CuO through coprecipitation method. The synthesized adsorbent was characterized and analyzed using the analytical techniques. The effect of extraction parameters including desorption solvent (type and volume), pH, adsorbent amount, contact time, temperature, and the volume of analyte solution on the extraction efficiency was investigated and optimized. The operational parameters of IMS method were also investigated. Under the optimum conditions (DSPE–IMS), the proposed method provided a linear range 4.0–24.0 ng for BUP with a determination coefficient R2 ≥ 0.98. LOD and LOQ values were 0.7 and 2.2 ng for BUP. The repeatability of proposed method was evaluated and reported as relative standard deviation (RSD% ≤ 5.5). The developed method was applied to determine BUP in different biological samples, in which satisfactory results were obtained (93.0–98.0%).
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bupropion (BUP) drug is an inhibitor of norepinephrine and dopamine reuptake, and also it acts as a receptor of nicotinic antagonist. The combination BUP and naltrexone was also approved by the Food and Drug Administration for the controlling of human weight. Furthermore, based on the clinical results, BUP can be consumed and affected in the drug abuse therapy and hyperactivity disorder due to attention deficit [1,2,3,4]. Various methods (chromatography, spectrometry, and electrochemistry) were utilized and reported for the BUP determination in different samples [4,5,6,7,8,9,10]. Although the above-mentioned methods provide good quantitative information, these are usually time-consuming, costly, require harmful solvents, and well-equipped laboratories with expert persons to the drug analysis. Therefore, there is still a necessary to develop sensitive, rapid, and simple methods for the determination of BUP in different matrices.
Magnetic solid-phase extraction (MSPE) is a new strategy of dispersive solid-phase extraction (DSPE) which has been significantly used in the sample preparation scope. In this technique, the magnetic nanoparticle (MNP) adsorbents are gathered simply by using a magnet [11]. Fe, Co, or Ni compounds are exploited [11, 12], as MNPs which among them Fe3O4 is the most commonly interested due to its suitable bioapplications and wastewater treatments, excellent paramagnetic properties, low toxic effect, low cost, and simple preparation [13, 14]. However, Fe3O4 MNPs easily accumulate in aqueous samples and this can decrease their surface areas and adsorption efficiency [15, 16]. Moreover, the oxidation reaction of Fe3O4 naked NPs leads to a phase change [17]. Combining Fe3O4 NPs with other metal oxides can be employed to eliminate or reduce the stated limitations [11, 18]. For example, Peng et al. combined Cu(OH)2 to Fe3O4 NPs through coprecipitation method, so that the new composite gave better results than Fe3O4 alone [19].
Nowadays, nanocarbon compounds have been widely entered as efficient adsorbents in solid-phase extraction (SPE) techniques because of properties such as their high surface area, and appropriate physical and chemical structure. Among that, graphene is a key and remarkable compound in material field due to its unique properties. To develop its hydrophilic properties and applications in water samples, graphene is converted to graphene oxide (GO) via an oxidation reaction. GO has been combined with some magnetic nanoparticles and widely used as a promising adsorbent in SPE studies [20].
Ion mobility spectrometry (IMS) is an analytical method for the identification and determination of trace analytes existing in various samples. Briefly, IMS is a specific separation technique for chemical compounds based on the ionic mobility of gases in a weak electric field at atmospheric pressure. The ion mobility is determined regard to its velocity in drift tube where the electric field is applied. During drift tube, the analyte ions separate according to their size, mass, shape, and charge. Consequently, the ions spread in drift tube and achieve to faraday cup (detector) at a range of drift times. The total numbers of ions are calculated for the analytes quantitative assay. IMS is applied to detect a wide ranging of compounds such as drugs, pollutants, and explosives. The rapid analysis time, simplicity, inexpensive, and high sensitivity are the major advantages of IMS [21, 22].
In this study, we proposed the DSPE technique for the extraction and preconcentration of BUP. The strategy of combining CuO with Fe3O4 NPs to place GO as adsorbent has been exploited for improving adsorption performance. The IMS method equipped with a corona discharge source was applied to determine the drug analyte. Various variables affecting the performance of DSPE and also IMS were studied and optimized. The developed method was validated, and finally, its capabilities were successfully confirmed through the BUP analysis in biological samples.
Experimental
Materials
The BUP tablets (150 mg) were obtained from Exir Pharmaceutical Co (Iran). The stock solution (100 µg/mL) was prepared in methanol. Iron (III) chloride (FeCl3), iron (II) sulfate (FeSO4), copper (II) chloride (CuCl2), sodium hydroxide (NaOH), and ethanol were purchased from Merck Co.
Instrumentation
In this research, an ion mobility spectrometer in positive mode (IMS − 400, TOF Tech. Pars Company, Iran) was used for quantitative drug analysis. An IMS cell (drift tube and a needle for generating the corona in ionization region), two high-voltage power supplies (drift and corona), a pulse generator, an analog-to-digital converter, and a computer for recording spectra are the main components of the IMS apparatus. The drift tube contained 11 Al–rings (OD: 55 mm and ID: 36 mm), and the ionization region also contained 5 Al–rings (OD: 55 mm and ID: 20 mm). The rings were connected to each other via a resistor (5 MΩ). The IMS cell is installed in an oven so that its temperature could be adjusted from 25 to 200 ºC. The injection port could be heated up to 250 ºC. Nitrogen was used as carrier and drift gases with flow rate of 300 mL/min and 600 mL/min, respectively [21]. The total peak area of analyte was integrated over the acquisition time (from the first to final peaks) and was considered as IMS response. The operational conditions of IMS (temperatures: injection and oven, flow rates: carrier and drift gas, voltages: corona and drift, and pulse width) were reported in the results section.
The adsorbent preparation (Fe 3 O 4 @CuO&GO)
The Hummers procedure with a minor change was served to synthesize GO [23]. Fe3O4@CuO&GO nanocomposite was prepared via a modified precipitation method in a N2 atmosphere. In accordance with the initial tests and by using digestion method, it is found that the mass ratio of 9% GO to 91% Fe and Cu was the optimal value for the preparation of composite adsorbent. Fe3O4@CuO&GO adsorbent was prepared as follows: GO was added to deionized water and mixed by ultrasonic vibration for 2 h. Then, the solutions of FeCl3 (0.1 M), FeSO4 (0.2 M), and NaOH (1.5 M) simultaneously and gradually were added into the reactor vessel. The mixture was stirred for 1 h, and then, the CuCl2 (0.1 M) and NaOH (1.5 M) solutions were simultaneously poured into the reactor. Next, the above solution was mixed for 1 h. After that, precipitation was separated and washed continuously with the solvent of deionized water and ethanol and freezing in half a day. Finally, solid products were crushed, sieved, and kept in a desiccator [24]. The prepared nanocomposite adsorbent was characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared (FT–IR) analysis methods.
DSPE procedure
In the preliminary testing of DSPE, 40 mg of adsorbent (Fe3O4@CuO&GO nanocomposite) was washed with 5 mL of methanol and 5 mL of deionized water, respectively. It was then added to 10 mL of a solution containing 10 ng of BUP at pH 8–9 and a homogenous suspension was established. The solution was mixed for 10 min and, after reaching to equilibrium, the adsorbents were collected using an external magnet at the bottom of a laboratory beaker. The drugs adsorbed on the nanocomposite materials were separated by 1 mL methanol (desorption solvent), and also it was shaken in an ultrasonic bath for 3 min. The adsorbents were then collected (as above) from the solution, and 1 µL of the extract liquid was injected into the IMS for drug analysis. In order to obtain the best extraction efficiency for BUP via DSPE method, various variables (desorption solvent, pH, adsorbent amount, contact time, temperature, and the analyte solution volume) affecting the performance of method were studied and optimized. Each analysis was repeated in three times.
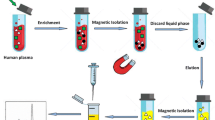
The real sample preparation
The drug-free human plasma and serum samples were obtained from Pars Laboratory (Yazd, Iran). For plasma sample preparation, first, the spiked sample (1 mL) was mixed with methanol (1 mL) that protein to precipitate out of solution. Then, the solution was vortexed and centrifuged at 4000 rpm for 6 min, and the supernatant was diluted with water (1:3, v/v). The solution pH was set by adding the solutions of HCl or NaOH (0.1 M), and the sample was subsequently treated with DSPE [25].
To prepare serum sample, briefly, 100 μL of the spiked sample was mixed well with 900 μL of methanol and followed by centrifugation for 10 min at 4000 rpm. The supernatant of the sample solution (containing the analytes) was taken and diluted with 3 mL of deionized water. Finally, the sample was treated by DSPE for the cleanup and extraction [26].
Results and discussion
Characterization of Fe 3 O 4 @CuO&GO
The XRD technique was used to confirm the crystalline structure of Fe3O4@CuO&GO; this pattern (Fig. 1) showed the diffraction peaks at 2θ values: 30.1°, 35.6°, 43.1°, 53.9°, 57.0°, 62.7° which agreed to (220), (311), (400), (422), (551), and (440) diffraction planes, respectively (JCPDS card no. 19–0629) [27]. The XRD results also showed that the core of Fe3O4 nanoparticles did not change during anchoring onto the surface of GO. Because the CuO species are highly dispersed, its peaks were not appeared in XRD pattern. [28]
The surface morphology of Fe3O4@CuO&GO was studied by using SEM (Fig. 2). This nanocomposite presented a flake-like structure in which CuO and Fe3O4 nanoparticles were distributed homogeneously all over the surface of the GO. In this figure, the Fe3O4 and CuO were seen as the dark and lighter color points on the GO surface [29].
The FT-IR spectra of Fe3O4@Cu&GO (before and after adsorption) were obtained (Fig. 3). The absorption band at 3420 cm−1 was attributed to stretching mode of O–H, and also a band was observed at 1126 cm−1 for C-O groups [30]. The peak at 1625 cm−1 may be assigned to the skeletal vibrations of graphitic domains of GO. The absorption band at 566 cm−1 can be attributed to Fe–O/Cu–O; it proved the presence of Fe3O4/CuO [31]. As seen in FT-IR spectra (Fig. 3), after adsorption, the absorption band in 1625 cm−1 has been shifted to 1636 cm−1. Regard to this change and also the molecular structure of drug and adsorbent, the mechanism of sorption can be related to π–π interactions among the oxygen and nitrogen groups between π electron donors and π electron acceptors from the aromatic parts (C = C groups) of BUP and Fe3O4@CuO&GO adsorbent. A slight shift of peak at 1126 to 1128 cm−1 by FT-IR investigation in before and after adsorption was attributed to C–OH bond that confirmed the H-bonds. Furthermore, this migration was also indicated the involvement of OH groups in the adsorption.
IMS analysis
In the present IMS work, the spectral behavior of BUP was studied at different instrumental conditions (corona voltage, 2200–2500 V; drift voltage, 5000–8000 V; injector port temperature, 170–230 ºC; IMS cell temperature 150–200 ºC). In the positive mode of IMS, the ionization mechanism is based on proton transfer from (H2O)nH3O+ (main reactant ion) to analytes that have at least one basic atom as proton acceptor. Based on observations, it is revealed that corona discharge source generated only the protonated molecule [M + H]+ from BUP in IMS and did not appear any the fragment ion peak. To achieve the best sensitivity, the all variables (Table 1) were investigated and optimized. The optimum conditions for the determination of drug are present in Table 1. According to Fig. 4a (the standard IMS spectrum), the product ion peak of BUP (pure) appeared in 8.8 ms with the reduced mobility value (Ko = 1.32 cm2 V−1 s−1, conditions: Table 1). It was completely separate and far from the reactant ion peaks of NH4+, NO+, and H3O+ (4.0–6.2 ms), respectively. [21, 32] There is not any reported Ko for product ion originated from BUP in research articles. The Ko value of (NH4+) [33, 34] was used as a reference to compare the drift times. According to our calculations, it was confirmed that the observed peak in 8.8 ms was attributed to the product ion of BUP in IMS. The standard addition method was also performed using the high-purity BUP. The peak height in 8.8 ms was increased, and any other product ion peak was not appeared.
DSPE optimization
In order to achieve the efficient extraction recovery using the proposed method, the experimental variables affecting the recovery including desorption solvent (type and volume), pH, adsorbent amount, extraction time and temperature, and the volume of analyte solution must be optimized. The extraction recovery is defined as the ratio of extracted analyte (found) to the amount of added analyte. The initial investigations showed that the parameters (variables) of DSPE technique were not coupling each other and can be optimized independently using one factor at a time.
Effect of desorption solvent
The type of desorption solvent is an important parameter in the extraction studies. The common organic solvents including acetonitrile, methanol, dichloromethane/acetonitrile, acetonitrile/methanol were used as the desorption solvents to elute the target analyte from adsorbent. The best extraction recovery of BUP was obtained with acetonitrile or methanol. In the reported conditions (Table 1), acetonitrile shows peak in IMS which can interfere to determine analytes. Methanol is a suitable solvent in the IMS measurements (without peak); therefore, it was chosen as desorption solvent for the present work. The effect of methanol volume on the extraction efficiency was investigated in range of 0.5–2 mL. As shown in Fig. 5, the recovery was increased until 1 mL, and then, it was decreased due to dilution effect. Therefore, 1 mL was chosen as the optimum volume that can be used to desorb analyte completely from adsorbent in the studied concentration range.
Effect of pH value
The pH of solution is an important parameter in adsorption process for the reason that it affects the form of analytes. Figure 6 shows the extraction recovery results of BUP in the pH range of 5.9 to 10.9. The improving recovery of BUP was seen with increasing the pH, and the maximum result was obtained at about pH = 7.9. This trend has been also reported by Stumm et al. [35] They concluded that the maximum adsorption between the anions of weak acid and metal oxides was observed usually about pKa1 (for BUP = 7.9). As shown in Fig. 6, when low pH values were used, the low recovery observed may be due to the nitrogen or oxygen atoms of BUP protonated. Also, in the high pH values, the low results can be related to compete OH− anions and BUP for capturing the active sites of adsorbent. [36, 37]
Effect of the amount of adsorbent
It is an importance matter to reach the best extraction recovery with low adsorbent amount as possible. Therefore, the most efficient adsorbent amount of Fe3O4@CuO&GO suspension for DSPE processes is necessary. To test this parameter and to obtain the optimum value, a series of experiments were performed with different amounts of adsorbent (15 to 50 mg). As shown in Fig. 7, the analyte adsorption was increased with increasing the amount of adsorbent until to the 40 mg. After that, the extraction recovery of BUP was no more increased. Therefore, 40 mg of Fe3O4@CuO&GO was selected as the optimum value for further experiments.
Effect of extraction time
In DSPE technique, the extraction (adsorption) time is an important parameter. The effect of this parameter was also studied over a period of 5 to 20 min. As shown in Fig. 8, the maximum extract of BUP has been obtained in 15 min. This suggested a rapid extraction of BUP using DSPE. Then, a decrease in adsorption recovery was observed with increasing the extraction time due to competition between adsorption and desorption processes. Therefore, 15 min was selected as the optimum extraction time in which the adsorption process has been achieved to the equilibrium.
Effect of extraction temperature
The effect of extraction temperature on the recovery was investigated in range of 20 to 40 ºC. The results shown in Fig. 9 explained that with increasing temperature up to 25 ºC, due to increase in the kinetic energy of analytes, the adsorption recovery was enhanced. Then, it was reduced due to the analyte desorption from the adsorbent surface. Therefore, this value was selected as the suitable temperature for next experiments to maintain high extraction efficiency.
Effect of the volume of analyte solution (enrichment factor)
The enrichment factor of the proposed method was considered by changing the volume of solution containing the BUP drug (10 ng) in range of 10 to 50 mL. According to Fig. 10, it was clear that with increasing the volume of analyte solution from 10 to 20 mL, the recovery results stayed constant, and then, at higher volumes it decreased. Therefore, 20 mL was chosen as the maximum volume of analyte solution in which the DSPE recovery was still acceptable. This value was also used to calculate the enrichment factor (= 20) of the proposed method.
Method validation
Under optimum conditions (Table 1), a number of parameters have been studied for evaluating the developed DSPE–IMS method, including linear dynamic range (LDR), determination coefficient (R2), detection limit (LOD), quantitative limit (LOQ), repeatability or precision (RSD), and accuracy. The evaluation results are present in Table 2. The calibration curve of BUP was linear in the range of 4.0–24.0 ng, with a good coefficient of determination (R2 > 0.98) in two segments (Fig. 11). In higher amounts (> 24 ng), the signal saturation and also the dimerization of analytes can happen, and this condition should be avoided in quantitative studies. The LOD and LOQ values of the proposed method were calculated using Eqs. 3 Sb/m and 10 Sb/m, respectively, where Sb was the standard deviation of the blank signal intensity and m was the slope of the calibration curve. The LOD and LOQ values were 0.7 and 2.2 ng for BUP, respectively. The repeatability of the proposed method for the analyte determination was also valued that was obtained 5.5%. The result of recovery test (accuracy) was 98.0%; in comparison with the synthesized nanocomposite Fe3O4@GO, our previous work, it has been increased about 8.0%. This improvement can be related to the synergetic coupling effect of GO and CuO in the magnetic nanocomposite. Finally, the reusability of Fe3O4@CuO&GO nanosorbent was done using methanol (as desorption solvent) and confirmed until 5 successive adsorption–desorption cycles.
Graph of the calibration curve for BUP (conditions: Table 1)
Method applications
The sample matrix effects reduced the selectivity of IMS. Therefore, a separation technique (DSPE) was necessary in quantitative analysis of BUP in real samples before the IMS analysis. Furthermore, the standard addition method was used to deal with the matrix effect imposed by the proposed DSPE.
In order to assign the potential and accuracy of the proposed method, the recovery tests of drug were followed by adding various amounts (6.0 to 20.0 ng, n = 3) of the standard solutions from BUP to the real samples (plasma and serum). Under optimum conditions (DSPE–IMS), the drug analyte was extracted and then was injected into the IMS. The blank samples (plasma and serum) were tested and analyzed that was demonstrated the proposed DSPE procedure could provide the clean extracts with no extra peaks being observed within the region where the analyte peak appeared. The ion mobility spectrum of the extracted BUP of the spiked biological samples is shown in Fig. 4b (for plasma). Figure 4 displays that the IMS spectra of BUP in standard and sample solutions are similar and the product ion (for both) is appeared in 8.8 ms. The standard addition method also demonstrated that BUP can be selectively adsorbed and concentrated by Fe3O4@CuO&GO in real samples. The recovery data reported in Table 3 (recovery: 93.0–98.0%, RSD% = 6–8%) confirmed the capability of developed method for the quantitative determination of BUP in real samples.
The analytical parameters of DSPE–IMS method were comparable to those of other existing methods for the determination of BUP in various matrices (Table 4). According to Table 4, the analytical parameters of the DSPE–IMS method are better or comparable to those of other methods introduced for the determination of BUP. Furthermore, this method is simple, fast, and low-consuming hazardous solvents and also inexpensive equipment.
Conclusions
In this work, off-line coupling a magnetic Fe3O4@CuO&GO nanocomposite as an adsorbent for DSPE and IMS was developed to determine BUP. The DSPE–IMS method offered advantages including the use of small amounts of adsorbent, low organic solvent consumption, good LOD (0.7 ng) and LOQ (2.2 ng) values, a relatively wide LDR (4.0–24.0 ng), and acceptable recovery results (98.0%) in comparison with other methods. Also, the proposed method could be successfully exploited to determine BUP in human plasma and serum as biological samples (recovery: 93.0 − 98.0%, RSD: 6–8%).
Data availability
All data underlying the results are available as part of the article and no addition source data are required.
References
S.M. Stahl, J.F. Pradko, B.R. Haight, J.G. Modell, C.B. Rockett, S. Learned-Coughlin, Prim Care Companion J. Clin. Psychiatry 6(4), 159 (2004)
L.P. Dwoskin, A.S. Rauhut, K.A. King-Pospisil, M.T. Bardo, CNS Drug Rev. 12(3–4), 178 (2006)
H.J. Aubin, A. Luquiens, I. Berlin, Br. J. Clin. Pharmacol. 77(2), 324 (2014)
A.R. Masters, M. McCoy, D.R. Jones, Z. Desta, J. Chromatogr. B. 1015, 201 (2016)
D.H.K. Reddy, Y.S. Yun, Coord. Chem. Rev. 315, 90 (2016)
K.K. Loboz, A.S. Gross, J. Ray, A.J. McLachlan, J. Chromatogr. B. 823(2), 115 (2005)
M. Castro-Puyana, M.Á. García, M.L., J. Chromatogr. B. 875(1), 260 (2008)
S. Jafari, M. Dehghani, N. Nasirizadeh, M. Azimzadeh, F.D. Banadaki, Bull. Mater. Sci. 44(1), 1 (2021)
I. Halkevych, L. Ivanauskas, Y. Bidnychenko, Z. Barsteigiene, Chem. Chem. Technol. 9(2), 245 (2015)
S.A. Abdel-Gawad, R.M. El-Gamal, Saudi Pharma. J. 26(2), 169 (2018)
K.K. Kefeni, B.B. Mamba, T.A.M. Msagati, Sep. Purif. Technol. 188, 399 (2017)
J. Gómez-Pastora, E. Bringas, I. Ortiz, Biochem. Eng. J. 256, 187 (2014)
J. Gómez-Pastora, S. Dominguez, E. Bringas, M.J. Rivero, I. Ortiz, D.D. Dionysiou, Chem. Eng. J. 310, 407 (2017)
S. Lata, S.R. Samadder, J. Environ. Manage. 166, 387 (2016)
D. Ramimoghadam, S. Bagheri, S.B. Abd Hamid, Colloids Surf. 133, 388–411 (2015)
K. Kalantari, M.B. Ahmad, K. Shameli, M.Z.B. Hussein, R. Khandanlou, H. Khanehzaei, J. Nanomater. 2014, 181 (2015)
L. Chen, C.H. Zhou, S. Fiore, D.S. Tong, H. Zhang, C.S. Li, S.F. Ji, W.H. Yu, Appl. Clay Sci. 127–128, 143 (2016)
B. Peng, T. Song, T. Wang, L. Chai, W. Yang, X. Li, C. Li, H. Wang, Chem. Eng. J. 299, 15 (2016)
C. Cha, S.R. Shin, N. Annabi, M.R. Dokmeci, A. Khademhosseini, ACS Nano 7(4), 2891 (2013)
R. Sitko, B. Zawisza, E. Malicka, TrAC-Trends. Anal. Chem. 51, 33 (2013)
M. Tabrizchi, V. ILbeigi, J. hazard. Mater. 176(1–3), 692 (2010)
T. Keller, A. Keller, E. Tutsch-Bauer, F. Monticelli 161(2–3), 130 (2006)
W.S. Jr Hummers, R.E. Offeman, J. Amer. Chem. Soc. 80(6), 1339 (1958)
K. Wu, C. Jing, J. Zhang, T. Liu, S. Yang, W. Wang, Appl. Surf. Sci. 466, 746 (2019)
M. Safari, M. Shahlaei, Y. Yamini, M. Shakorian, E. Arkan, Anal. Chim. Acta 1034, 204 (2018)
T.M. Alshammari, A.A. Al-Hassan, T.B. Hadda, M. Aljofan, Saudi Pharm. J. 23(6), 689 (2015)
S. Liu, Q. Xu, S. Latthe, A. Gurav, R. Xing, R.S.C. Adv. 5, 68293 (2015)
Y. Jain, M. Kumari, R.P. Singh, D. Kumar, R. Gupta, Catal. Lett. 150(4), 1142 (2020)
A.Q. Alorabi, M.S. Hassan, M. Azizi, Arab. J. Chem. 13(11), 8080 (2020)
S. Stankovich, R.D. Piner, S.T. Nguyen, R.S. Ruoff, Carbon 44(15), 3342 (2006)
M. Kidwai, V. Bansal, N.K. Mishra, A. Kumar, S. Mozumdar, Synlett 2007(10), 1581 (2007)
T. Khayamian, M. Tabrizchi, M.T. Jafari, Talanta 69, 795 (2006)
Y. Valadbeigi, V. Ilbeigi, A. Afgar, M. Soleimani, Int. J. Mass Spectrom. 470, 116699 (2021)
T. Sun, M. Mujahid Ali, D. Wang, Z. Du, J. Chromatogr. A. 1610, 460547 (2020)
W. Stumm, J. Morgan, Particle-particle interaction: colloids, coagulation, and filtration Aquatic Chemistry, 3rd edn. (John Wiley, New York, 1996), p.818
Z. Ren, G. Zhang, J.P. Chen, J. Colloid Interf. Sci. 358(1), 230 (2011)
B. Shao, Y. Guan, Z. Tian, X. Guan, D. Wu, Colloids Surfac. A. 506, 703 (2016)
Acknowledgements
This work was financially supported by Yazd Branch, Islamic Azad University (IAU), Yazd, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shamsi, F., Sheibani, A. & Shishehbore, M.R. Determination of bupropion by off-line coupling Fe3O4@CuO&GO nanocomposite and ion mobility spectrometry with application to biological samples. ANAL. SCI. 39, 1521–1529 (2023). https://doi.org/10.1007/s44211-023-00371-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-023-00371-z