Abstract
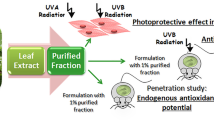
In this work, we studied the potential photoprotective effect of Ipomoea horsfalliae Hook., Convolvulaceae, flower extract. Ipomoea horsfalliae is a plant that grows in tropical and subtropical regions. I. horsfalliae ethanolic extracts were analyzed by ultra-high efficiency liquid chromatography–high-resolution mass spectrometry. Dicaffeoylquinic acid, chlorogenic acid, scopoletin, glycosylated cyanidin, pelargonidin, and kaempferol were identified as major components of I. horsfalliae flower extract. In vitro biossays were used to evaluate cytotoxic and sensitizing effects of the extracts, and their photoprotective effect was evaluated in BALB/c mice. Morphological and histopathological observation of the skin tissues from mice suggested that UV-B-induced edema was significantly inhibited by treatment with I. horsfalliae flower extract. It was not cytotoxic for both cancerous and normal cells, and no sensitizing effect was observed. I.horsfalliae flower extract appears to be a good starting point for research programs leading to the development of natural skin care products.

.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultraviolet (UV) radiation can cause cancer, premature aging, sunburns, and wrinkles. Skin cancer is a malignant tumor disease common in fair-skinned, light-eyed people, with blond hair (Ferlay et al. 2019). There is a permanent need for protection from UV radiation and prevention from its side effects. Currently, there is a strong tendency to use biodegradable and safe natural products in formulations that could prevent skin cancer. Herbal preparations have a high potential due to their antioxidant activity. Their phenolic compounds can scavenge reactive oxygen species, reduce skin alterations caused by UV exposure, and prevent aging (Estrella-Parra et al. 2019).
Ipomoea genus comprises ca. 700 species distributed in tropical and subtropical regions (Meira et al. 2012). Ipomoea horsfalliae Hook., Convolvulaceae, called “morning glory,” has simple and dark green alternate leaves; its inflorescences have deep fuchsia color and grow on the terminal part of the branches (Delgado et al. 2014). Ipomoea spp. have applications in medicine, due to their hypotensive, antimicrobial, anticancer (Bieber et al. 1986), and antidiabetic properties (Kusano and Abe 2000). The compounds responsible for the biological activity of these species include flavonoids, coumarins, isocoumarins, benzenoids, anthocyanins, glycolipids, and lignans (Truong et al. 2007; Meira et al. 2012; Batiga et al. 2019).
There is a high incidence of skin cancer in Colombia, and melanoma cancer (MC) is the most common type. In 2018, MC caused 518 deaths and 1907 new cases were registered (The Global Cancer Observatory 2019). Twenty-one species of Ipomoea genus are recognized as medicinal plants in Colombia (Bernal et al. 2011); their potential as primary sources of chemicals with protective effect against UV has not been studied yet. This study evaluated the potential of I. horsfalliae flower extract (IHFE) as a starting point for research programs that led to the development of natural skin care products. Chemical characterization, in vitro cytotoxic and sensitizing effects, and in vivo photoprotective effect of IHFE were evaluated.
Materials and Methods
Chemicals
Cyanidin-3-rutinoside, delphinidin-3-glucoside, and kaempferol-3-glucoside were purchased from PhytoLab (Vestenbergsgreuth, Germany). Kaempferol, chlorogenic acid, MTT 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, 2-aminophenol, p-formaldehyde, and eosin were purchased from Sigma-Aldrich (St. Louis, USA). Parsol® was purchased from Alfadelta S.A. of C.V. (Naucalpan de Juárez, Mexico).
Plant Material
Ipomoea horsfalliae Hook., Convolvulaceae, flowers were collected from an experimental plot at CENIVAM (N 07° 08,422′ W 073° 06,960′) in March 2017. Voucher specimen (COL 587134) was deposited at the Colombian National Herbarium, National University of Colombia. Undamaged, fully developed flowers were dried in a VirTis AdVantage Plus tray lyophilizer (Gardiner, USA).
Solvent Extraction
Dried flowers (1 g) were mixed with an ethanol solution (20 ml, 0.5% HCl, 1:1 v/v) and deposited for 5 min in an ultrasound bath (Elmasonic S15H, Singen, Germany). The mixture was filtered, and the residue was extracted twice more. IHFE was rotoevaporated and then dried in a VirTis AdVantage Plus tray lyophilizer.
UHPLC-ESI+-Orbitrap-MS Analysis
Flower extracts were analyzed on an UHPLC Dionex™ UltiMate™ 3000 (Thermo Fisher Scientific TFS, Bremen, Germany), coupled to an Orbitrap™ mass detector (Exactive Plus, TFS, Bremen, Germany), using a heated-electrospray interface (HESI-II), operated in positive ion mode (350 °C). Separation used a Hypersil GOLD™ aQ column (TFS, Sunnyvale, USA), of 100 mm × 2.1 mm id, × 1.9 μm of particle size, at 30 °C. The mobile phase was as follows: A: water (0.2% formic acid) and B: acetonitrile (0.2% formic acid). Analysis started with 100% A and changed linearly up to 100% B in 8 min, remained for 4 min, then returned to 100% A in 1 min, where it remained in equilibrium for 3 min. Flow was 0.3 ml/min and the injection volume 1 μl. Capillary voltage (3.5 kV, 320 °C) and higher energy collisional dissociation (HCD) were used. Mass range in all experiments was set at m/z 80–1000. The data obtained was processed with the Thermo XCalibur™ Roadmap software, version 3.1.66.10. Compound identification was based on the extracted ion current (EIC), the exact masses of the protonated target compounds, and by comparison with certified standards.
Cytotoxicity Assay
MTT assay (Mosmann 1983) was used to evaluate the potential of IHFE to reduce the viability of human cells. Six cell lines were selected (Table S1, Supplementary material), which represented cells from hepatocellular carcinoma (HepG-2), leukemia (THP-1), normal kidney (HEK-293), normal lung (MCR-5), and xeroderma pigmentosum (XP4PA and XP12RO-SV). Confluent monolayers of cells in 96-well cell culture microplate were treated with IHFE at concentrations in the range of 50 to 500 μg/ml for 3 days at 37 °C in a 5% CO2 humidified atmosphere. Non-treated cells were run in parallel. MTT (5 mg/ml) was added to each well (20 μl), and the microplate was kept at 37 °C for 4 h. Dimethylsulfoxide (100 μl) was added, and absorbances were measured at λ = 580 nm using a plate reader. A dose-response curve was plotted, and the half-maximal cytotoxic concentration (CC50) was determined from the plot.
In Vitro Test to Screen Skin Sensitizers in IHFE
The production of interleukin (IL)-8 by stimulated THP-1 cells is used as a biomarker of sensitizing effect (Takahashi et al. 2011); in the present study, the protocol described by Parise et al. (2015) was followed. THP-1 cells were seeded at a density of 2.5 × 105 cells/ml in 24-well plates and were incubated in culture medium with or without IHFE at a non-cytotoxic concentration of 80 μg/ml for 24 h at 37 °C, 5% CO2. Cells incubated in culture medium with 50 μM of 2-aminophenol were run in parallel. 2-Aminophenol is recognized as a strong sensitizer (Parise et al. 2015). The supernatants from cell cultures were collected for the determination of IL-8 by using an ELISA Kit (Invitrogen™). Three independent experiments in duplicate were carried out.
Protective Effect Against Skin Damage Induced by UV-B
Photoprotective activity was measured, according to Estrella-Parra et al. (2019), with some modifications. The procedure was performed with 20 BALB/c mice, from 4 to 6 weeks of age, and a weight of 16 ± 2 g. The mice were depilated with Nair™ sensitive skin cream (Church and Dwight, Princeton, USA) 24 h before starting the experiments.
UV-B exposure acute effect on mouse skin was studied in A, B, C, and D groups of five individuals. Groups A and B were treated with ethanol applied topically (100 μl, 70% v/v). Groups A and C were not irradiated. In groups C and D, the dorsal area of each mouse was divided into two and Parsol® (100 μl, 30 mg/ml) and IHFE (100 μl, 30 mg/ml) were applied on the left and right zones, respectively.
Groups B and D were irradiated for 3 min with a Spectroline EB-280C UV-B lamp (λ = 312 nm), located 15 cm away, with an irradiation dose of 6 mJ/cm2. This procedure was repeated three times, every 24 h. All mice were sacrificed by asphyxia in a CO2 chamber, 48 h after receiving their last radiation dose.
Histological Analysis
Histological analysis was performed according to Estrella-Parra et al. (2019). Samples were placed on cassettes for histology and suspended in a p-formaldehyde solution (500 ml, 2% w/v in phosphate buffer, 0.1 M, pH 7.2) for 24 h. They were washed with water (3 h), dehydrated in ethanol solutions (500 ml, 70, 80, 96, or 100% v/v) for 2 h, and included in paraffin. Samples of 5 μm thickness were cut in a Leica® RM2125RT rotation microtome (Leica Biosystems, Wetzlar, Germany). Histological sections were stained with hematoxylin and eosin (H&E) and were observed in a Leica® DM500 optical microscope.
Data Analysis
R software for Windows (version 3.5.2, http://www.R-project.org) was used. The dose-response curve was plotted, and the half-maximal cytotoxic concentration (CC50) was calculated. Results were expressed as the means ± standard error of the mean (SEM) from duplicate assays of independent experiments. Levels of significance were calculated by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc and Student’s t tests.
Results
UHPLC-ESI+-Orbitrap-MS Analysis of IHFE
The extracted ion currents of the exact masses of [M]+ or [M + H]+ of compounds present in IHFE were obtained from the total ion current (TIC) (Fig. 1). In Table 1, the experimental exact masses of M+ and [M + H]+ identified in IHFE are shown together with the criteria used for compound identification, and with the main ion-fragments obtained at the HCD under different collisional energies. In Fig. 2, mass spectra of kaempferol-diglucoside and pelargonidin-sophoroside-glucoside are shown. The fragmentation pattern of these compounds is characterized by the consecutive loss of sugar moieties from [M + H]+ ions.
Ipomea horsfalliae flower extract chromatogram (extracted ion chromatogram, EIC), obtained by UHPLC-ESI+-Orbitrap-MS. Peak identification appears in Table 1
Table 2 presents the linearity and sensitivity of the method used to quantify phenolic compounds by UHPLC-ESI+-Orbitrap-MS. Cyanidin-3-rutinoside, delphinidin-3-glucoside, kaempferol, kaempferol-3-glucoside, and chlorogenic acid were used as external standards. The analytical method had low detection (LOD = 0.1–0.3 μg/ml) and quantification (LOQ = 0.2–1 μg/ml) limits. Coefficients of determination (R2 = 0.9896–0.9990) demonstrated good method linearity in the range of concentrations evaluated (2–10 μg/ml).
Cytotoxicity
IHFE did not exhibit relevant cytotoxicity for both cancerous and normal cells according to dose-response curves (Fig. S1, Supplementary material) and the World Health Organization parameter (WHO 2019). CC50 values were higher than 200 μg/ml for cells derived from hepatocellular carcinoma (HepG-2, 270 ± 80 μg/ml), monocytic leukemia (THP-1 > 500 μg/ml), normal kidney (HEK-293, 340 ± 110 μg/ml), and normal lung (MRC-5, 250 ± 50 μg/ml). For XP cells, CC50 values were lower compared with normal cells (XP4PA, 110 ± 20 μg/ml; XP12RO-SV, 63 ± 5 μg/ml), which was expected since the cells were deficient in a key DNA repair protein, and therefore, they were more sensitive to treatment with IHFE (Maher et al. 1977).
Sensitizing Effect
Allergic contact dermatitis results from T cell–mediated immune responses induced by compounds called sensitizers. When THP-1 cells were stimulated with the representative sensitizer, 2-aminophenol, the level of IL-8 in the culture medium increased (from 1.079 ± 44 to 247 ± 77 pg/ml in non-stimulated cells; p < 0.01, ANOVA). In contrast, THP-1 cells stimulated with IHFE at a concentration of 80 μg/ml did not increase the IL-8 production (204 ± 54 pg/ml) which suggests the lack of potential sensitizing effect at the concentration tested.
Protective Effect of IHFE Against Skin Damage Induced by UV-B
Figure 3 illustrates the extent of histological skin lesions of BALB/c mice from group B, treated with ethanol (70% v/v) and UV-B. The number of lesions with an incidence of 100% was higher than that in group A, treated with ethanol and no UV-B exposure. Fusiform nuclei with focal extension were evidenced in the stratum corneum. Cells of burn, hyperplasia, hypertrophy, atrophy, and pleomorphism were manifested in the epidermis of all organisms (Fig. 4). Skin, exposed to UV radiation, accelerated mitotic activity, and the number of layers in the epidermis increased. A higher number of congested blood vessels, extravasation of erythrocytes, and polymorphonuclear cells were observed in the dermis.
Extension of lesions evaluated in the skin of the BALB/c mice treated with ethanol (70%, v/v), Parsol®, or Ipomea horsfalliae flower extract (3 mg/ml), exposed to UV-B. The extension is focal (0 to 20%), multifocal (20 to 50%), or diffuse (50 to 100%). Pk, parakeratosis; Hk, hyperkeratosis; Sp, spongiosis; BC, burn cells; Hp, hyperplasia; Ht, hypertrophy; At, atrophy; Pl, pleomorphism; Ed, edema; Hg, hemorrhage; IF, inflammatory infiltrates; CG, congestion
BALB/c mouse skin histological sections of the group treated with ethanol (70% v/v, a and c) or Ipomea horsfalliae flower extract (3 mg/ml) and UV-B (d and e). H&E stain. SC, stratum corneum; E, epidermis; BC, burn cells; Ht, hypertrophy; At, atrophy; Pl, pleomorphism; D, dermis; SG, sebaceous gland; HF, hair follicle; RF, reactive fibroblasts; BV, blood vessel; M, muscle; IF, inflammatory infiltrates; H, hypodermis
The extent of the histological lesions in the individuals treated with Parsol® or IHFE was lower than that in the group without protection (Fig. 3). The group with IHFE or Parsol® showed significant differences, according to Student’s t test, in comparison with the group treated with ethanol and UV-B. Stratum corneum presented normal appearance, contained flattened cells that lacked nuclei and organelles. In the epidermis, round burn cells were detected, with contracted nuclei and eosinophilic cytoplasm; the extension of these cells was multifocal and smaller than that of the group without protective substance. IHFE caused changes in cell growth in the epithelium (Fig.4); multifocal atrophy (30%), hypertrophy (5%), and focal hyperplasia (11%) were observed. Parsol® changed the size and shape of epithelial cells, which caused focal pleomorphism (8%) and multifocal atrophy (24%). There was no edema in the dermis of individuals covered with IHFE or Parsol®. In connective tissue, congestion of blood vessels (50%), erythrocyte extravasation (30%), and polymorphonuclear number (54%) were higher in mice treated with Parsol® than in those treated with IHFE.
Discussion
Evaluation of the cytotoxic effect for cultured cells is a preliminary approach to predict organ-specific toxicity, and the first step of the discovery process ending with a new natural anticancer product (Zhang et al. 2007). A standardized reference value of CC50 is not available to estimate the cytotoxic potential of plant extracts. It has been proposed that extracts with CC50 > 90 μg/ml could be classified as non-cytotoxic, while extracts with CC50 < 90 μg/ml as moderately or highly cytotoxic according to the WHO (2019). The US National Cancer Program suggests a cut-off for the effective dose 50 of 30 μg/ml in cell-based assays used to select plant extracts with potential anticancer effect (WHO 2019). IHFE seems not to have the potential to kill the cancerous cells tested in this study since it showed CC50 values > 250 μg/ml. Nevertheless, the result does not exclude the potential effect on other cancer cells. In addition, IHFE did not exhibit a relevant cytotoxic effect on cells derived from both normal kidney and lung, which are target cell models used in predictive toxicology testing. Consequently, IHFE might be a good candidate for research on natural products for human health. We recognize that our preliminary cytotoxic analysis of IHFE is limited; further studies with accurate and relevant tests should be carried out.
Many compounds in plant extracts can function as sensitizers because of their immunomodulatory effect. In three independent experiments, the levels of IL-8 in IHFE-stimulated and non-stimulated THP-1 cells were similar, which suggests the absence of a sensitizing effect. We could speculate that phenolic acids in IHFE could be responsible for the absence of IL-8 production. These phytochemicals displayed anti-inflammatory properties downregulating the NF-κB pathway signaling involved in the IL-8 gene transcription (Liu et al. 2018). A standardized reference value of effective dose 50 in the THP-1/IL-8 assay is not available to estimate the sensitizing effect of plant extracts. Consequently, further analysis with accurate and relevant tests such as the mouse local lymph node assay (LLNA) and human the cell activation test (h-CLAT) are required to investigate the presence of chemical sensitizers in IHFE.
Some compounds isolated from plants are used as sunscreens because they are antioxidants and absorb UV radiation (Radice et al. 2016). In IHFE, several anthocyanins were detected; cyanidin, pelargonidin, and their glycosylated derivatives have been recognized as antioxidants and photoprotective agents (Afaq et al. 2005). Glycosylated compounds of cyanidin, pelargonidin, and delphinidin were found in pomegranate fruits and showed a protective effect against skin changes in SKH-1 mice, induced by UV-B, and acted as modulators of photocarcinogenesis biomarkers (Afaq et al. 2005; Afaq et al. 2010).
Dicaffeoylquinic acid, a major component of IHFE, is an ester of caffeic and quinic acids (Truong et al. 2007). Caffeic acid protects the cells from the cytotoxic effect caused by UV-C; its photoprotective activity was evidenced in fibroblasts and epidermoid carcinoma cells. Proliferation of these cells exposed to UV-C was higher in the presence of caffeic acid (56 or 167 μM) compared with the control (Neradil et al. 2003). Caffeic acid esters act as sunscreens; they are stable against UV-A or UV-B radiation and have a sun protection factor of ca. 93% (Rivelli et al. 2010).
Scopoletin, found in IHFE, has been recognized for its anti-inflammatory (Moon et al. 2007), antioxidant (Parra et al. 2018), and vasodilator (Kwon et al. 2002) activities. Coumarin derivatives reduced the embryotoxic effects of UV-B radiation in sea urchin gametes and have been considered as possible photoprotectors (de Araujo Leite et al. 2015).
Results from this study suggest that the topical application of IHFE can prevent acute inflammatory response, reducing the number of burn cells, inflammatory infiltrates, and can prevent also intercellular edema. Some studies have shown the protective effect of phenolic compounds detected in IHFE, on the skin carcinogenesis induced by UV. Caffeoylquinic acid, quercetin-rhamnoside, and glycosylated compounds of kaempferol, present in Prunus persica (L.) Batsch flower extract, inhibited the increase in the number of layers of the epidermis and prevented oxidative deterioration by enzymatic modulation of superoxide dismutase and glutathione peroxidase (Kwak et al. 2018).
Conclusion
IHFE could be considered a good starting point for research programs which seek to develop natural skin care products. Topical application of IHFE on mouse skin reduced acute inflammation caused by exposure to UV-B. Phenolic acids, coumarins, flavonols, and anthocyanins could be responsible for the photoprotective effect of IHFE. Some limitations of this study need to be considered. IHFE toxicity and skin sensitization potentials need to be investigated using accurate and relevant tests, and the mechanistic explanation of the photoprotective effect should be provided. Despite these limitations, the present study gives valuable information on the potential use of I. horsfalliae flower extracts.
References
Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H (2005) Anthocyanin and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-B pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer 113:423–433. https://doi.org/10.1002/ijc.20587
Afaq F, Khan N, Syed DN, Mukhtar H (2010) Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem Photobiol 86:1318–1326. https://doi.org/10.1111/j.1751-1097.2010.00815.x
Barnes JS, Schug KA (2011) Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometry. Int J Mass Spectrom 308:71–80. https://doi.org/10.1016/j.ijms.2011.07.026
Batiga S, Valli M, Zeraik ML, Fraige K, Leme GM, Pitangui NS, Almeida AMF, Michel S, Young MCM, Bolzani VS (2019) Chemical composition and biological properties of Ipomoea procumbens. Rev Bras Farmacogn 29:191–197. https://doi.org/10.1016/j.bjp.2018.08.010
Bernal HY, García MH, Quevedo SF (2011) Guidelines for knowledge, conservation and sustainable use of native medicinal plants in Colombia: national strategy for plant conservation, first ed. Ministry of Environment, Housing and Territorial Development and Research Institute of Biological Resources Alexander von Humboldt, Bogotá
Bieber LW, Da Silva Filho ÁA, Corréa RM, Chiappeta ADA, Do Nascimento SC, De Souza IA, De Méllo JF, Veith HJ (1986) Anticancer and antimicrobial glycosides from Ipomoea bahiensis. Phytochemistry 25:1077–1081. https://doi.org/10.1016/S0031-9422(00)81557-5
de Araujo Leite JC, de Castro TM, Barbosa-Filho JM, de Siqueria-Junior JP, Marques-Santos LP (2015) Photoprotective effect of coumarin and 3-hydroxycoumarins in sea urchin gametes and embryonic cells. J Photochem Photobiol B Biol 146:44–51. https://doi.org/10.1016/j.jphotobiol.2015.02.024
Delgado GC, Buril MT, Alves M (2014) Convolvulaceae do Parque Nacional do Catimbau, Pernambuco, Brasil. Rodriguésia 65:425–442. https://doi.org/10.1590/S2175-78602014000200008
Estrella-Parra EA, Espinosa-González AM, García-Bores AM, Zamora-Salas SX, Benítez-Flores JC, González-Valle MR, Hernández-Delgado CT, Peñalosa-Castro I, Avila-Acevedo JG (2019) Flavonol glycosides in Dyssodia tagetiflora and its temporal variation, chemoprotective and ameliorating activities. Food Chem Toxicol 124:411–422. https://doi.org/10.1016/j.fct.2018.12.024
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941–1953. https://doi.org/10.1002/ijc.31937
Harborne JB, Baxter H (1999) The handbook of natural flavonoids, 2nd edn. John Wiley and Sons, Hoboken
Kusano S, Abe H (2000) Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rats. Biol Pharm Bull 23:23–26. https://doi.org/10.1248/bpb.23.23
Kwak CS, Yang J, Shin CY, Chung JH (2018) Topical or oral treatment of peach flower extract attenuates UV-induced epidermal thickening, matrix metalloproteinase-13 expression and pro-inflammatory cytokine production in hairless mice skin. Nutr Res Pract 12:29–40. https://doi.org/10.4162/nrp.2018.12.1.29
Kwon EK, Jin SS, Choi MH, Hwang KT, Shim JC, Hwang IT, Han JH (2002) Mechanism of relaxation of rat aorta by scopoletin; an active constituent of Artemisia capillaris. J Physiol Pathol Korean Med 16:389–396
Liu H, Ma S, Xia H, Lou H, Zh F, Sun L (2018) Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J Ethnopharmacol 222:201–207. https://doi.org/10.1016/j.jep.2018.05.008
Lu TS, Saito N, Yokoi M, Shigihara A, Honda T (1992) Acylated pelargonidin glycosides in the red-purple flowers of Pharbitis nil. Phytochemistry 31:289–295. https://doi.org/10.1016/0031-9422(91)83056-Q
Maher VM, McCormick JJ, Grover PL, Sims P (1977) Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat Res-Fund Mol M 43:117–137. https://doi.org/10.1016/0027-5107(77)90137-3
March RE, Miao XS (2004) A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int J Mass Spectrom 231:157–167. https://doi.org/10.1016/j.ijms.2003.10.008
Meira M, Silva EP, David JM, David JP (2012) Review of the genus Ipomoea: traditional uses, chemistry and biological activities. Rev Bras Farmacogn 22:682–713. https://doi.org/10.1590/S0102-695X2012005000025
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and citotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Moon PD, Lee BH, Jeong HJ, An HJ, Park, SJ, Kim HR, Ko SG, Um JY, Hong SH, Kim HM (2007) Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IĸB/NF-ĸB signal cascade in the human mast cell line HMC-1. Eur J Pahrmacol 555:218–25. https://doi.org/10.1016/j.ejphar.2006.10.021
Neradil J, Veselská R, Slanina J (2003) UVC-protective effect of caffeic acid on normal and transformed human skin cells in vitro. Folia Biol (Praha) 49:197–202
Otsuki T, Matsufuji H, Takeda M, Toyoda M, Goda Y (2002) Acylated anthocyanins from red radish (Raphanus sativus L.). Phytochemistry 60:79–87. https://doi.org/10.1016/S0031-9422(02)00063-8
Parise CB, Sá-Rocha VM, Moraes JZ (2015) Skin sensitizer identification by IL-8 secretion and CD86 expression on THP-1 cells. Toxicol In Vitro 30:318–324. https://doi.org/10.1016/j.tiv.2015.10.004
Parra C, Soto E, León G, Salas CO, Heinrich M, Echiburú-Chau C (2018) Nutritional composition, antioxidant activity and insolation of scopoletin from Senecio nutans: support of ancestral and new uses. Nat Prod Res 32:719–722. https://doi.org/10.1080/14786419.2017.1335726
Radice M, Manfredini S, Ziosi P, Dissette V, Buso P, Fallacara A, Vertuani S (2016) Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 114:144–162. https://doi.org/10.1016/j.fitote.2016.09.003
Rivelli DP, Filho CA, Almeida RL, Ropke CD, Sawada TC, Barros SB (2010) Chlorogenic acid UVA-UVB photostability. Photochem Photobiol 86:1005–1007. https://doi.org/10.1111/j.1751-1097.2010.00776.x
Takahashi T, Kimura Y, Saito R, Nakajima Y, Ohmiya Y, Yamasaki K, Aiba S (2011) An in vitro test to screen skin sensitizers using a stable THP-1-derived IL-8 reporter cell line, THP-G8. Toxicol Sci 124:359–369. https://doi.org/10.1093/toxsci/kfr237
The Global Cancer Observatory 2019 Colombia 2018. https://gco.iarc.fr/today/data/factsheets/populations/170-colombia-fact-sheets.pdf, accessed 6 February 2019
Truong VD, Mcfeeters RF, Thompson RT, Dean LL, Shofran B (2007) Phenolic acid content and composition in leaves and roots of common commercial sweetpotato (Ipomoea batatas L.) cultivars in the United States. J Food Sci 72:343–349. https://doi.org/10.1111/j.1750-3841.2007.00415.x
WHO–Tropical Diseases 2019. Cytotoxicity: in vitro determination. http://www.who.int/tdr/grants/workplans/en/cytotoxicity_invitro.pdf. (accessed 24 March 2019)
Yoshida K, Osanai M, Kondo T (2003) Mechanism of dusky reddish-brown “kaki” color development of Japanese morning glory, Ipomoea nil cv Danjuro. Phytochemistry 63:721–726. https://doi.org/10.1016/S0031-9422(03)00273-5
Zhang L, Mu X, Fu J, Zhou Z (2007) In vitro cytotoxicity assay with selected chemicals using human cells to predict target-organ toxicity of liver and kidney. Toxicol in Vitro 21:734–740. https://doi.org/10.1016/j.tiv.2007.01.013
Funding
This work was supported by COLCIENCIAS [Convocations 617 and 647 Doctorados Nacionales]; Ministerio de Educación Nacional, Ministerio de Industria, Comercio y Turismo, ICETEX, Convocatoria Ecosistema Científico-Colombia Científica; COLCIENCIAS [Grant RC-FP44842-212-2018].
Author information
Authors and Affiliations
Contributions
LJS, YC, and JJM contributed to running the laboratory work and chromatographic and data analysis. LJS drafted the manuscript. EQ and REO contributed to evaluating cytotoxic and sensitizing activities. REO supervised the laboratory work and aided in the drafting of the paper. JGA, AMG, and AME contributed to evaluating photoprotective activity and critical reading of the manuscript. JCB and MRG contributed to histological analysis. JRM and EES contributed to the analytical study, supervised the laboratory work, and carried out critical reading and manuscript drafting.
Corresponding author
Ethics declarations
Ethical Disclosures
Plant material was collected according to a Contract for Access to Genetic Resources and Derivative Products (No. 101, June 3, 2014, Colombian Ministry of Environment and Sustainable Development).
Protection of Human and Animal Subjects
The photoprotective test was endorsed by the Ethics Committee (September 5, 2017) and the Biosafety Commission (September 13, 2017) of the National Autonomous University of Mexico. All procedures were carried out in accordance with Official Mexican Regulation (NOM-062-ZOO-1999).
Declaration of Interest
None.
Electronic Supplementary Material
ESM 1
(DOCX 76.3 kb)
Rights and permissions
About this article
Cite this article
Sierra, L.J., Córdoba, Y., Mejía, J.J. et al. Photoprotective Activity of Ipomoea horsfalliae Flower Extract. Rev. Bras. Farmacogn. 30, 69–79 (2020). https://doi.org/10.1007/s43450-020-00024-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-020-00024-6