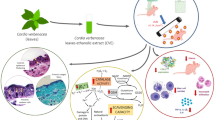
Abstract
Natural antioxidants have attracted attention for their therapeutic use as photochemopreventive agents. Inga edulis leaves extract and its purified fraction have high polyphenolic content and high antioxidant capacity. In addition, they presented UV photostability and low citotoxicity in fibroblast cells. In this context, this study first aimed at development of topical formulation containing purified fraction of I. edulis extract and the evaluation of skin penetration of the compounds. Moreover, the photoprotective/photochemopreventive potential of the formulation containing I. edulis purified fraction were investigated in vitro and in vivo. The topical formulation containing 1% of the purified fraction of I. edulis increased the endogenous antioxidant potential of the skin, and vicenin-2 and myricetin compounds were able to penetrate the epidermis and dermis. Additionally, the purified fraction (25 and 50 mg/mL) showed a photoprotective effect against UVA and UVB radiation in L929 fibroblast cells. In vivo studies have shown that the formulation added with purified fraction provided an anti-inflammatory effect on the skin of animals after UVB exposure, since it was observed a reduction in MPO activity, IL-1β and TNF-α cytokines, and CXCL1/KC chemokine concentrations. In conclusion, the purified fraction of I. edulis, rich in phenolic compounds, when incorporated in topical formulation, appears as an alternative to prevent skin damages induced by UV radiation, due to its antioxidant and anti-inflammatory properties.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human skin is the largest organ in the body and is a barrier between the internal and external environment. It plays a significant role in physiological functions of sensation, protection, thermoregulation, defense system, and metabolic mechanisms to help in maintaining innate defense system [1]. However, it is directly exposed to the harmful effects of ultraviolet radiation (UVR), toxic chemicals and pathogens [2].
Solar radiation is the main source of human exposure to UVR, which is divided in UVC (200-280 nm), UVB (280-320 nm) and UVA (320-400 nm). UVC radiation is a potent mutagenic agent, but it practically does not reach the earth's surface since it is absorbed by the ozone layer. On the other hand, UVB radiation (5%) and especially UVA (95%) are capable of reaching the earth causing skin damage [3,4,5].
UVB, because it is highly energetic, is able to penetrate the epidermis causing direct damage to deoxyribonucleic acid (DNA), inflammation (erythema or sunburn), immunosuppression, melanogenesis, photoaging and skin cancer, as well as indirect damage caused by the production of reactive oxygen species (ROS). UVA radiation, less energetic than UVB, penetrates more into the skin reaching the dermis, indirectly causing biological damage through ROS that are generated by endogenous photosensitization. These ROS, in turn, affect biomolecules such as lipids, proteins and DNA, inducing oxidative stress and inflammation through activation of mitogen-activated protein kinases (MAPK), induction of constitutive expression of cyclooxygenase 2 (COX-2) and expression of pro-inflammatory proteins tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), inducible nitric oxide synthase (iNOS) that mainly trigger photocarcinogenesis and photoaging [4, 6, 7].
Natural antioxidants have attracted attention for their therapeutic use as photochemopreventive agents, since they have been shown to be associated with a reduced incidence of ROS-mediated photocarcinogenesis and photoaging [6, 8, 9].
Inga edulis (I. edulis) is a fruit tree native from Central and South America that have demonstrated high polyphenolic content and high antioxidant capacity. Several phenolic compounds have been identified in I. edulis leaves such as 5,7,3′,4′-tetrahydroxy-3-methoxyflavone; 6,3′,4′-trihydroxyaurone; and 5,7,4′-trihydroxy-6,8-dimethylflavonone; epicatechin; apigenin C-di-hexoside; myricetin-O-hexose-deoxyhexose; myricetin-O-deoxyhexose and vicenin-2 [10, 11]. Being demonstrated the high polyphenol and flavonoid contents, photostability in response to UVA and UVB radiations and the low cytotoxicity in L929 fibroblast cells, I. edulis leaves extract and purified fraction are potential candidates to be safely incorporated in topical photochemopreventive formulations [10].
In this context, this study first aimed at development of topical formulation containing purified fraction of I. edulis leaves extract and the evaluation of skin penetration of the compounds. Moreover, the photoprotective/photochemopreventive potential of the formulation containing I. edulis purified fraction were investigated in vitro and in vivo aiming the photochemoprevention of skin.
2 Materials and methods
2.1 In vitro evaluation of the antioxidant capacity of the I. edulis purified fraction by the dichlorofluorescein assay
2.1.1 The cell culture
The L929 fibroblast cells were purchased from the Cell Bank of Rio de Janeiro (BCRJ). They were routinely grown in 150 cm2 tissue culture flasks in Dulbecco's modified Eagle’s medium (DMEM) (Gibco®, Mandaluyong, Philippines), supplemented with 10% fetal bovine serum (FBS) (Gibco®, Mandaluyong, Philippines), and a 1.0% antibiotic/antimycotic solution (10,000 U of penicillin, 10 mg of streptomycin and 25 μg amphotericin in 1 mL) (Sigma-Aldrich, Saint Louis, USA). The cells were grown at 37°C in a humidified incubator with 5% carbon dioxide (CO2) and were subcultured every 2-3 days by 0.25% trypsin-treatment after confluency was achieved [10].
2.1.2 The dichlorofluorescein assay
Solutions of different concentrations of the purified fraction of I. edulis leaves extract (2, 5, 10, 20, 40, 60, 80 and 100 µg/mL) were prepared from a stock solution (1 mg/mL) solubilized in phosphate-buffered saline (PBS) (pH 7.4). The concentrations of the solutions added into the wells were chosen since they are not cytotoxic (100% of cell viability at 156 µg/mL), according to results of previous studies performed with this fraction in L929 fibroblast cells using neutral red as a marker of cell [10].
To carry out the assay, the cells were cultured in 96-well microplates at a confluence of 0.5 × 105 cells/well and incubated at 37°C with 5% CO2. Twenty-four hours later, the culture medium was discarded and 100 µL of a 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) solution (5 µM) was added to each well. After 30 minutes of incubation, the solution was discarded, and a solution of PBS was added to each well. Subsequently, samples from the purified fraction (20 µL/well) at different concentrations were added, with the exception of control wells, remaining for 30 minutes in the incubator at 37 ℃ with 5% CO2.
After this time, part of the microplates was subjected to 20 J/cm2 of UVA radiation. This dose was defined by a previous study performed in the same laboratory [12]. The dose corresponds to the average UVA radiation emitted by the sun on two days in October between 11 am and 2:30 pm (38 ℃), in the city of Ribeirão Preto, SP, Brazil. The same radiometer was used in both studies. Figueiredo et al. [12] demonstrated that 20 J/cm2 of UVA corresponds to 100% of cell viability. The other part of the microplates was added with 20 µL of a solution of hydrogen peroxide (H2O2) (100 mM), except the negative controls, which remained in the incubator for 1 hour at 37 ℃ with 5% CO2. H2O2 generates intracellular ROS and plays a central role in oxidative stress-mediated cellular toxicity. However, the toxic effect of H2O2 and its role in redox signaling, now known, are dependent on the physicochemical properties of biomembranes, since their hydrophilicity can impair their passage across the cell membrane [13]. After cellular uptake the fluorogenic DCFH-DA dye is deacetylated and oxidized by esterase and ROS, respectively, into 2′-7′ dichlorofluorescein (DCF), a fluorescent compound [14]. This assay was used to evaluate the antioxidant capacity of the components of the purified fraction of I. edulis. The sequestration of ROS by these components decreases the oxidation of the deacetylated DCFH, consequently, with the decrease of the fluorescence of DCF. All microplates, irradiated and treated with hydrogen peroxide, were read in a fluorimeter (BioTek Synergy 2, USA), using excitation and emission wavelengths of 488 and 525 nm, respectively [15].
2.2 The topical formulation containing purified fraction of I. edulis leaves extract
The topical formulation was prepared using 6% of a self-emulsifying base (Hostacerin® SAF); 5% of propylene glycol; 0.05% of chloromethylisothiazolinone/methylisothiazolinone (Nipaguard® CG) preservatives; 1% of the I. edulis purified fraction and purified water. The purified fraction was previously solubilized in a solution of propylene glycol and water at a ratio of 1:1 (w/w). Then, the purified fraction was incorporated into the formulation (aqueous phase) during its cold preparation (room temperature 25 ± 2°C), under agitation at 300 rpm in a mechanical shaker (Fisatom, model 713D) for approximately 15 minutes. The choice of formulation and the concentration of the incorporated purified fraction (1%) was based on previous studies of stability which showed that this formulation presented physical stability and maintenance of antioxidant activity [16]. A placebo formulation was also prepared, which consists of the same formula without the purified fraction. The formulations were kept in opaque plastic pots.
2.3 The in vivo skin penetration
The in vivo skin penetration studies were performed on 3-month-old hairless mice (lineage HRS/J Jackon Laboratory—BarlHarbor), male or female, weighing approximately 30 g. The animals were housed in a temperature-controlled room with access to water and food ad libitum until use. They were housed in cages with a 12-h light and 12-h dark cycle. All experiments were conducted in accordance with the National Institutes of Health guidelines for the welfare of experimental animals and with the approval of the Ethics Committee of the Faculty of Pharmaceutical Science of Ribeirão Preto (University of São Paulo, Ribeirão Preto, SP, Brazil—Process n° 13.1.525.53.1).
The animals were divided into three groups (n = 3 each group): a group treated with a formulation containing the purified fraction of I. edulis, a group treated with a placebo formulation, and a non-treated group (control). Three hundred milligrams of the formulations were applied to the back of the animals. One hour after application, time necessary to allow the compounds to penetrate the skin, the animals were sacrificed by inhalation of CO2. The treated area of skin was removed (1.77 cm2) and subjected to tape stripping, in which the skin was stripped with 15 pieces of adhesive tape to remove the stratum corneum [17].
The remaining epidermis and dermis were cut into small pieces, sonicated for 30 min in 2.5 mL of methanol/water (80:20 v/v), vortex mixed for 1 min, and centrifuged for 15 min at 15,000 rpm. The supernatant was transferred to a 5-mL volumetric flask. The remaining precipitate was added to 2.5 mL of methanol/water (80:20 v/v) and the extraction procedure was repeated. The supernatant of the second extraction was added to that of the first in a volumetric flask, and the volume was adjusted with the same solvent [17]. To verify the amount of antioxidant compounds that penetrated the skin, the samples were dried in air flow, resuspended in ethanol/water (50:50 v/v) solution, and submitted to chemiluminescence analysis (xanthine/xanthine oxidase/luminol system), and high-performance liquid chromatography (HPLC) analysis.
2.3.1 The xanthine/xanthine oxidase/luminol assay
To determine the antioxidant capacity of the samples the xanthine/xanthine oxidase/luminol system was used. Into each test tube were added 400 µL of a solution of ethylenediamine tetra acetic acid (EDTA) (1 mM) in glycine buffer (0.1 M) pH 9.4, 150 µL xanthine (6 mM), 10 µL sample and 10 µL luminol solution (0.6 mM). The reaction was started with the addition of 100 µL of freshly prepared xanthine oxidase solution (20 mU/mL) (kept cooled on ice). The chemiluminescence measurement was performed in an Autolumat LB953 luminometer (EG&G Berthold) for 5 minutes at 25 ℃ [18]. The percentage of inhibition of chemiluminescence was calculated by measuring the area under the curve (AUC), using the following formula:
The reaction medium without the solution of the I. edulis purified fraction was considered as a positive control [19].
2.3.2 The chromatographic analysis
The chromatographic analysis was performed on a Shimadzu HPLC system (model LC-10AT), coupled with a UV/VIS detector (SPD-10A), an integrator (C-R6A) and a Rheodyne® injector (7725i) with a 20 µL loop. The quantification of compounds from the I. edulis purified fraction penetrated in the skin was performed using a Hypersil Gold® C18 column (Thermo SCIENTIFIC, 250 × 4.6 mm, particle size 3.0 µm), coupled to a pre-column (10 × 4 mm) with the same stationary phase. For the elution of the compounds, a gradient was used (Table 1) in which the mobile phases consisted of ultrapure water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B), with a flow rate of 0.8 mL/minute. The detection was performed at a wavelength of 280 nm and chromatographic peaks were identified by comparing the retention times of authentic standards: vicenin-2 (HWI Analytik GMBH® pharma solutions) and myricitrin (Sigma Aldrich®). Standards and samples were prepared in 50% ethanol [10].
2.4 Evaluation of the in vitro photoprotective/photochemopreventive potential of the purified fraction of I. edulis and of the formulation
2.4.1 Samples
In this assay, the photoprotective/photochemopreventive potential of the purified fraction of I. edulis and of the formulation containing the purified fraction were tested. For this, solutions of the purified fraction at concentrations of 25 and 50 mg/mL (propylene glycol and water 1:1, v/v); the formulation containing 1% of the purified fraction of I. edulis, and placebo formulation were tested. To compare the results, two commercial sunscreen formulations of the same brand with a sun protection factor (SPF) of 15 and 30 were also tested. Each sample was applied in triplicate.
2.4.2 Evaluation of the oxidative stress in cells exposed to UVA radiation
The L929 fibroblast cells were cultivated as described in item 2.1.1, seeded in 6-well plates with an initial density of 8 × 105 cells/well and incubated at 37 ℃ with 5% CO2 for 24h. After that, the culture medium was discarded, the plates were washed with 0.9% saline solution and a 5 µM DCFH-DA solution (2 mL/well) was added. The plates were incubated for 30 minutes, under the same conditions described above. After 30 minutes, the DCFH-DA solution was replaced with Hank’s buffer (2 mL/well) and the 6-well plates were covered with a quartz plate where the samples were applied at a concentration of 2 mg/cm2, as recommended by the European Cosmetics and Perfumery Association (COLIPA). Then, the set (6-well plate/quartz plate) was placed into the solar simulator (Vilber Lourmat, Mame-La-Vallée, France) where it was exposed to a dose of 20 J/cm2 of UVA radiation. This dose can induce the maximum formation of ROS, according to the previous study performed by Figueiredo et al. [12]. At the end of irradiation, the 6-well plates were read in a fluorimeter (BioTek Synergy 2, USA) at 485 nm excitation and 528 nm emission [7, 20, 21]. The photoprotective effect was evaluated by the amount of ROS that were reduced. The values obtained for the group of cells exposed to radiation without any type of protection (irradiated control), corresponds to 100% of ROS formation.
2.4.3 Assessment of the cell viability after UVB exposure
The L929 fibroblast cells were cultivated and seeded as described in item 2.4.2. After 24 hours of the cells were seeded, the culture medium was replaced by Hank’s buffer (2 mL/well). Hank's buffer was used to keep the cells viable during the short period of irradiation without stimulating their proliferation [22]. Then, the 6-well plates were covered by a quartz plate where the samples were applied at a concentration of 2 mg/cm2, as recommended by COLIPA. The set (6-well plate/quartz plate) was placed into the solar simulator in which it was exposed to a dose of 0.180 J/cm2 of UVB radiation. The selected dose was able to reduce cell viability by 50% in preliminary experiments performed in the present study (unpublished data).
At the end of the radiation, the Hank’s buffer contained in the 6-well plates was removed and replaced by DMEM cell culture medium supplemented with 2% Fetal Bovine Serum (FBS). Then, the plates were incubated at 37 ℃ with 5% CO2 for 48 hours [7, 20, 21].
After 48 hours, the DMEM was discarded, and the cells were washed with saline solution (0.9%). Subsequently, 2 mL of a resazurin solution (0.0001% w/v, diluted in supplemented DMEM) were added to each well. The plates were incubated for 4 hours under the same conditions described above. After 4 hours, the plates were read in fluorimeter at excitation of 485 nm and emission of 528 nm [7]. The photoprotective effect was evaluated by cell viability. The results obtained for the non-irradiated control group corresponded to 100% of cell viability.
2.5 The in vivo photoprotective/photochemopreventive potential of the formulation containing I. edulis purified fraction
The hairless mice were divided into six groups (n = 5 each group): untreated and non-irradiated control (NIC); untreated and irradiated control (IC); non-irradiated treated with placebo formulation (NIP); irradiated treated with placebo (IP); non-irradiated treated with formulation containing I. edulis purified fraction (NIF) and irradiated treated with formulation containing I. edulis purified fraction (IF). The treatment consisted of the topical application of 300 mg of formulations on the back of each animal, 2 hours before irradiation. The amount of formulation added (300 mg) was selected to cover the entire back of the animal.
The groups exposed to UVB radiation were placed inside a wooden enclosure containing the ultraviolet fluorescent lamp PHILIPS TL40W/12 (Medical, Netherlands) and were irradiated for approximately 45 minutes, which corresponds to a total dose of 2.87 J/cm2 of UVB radiation. Previous studies performed in the same laboratory showed that this dose was able to induce an inflammatory process, which was confirmed by an approximately fivefold increase in interleukin-1beta (IL-1β) concentration in comparison with non-irradiated control mice. The animals could move freely in the box, with the dorsal region of each one being directly exposed to UVB radiation. After 3h post-irradiation the mice were sacrificed by inhalation of carbon dioxide. The skin tissue on the animals' back was collected, cleaned with a 0.9% (w/v) sodium chloride (NaCl) solution, and stored at −80 ℃ [23,24,25].
2.5.1 Evaluation of the myeloperoxidase (MPO) activity
The skin samples taken 3 h post-irradiation were thawed, minced, and placed in 50 mM dibasic potassium phosphate (K2HPO4) buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) at the concentration of 50 mg/mL. The skin was homogenized in Turrax® and centrifuged for 10 minutes at 12,000 rpm at 4 ℃. The supernatant from each sample (60 μL) was transferred to a 96-well plate and mixed with 200 µL of phosphate buffer (50 mM, pH 6.0) containing o-dianisidine dihydrochloride (52.64 mM) and H2O2 (0.05%). The spectrophotometer reading was performed after 15 minutes at 450 nm. The enzyme activity was calculated considering that a unit of MPO activity is capable of degrading one µmol of H2O2 per minute which corresponds to 0.0113 units of absorbance [26, 27].
2.5.2 IL-1β, TNF-α and CXCL1/KC measurements
IL-1β and TNF-α cytokines and C-X-C motif chemokine ligand-1/keratinocyte-derived chemokine (CXCL1/KC) were quantified by enzyme-linked immunosorbent assay (ELISA) using skin samples taken 3 h post-irradiation (stored at −80 ℃). The samples were processed and homogenized in the Polytron® PT 3100 equipment, with PBS buffer containing 0.2% Tween 20 and protease inhibitors, with subsequent centrifugation at 12,000 rpm for 10 minutes at 4 ℃. The supernatant obtained from the centrifugation was collected and tissue levels of IL-1β, TNF-α and CXCL1/KC were quantified by colorimetric assay according to the manufacturer’s instructions (R&D System; Quantikine® Elisa Mouse IL-1β; TNF-α; CXCL1/KC Immunoassay).
2.6 Statistical analysis
Data were statistically analyzed using GraphPad Prism® (version 5.01, 2007). Results were expressed by means ± standard error of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s test of multiple comparisons, and the level of significance was set to p < 0.05.
3 Results and discussion
3.1 The purified fraction of I. edulis reduces oxidative stress induced by H2O2 and UVA irradiation
Inga species show high content of polyphenolic compounds and, consequently, high antioxidant capacity. In the leaves of the I. edulis, epicatechin, apigenin C-di-hexoside, myricetin-O-hexose-deoxyhexose, myricetin-O-deoxyhexose and vicenin-2 were identified [10, 11]. The purified fraction of the leaves extract obtained using macroporous resin also exhibited high polyphenol and flavonoid contents. HPLC analysis revealed the majority compounds as myricitrin (myricetin 3-rhamnoside) and vicentin-2 in the purified fraction of I. edulis leaves extract [10].
The results showed that different concentrations of the purified fraction (2 to 10 µg/mL) were able to promote a dose-dependent reduction in the fluorescence produced by DCF (Fig. 1A). The 2 µg/mL concentration of the fraction decreased the fluorescence intensity of the probe in the intracellular medium by 60.5%, while at the 10 µg/mL concentration the decrease in intensity was 79.86%, relative to the positive control group (Fig. 1B). Starting at the 20 µg/mL concentration, the observed reduction in DCFH probe oxidation was statistically similar to the negative control (cells without H2O2 and not treated with the purified fraction, NC) (Fig. 1A). The obtained results suggest that the components of the purified fraction penetrating inside the cells are able to scavenging ROS generated by H2O2. Furthermore, by the design of the experimental procedure, hydrogen peroxide could be sequestered by the components of the purified fraction outside the cells, decreasing the amount of H2O2 inside the cell, which could provide the decrease of DCF probe fluorescence.
In vitro antioxidant potential evaluation of the purified fraction of I. edulis by the dichlorodihydrofluorescein assay in cell culture and using the addition of H2O2 as oxidant. A Measurement of the fluorescence intensity generated by DCFH in cultured L929 fibroblast cells treated or not with different concentrations of solutions of the purified fraction. B Decrease in fluorescence intensity (%) generated by DCFH in L929 fibroblast cells in the presence of solutions of the purified fraction at different concentrations, compared to the positive control (PC). Results represent the mean of 6 determinations ± SD, with p < 0.05, using one-way ANOVA, followed by Tukey’s multiple comparisons test. a.b.c.dThe same letters are not significantly different. H2O2 hydrogen peroxide, DCFH Dichlorodihydrofluorescein, SD standard deviation, NC Negative control, IC50 mean inhibitory concentration
These results were consistent with that found by Zou et al. [28]. The polyphenols (mostly flavan-3-ols) presented in persimmon vinegar, obtained from fermented persimmon fruit, reduced H2O2-induced ROS production in a dose-dependent manner. However, they used concentrations ranging from 25 to 100 µg/mL. Majority flavonols from I. edulis showed similar antioxidant potential at much lower concentration.
The decrease in fluorescence may have occurred due to sequestration of the oxidizing agent by the antioxidant compounds present in the purified fraction of I. edulis. In studies performed by Domitrović et al. [29], myricitrin showed H2O2 sequestering activity in in vitro assays with a method of quantifying the oxidizing agent at 230 nm. The IC50 value was 28.46±0.67 µg of myricitrin per mL of reaction medium. Thus, these data indicate that the myricitrin present in the purified fraction may have sequestered the H2O2, since the amount of 20 µg of purified fraction, which contains 2.92 µg of myricitrin in the reaction medium, provided the decrease of 81.2% of fluorescence. The calculated IC50 value for the inhibition of this oxidizing agent by the purified fraction in cell culture was 0.849 µg per mL of reaction medium.
The antioxidant effect of the purified fraction of I. edulis was also evaluated in culture of L929 fibroblast cells, using UVA radiation as an oxidizing agent. When the cells were irradiated with a dose of 20 J/cm2 (PC), it was observed that there was a 4717-fold increase in DCF fluorescence compared to the negative control group (non-irradiated cells, NC), indicating the oxidation of DCFH by ROS or oxidizing substances generated in the cells by the radiation (Fig. 2A). However, when the cells were treated with solutions of the purified fraction at different concentrations, a decrease in the fluorescence generated by DCFH was observed. At the concentration of 2 µg/mL, a 47.2% reduction in the fluorescence intensity of the probe was observed when compared to the positive control group. The other concentrations of the purified fraction (5–100 µg/mL) presented reduction of approximately 78% (Fig. 2B).
In vitro antioxidant potential evaluation of the purified fraction of I. edulis by the dichlorodihydrofluorescein assay in cell culture and using UVA radiation (20 J/cm2) as an oxidant. A Measurement of the fluorescence intensity generated by DCFH in cultured L929 fibroblast cells treated or not with different concentrations of solutions of the purified fraction. B Decrease in fluorescence intensity (%) generated by DCFH in L929 fibroblast cell culture in the presence of solutions of the purified fraction at different concentrations, compared to the positive control. Results represent the mean of 6 determinations ± SD with p < 0.05, using one-way ANOVA, followed by Tukey’s multiple comparisons test. a.b.c.dThe same letters are not significantly different. DCFH Dichlorodihydrofluorescein, SD standard deviation, IC50 mean inhibitory concentration
Petruk et al. [30] showed that the pre-treatment of BALB/3T3 fibroblasts with açai extract (containing malvidin and cyanidin derivatives as the most active molecules) was able to protect cells from UVA induced oxidative stress by reducing ROS production, as well as decreasing intracellular glutathione (GSH) and lipid peroxidation levels. This shows that phenolic compounds can act on different pathways of oxidative stress.
Ryšava et al. [31] demonstrated the increase of ROS, especially the superoxide radical, inside human fibroblasts under UVA radiation. The generated ROS can oxidize DCFH to fluorescent DCF. However, the decrease in DCF fluorescence in cells previously treated with the solutions of purified fraction of I. edulis suggests that the fraction components can have scavenge the free radicals generated by UVA. By experimental procedure, cells were treated with the solutions of the purified fraction of I. edulis 30 minutes before being irradiated with UVA. Thus, the possibility of the absorption of UVA radiation by the components of the purified fraction cannot be ruled out. This is supported by previous studies that demonstrated that UVR is able to increase the fluidity of lipids and lead to a disorganization of the cell membrane, impairing the barrier function and increasing the penetration of compounds into the cell [32, 33].
The IC50 value obtained for UVA radiation (IC50 = 2.3 ug/mL) was 2.7 times higher than the IC50 value obtained for H2O2 (IC50 = 0.849 ug/mL). These data suggest that direct contact of the compounds in the purified fraction with H2O2 facilitated the sequestration of the radical. In addition, the compounds in the purified fraction may be more efficient in scavenging H2O2 than in scavenging radicals generated by UVA radiation. However, for this reduction to occur in the experiment performed with UVA radiation, there was a need for penetration of the antioxidant compounds of the fraction into the cell, and this may not be as effective, requiring a larger amount of fraction to reduce by 50% the oxidation of DCFH and, consequently, the fluorescence.
3.2 Antioxidant compounds of the purified fraction of I. edulis penetrated the skin
The ability of the topical formulation to provide the penetration of compounds from the purified fraction of I. edulis into the epidermis/dermis was evaluated in vivo on the hairless mice. The in vivo skin penetration, using the animal’s organism as a whole, makes it possible to observe the influence of various parameters on the absorption of the active substances, such as skin metabolism and the presence of blood circulation [34].
A topical formulation must provide the penetration and retention of the antioxidant compounds in the skin to exert a photochemoprotective effect. These compounds must be released from the vehicle, penetrated through the stratum corneum and retained in the epidermis and dermis [35]. Thus, cutaneous penetration and retention studies are important for the choice of formulation capable of allowing the highest bioavailability of the actives in the skin [36].
Due to the antioxidant potential that the I. edulis purified fraction showed in the in vitro studies, the 1% concentration was incorporated into a formulation. The formulation corresponds to a hydrophilic polymer stabilized emulsion. It is a moisturizing base constituted by the association of viscosity donors, emulsifiers and emollients, which give the emulsion an anionic character. The phosphoric ester component present in the formulation (ethoxylated lauryl alcohol phosphoric triester), by presenting great affinity for the skin phospholipids, could facilitate the penetration of the active agents. Being cold-prepared, this formulation allowed the purified fraction of I. edulis to be incorporated into the aqueous phase, providing security in the homogeneity of its hydrophilic compounds.
A study carried out by Silva [16] demonstrated that the formulation used in this study presented physical stability after the centrifugation test (cycles of 24 hours at 40±2 ℃ and 24 hours at 4±2 ℃ for 12 days), and maintenance of antioxidant stability measured by DPPH● radical scavenging test and the chemiluminescence inhibition test (xanthine/xanthine oxidase/luminol system). This formulation, among others evaluated, was the one that allowed the highest penetration of antioxidant compounds of the purified fraction of I. edulis in pig ear skin, being vicenin-2 one of these compounds [16]. Therefore, these results justify the choice of this formulation for in vivo studies.
The data obtained using xanthine/xanthine oxidase/luminol system showed that the skin of the animals in the control group reduced by 22.7% the total chemiluminescence generated in the system, represented by the blank of the reaction in which the skin sample was replaced by buffer (Fig. 3B). This suggests that the skin of hairless mice is provided with an endogenous antioxidant system. The chemiluminescence of the skin treated with the placebo formulation showed no statistically significant difference (p < 0.05) when compared to the control group (Fig. 3). It was observed that the components of the base formulation (placebo) were not able to interfere in the enzymatic and chemical reactions involved in this assay. Skin samples treated for 1 hour with the formulation added to the purified fraction showed a reduction in chemiluminescence of 37.8% (Fig. 3B), 15% higher than the value found in the control group (22.7%). This proves that the formulation allowed the penetration of antioxidant compounds from the purified fraction into the animals’ skin. The penetrated compounds contributed to the increase of the endogenous antioxidant potential of the skin by their ability to sequester the superoxide anion formed in the reaction or to reduce the oxidized luminol, thus decreasing the chemiluminescence generated by luminol.
Quantification of the chemiluminescence generated by the xanthine/xanthine oxidase/luminol system obtained in the in vivo penetration study with the formulation added to 1% of the purified fraction of I. edulis after 1 hour of application. A Reduction in chemiluminescence (area under the curve) and B reduction in chemiluminescence (%) relative to blank (100% chemiluminescence). Control Group: animals not treated with formulation. Placebo Group: animals treated with the formulation free of the purified fraction. Formulation Group: animals treated with the formulation added to 1% of the purified fraction. The percentage of chemiluminescence reduction for each group was calculated taking into account the total chemiluminescence generated in the system, represented by the blank. Results represent the mean of 3 animals per group ± SD, with p < 0.05, using one-way ANOVA, followed by Tukey’s multiple comparisons test. a.b.cThe same letters are not significantly different. SD standard deviation
The amount of purified fraction penetrated into the epidermis/dermis was 3.4 µg/cm2 of skin, calculated based on the calibration curve of chemiluminescence inhibition (%) versus mass of purified fraction in the reaction medium (µg) (y = 1325x + 7.066, R2 = 0.990).
The majority compounds of the purified fraction of I. edulis that penetrated the skin of the animals were quantified by the HPLC technique. The substances that penetrated and were extracted from the skin of the mice treated with the placebo formulation are represented in chromatogram A (Fig. 4A) and with the formulation added the purified fraction of I. edulis are mentioned in chromatogram B (Fig. 4B). In chromatographic profile B, the compounds identified were the polyphenols vicenin-2 (retention time of 31.7 minutes) and myricitrin (retention time of 45.87 minutes). According to [10], these two substances were found to be majority in the purified fraction of I. edulis. The amounts of vicenin-2 and myricitrin penetrated were 0.05 and 0.5 µg/cm2 of skin, respectively, calculated based on the calibration curves of the standards of these polyphenols [10].
Chromatographic profiles obtained by the HPLC technique of the skin samples of the animals treated with the placebo formulation (A) and with the formulation added with 1% of the purified fraction of I. edulis, after 2 hours of the application and identification of the penetrated myricitrin (MRT) and vicenin-2 (VCN) compounds (B)
3.3 The purified fraction of I. edulis presents in vitro photoprotective/photochemopreventive potential
The methanol/water extract of I. edulis leaves consists of some flavonoids, from the flavonols class, which are involved in several physiological functions in higher plants [11, 37]. Some lines of evidence suggest a photoprotective role of these compounds against damages induced by excessive exposure to UV and visible radiation [38]. Merzlyak et al. [39] showed the sunlight reflectance ability in the UV range of 300 to 400 nm of flavonols from anthocyanin-free plants. Thus, the purified fraction of I. edulis may have a photoprotective effect due to its flavonol content, and a photochemoprotective effect due to the antioxidant and free radical scavenging potential of its polyphenolic compounds.
The photoprotective potential of the purified fraction of I. edulis in solution and incorporated in the formulation was evaluated in vitro, using cell culture in which L929 fibroblast cells were exposed to UVA (20 J/cm2) and UVB (0.180 J/cm2) radiations.
The results were expressed considering that the maximum production of ROS (100%) occurred in the group of cells exposed to UVA radiation without any type of protection (irradiated control group, IC) (Fig. 5A). The cells treated with commercial sunscreens with SPF 15 and SPF 30 (applied on the quartz plate during radiation) showed a reduction of 58% (using SPF 15) and 75% (using SPF 30) of the ROS formed in comparison to the IC group (Fig. 5A). The commercial products were able to absorb or reflect UVA radiation.
Evaluation of the photoprotective/photochemoprotective potential in vitro of purified fraction from I. edulis and the formulation. A Quantification of ROS formed in L929 fibroblast cells, exposed or not to 10 J/cm2 of UVA radiation. B Determination of the viability of L929 fibroblast cells exposed or not exposed to 0.180 J/cm2 of UVB radiation. NIC (non-irradiated control) = cells not exposed to radiation; IC (irradiated control) = cells not treated and exposed to radiation; SPF 15 and SPF 30 = cells treated with SPF 15 and SPF 30 commercial sunscreens and exposed to radiation; 25 mg/mL and 50 mg/mL = cells treated with solutions of the purified I. edulis fraction at concentrations of 25 and 50 mg/mL and exposed to radiation; Placebo = cells treated with the placebo formulation and exposed to radiation; Formulation = cells treated with the formulation added to 1% of the purified I. edulis fraction and exposed to radiation. Results represent the mean of 3 determinations per group ± SEM, with p < 0.05, using one-way ANOVA, following Tukey’s multiple comparisons test. a.b.c.dThe same letters are not significantly different. ROS Reactive oxygen species, SPF Sun protection factor
In the cells protected by solutions of the purified fraction at concentrations of 25 and 50 mg/mL during radiation, this photoprotective effect was 26.5 and 33%, respectively, in relation to the IC, which were not significant statistically (p < 0.05). These results suggest that there are compounds that absorb UVA radiation in the purified fraction. However, when it was used the formulation added with 1% of the purified fraction the photoprotective effect was similar to that of the placebo formulation.
On the other hand, in the photoprotection assays performed with UVB radiation at a dose of 0.180 J/cm2, the results were expressed as a function of cell viability after exposure to this radiation, because UVB rays have more energy per photon than UVA rays, therefore, are able to react directly with DNA, ribonucleic acid (RNA) and proteins [40, 41]. Therefore, UVB radiation is more cytotoxic and mutagenic than UVA.
L929 fibroblast cells exposed to UVB (IC) showed a 47.6% reduction in viability when compared to unexposed cells (NIC) (Fig. 5B). On the other hand, the same cells exposed to UVB radiation and protected by commercial sunscreens of SPF 15 and SPF 30, exhibited no loss of viability, suggesting that the commercial products were able to prevent cell death. The solutions of the purified fraction at concentrations of 25 and 50 mg/mL maintained cell viability by 68.5% and 88.3%, respectively, in relation to NIC. These data indicate that the viability of the irradiated cells under protection of the purified fraction solutions was increased by 30 and 68%, respectively, in comparison to IC, suggesting that the purified fraction possesses compounds capable of absorbing UVB radiation. When the formulation added with 1% of the purified fraction and the placebo formulation were applied to the 9.6 cm2 area of the quartz plate (well diameter), there was no statistically significant difference (p < 0.05) between the placebo (67%), formulation added the fraction (56%) and IC (52.4%). This fact suggests that the concentration of UVB-absorbing compounds present in the formulation was low, which did not allow visualization of cell protection.
Parameters, such as, cell viability and oxidative stress measured after UVA and UVB radiations contribute to the evaluation of the photoprotective potential of substances and plant extracts. These data corroborate the validation performed by Figueiredo et al. [12] during the evaluation of the photoprotective potential of natural products with different UV absorption profiles such as Garcinia brasiliensis extract (UVB absorber) and astaxanthin (UVA absorber). This validation shows that cell viability and ROS generation are models that allow researchers to evaluate the photoprotective ability of compounds when exposed to UVB and UVA radiation, respectively.
3.4 The formulation containing I. edulis purified fraction presents photoprotective/photochemopreventive potential in vivo
The in vivo evaluation of the photoprotective/photochemoprotective potential of the formulation containing the purified fraction was performed on hairless mice skin. Skin samples were subjected to myeloperoxidase enzyme activity assays and quantification of the cytokines IL-1β, TNF-α and the chemokine CXCL1/KC.
3.4.1 The formulation reduces MPO activity induced by UVB radiation
The initiation and maintenance of the acute inflammatory response is associated with the activation and recruitment of neutrophils to the injured tissue. The migration of neutrophils from the vascular lumen to the inflamed area involves a complex and dynamic process. The neutrophil needs to recognize the appropriate adhesion molecules on the surface of the activated endothelium, penetrate the endothelial cell monolayer and basement membrane, and follow chemotactic signals to the injured tissue [42]. Recent evidence suggests that the enzyme MPO, a heme peroxidase, whose expression occurs in neutrophils and monocytes, is a critical determinant of the inflammatory process. MPO enzyme catalyzes H2O2 oxidation of halide to hypohalite, e.g., chloride (Cl−) oxidation to hypochlorite (OCl−), resulting in the production of highly reactive intermediates that can induce damages to the inflamed tissue [43].
Exposure of the cells to 2.87 J/cm2 of UVB radiation resulted in a significant (p < 0.05) increase of 247% and 195% in MPO activity in the skin of animals in the irradiated control (IC) and irradiated placebo (IP) groups, respectively, by comparison with non-irradiated animals (NIC). There was no significant difference (p < 0.05) between the MPO activity found in the IC and IP groups (Fig. 6). These results corroborate the study of Divya et al. [6] whose MPO activity also had a significant increase after exposure of the skin of animals to UVB radiation compared to the non-irradiated control group.
Quantification of myeloperoxidase enzyme activity in hairless mice skin exposed or not exposed to 2.87 J/cm2 of UVB radiation. NIC = non-irradiated control (animals not treated with formulation and not irradiated), NIP = non-irradiated placebo (animals treated with placebo formulation and not irradiated), NIF = non-irradiated formulation (animals treated with formulation added of purified fraction and not irradiated), IC = irradiated control (animals not treated with formulation and irradiated), IP = irradiated placebo (animals treated with placebo formulation and irradiated), IF = irradiated formulation (animals treated with formulation added of purified fraction and irradiated). Results represent the mean of 5 animals per group ± SD. *p < 0.05 significant difference compared to non-irradiated control (NIC) using one-way ANOVA, following Tukey’s multiple comparisons test. a.b.cThe same letters are not significantly different. MPO myeloperoxidase, SD standard deviation
On the other hand, the skin of animals treated with the formulation containing the purified fraction (IF) for two hours, followed by UVB irradiation, showed a 48% reduction in MPO activity compared to the group of untreated and irradiated animals (IC). As for the non-irradiated groups (NIC, NIP, and NIF), there was no statistically significant difference (p < 0.05) between them.
The decrease in MPO activity in the group of animals treated with the formulation containing the purified fraction and exposed to UVB radiation can be explained by the inhibition of neutrophil migration to the irradiated area, or by the inhibition of the activity of this enzyme by the compounds of the purified fraction that penetrated the skin.
Lee et al. [44] showed that vicenin-2 significantly reduced neutrophil infiltration when measuring MPO levels in rat kidney tissues. According to Zhang et al. [45], myricitrin is also able to reduce inflammatory cell infiltration in the colon tissue. This suggests that the decrease in MPO activity in the group of animals treated with the formulation containing the purified fraction and exposed to UVB radiation may be related to the action of the flavonoids vicenin-2 and myricitrin, the main compounds of the purified fraction. These compounds might be responsible for reducing the activity of the synthesized MPO by neutrophils.
3.4.2 The formulation reduces cytokines IL-1β, TNF-α and the chemokine CXCL1/KC induced by UVB
Solar UV radiation disrupts the oxidant/antioxidant balance in the skin, leading to oxidative stress, which is accompanied by the progression of inflammatory disorders. Both UVA and UVB radiations induce the expression of COX-2 and nitric oxide synthase (NOS). The nitric oxide (NO) produced by NOS, acts as a secondary messenger, modulating the formation of endogenous ROS, which together prostaglandin E2 (PGE2), synthesized by the action of COX-2, with the released pro-inflammatory cytokines (IL-1β and TNF-α) and chemokines, orchestrate the inflammatory response. In addition, direct UVB radiation and the ROS generated by this radiation and by UVA radiation activate some signaling cascades that lead to the activation of nuclear factor kappa β (NF-ƙβ) and MAPK that mediate the expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, Interleukin (IL-8)) [41].
Studies have shown that the major cytokines released by skin cells after an exposure to solar UV radiation include IL-1 and IL-6, TNF-α, and the chemokine IL-8 [46]. IL-8 differs from all other chemokines in its ability to mediate chemotaxis of neutrophils which are its primary target. However, neutrophils are not only targets, but also synthesizers of this chemokine. Two receptors for IL-8 are expressed on the surface of neutrophils, C-X-C motif chemokine receptor 1 (CXCR1) and C-X-C motif chemokine receptor 2 (CXCR2) [47, 48]. Once recruited to the inflammatory focus, neutrophils exhibit an altered ability (usually up-regulated) for chemokine expression/production, thus contributing to the regulation of leukocyte accumulation [49].
In this study, UVB radiation exposure (IC group) provided an increase of approximately 532% in the level of IL-1β when compared with the NIC group. The other non-irradiated groups (placebo and formulation) showed no statistically significant difference compared to NIC. The animals exposed to UVB radiation and treated with the formulation containing the purified fraction of I. edulis were able to reduce approximately 37% the amount of the pro-inflammatory cytokine IL-1β (Fig. 7A).
Quantification of the cytokines IL-1β (A) and TNF-α (B) and of the chemokine CXCL1/KC (C) in skin of hairless mice unexposed or exposed to 2.87 J/cm2 of UVB radiation. NIC = non-irradiated control (animals not treated with formulation and not irradiated), NIP = non-irradiated placebo (animals treated with placebo formulation and not irradiated), NIF = non-irradiated formulation (animals treated with formulation added of purified fraction and not irradiated), IC = irradiated control (animals not treated with formulation and irradiated), IP = irradiated placebo (animals treated with placebo formulation and irradiated), IF = irradiated formulation (animals treated with formulation added of purified fraction and irradiated). Results represent the mean of 5 animals per group ± SEM with p < 0.05, using one-way ANOVA, following Tukey’s multiple comparisons test. a.b.cThe same letters are not significantly different. IL interleukin, TNF-α tumor necrosis factor-alpha, CXCL1/KC C-X-C Motif Chemokine Ligand 1/keratinocyte-derived chemokine
Animals untreated and exposed to UVB demonstrated a 26% increase in the pro-inflammatory cytokine TNF-α compared to the NIC group (Fig. 7B). Taking into account the average of the results of the NIP and NIF groups (2.067 pg/mg of skin), the increase in TNF-α provided by UVB radiation was 134.64%. The skin of the animals exposed and treated with the formulation added with the purified fraction of I. edulis showed a TNF-α content 32% lower than the IC group. The TNF-α level of the animals irradiated and treated with the placebo formulation was statistically similar to the IC, which shows the non-interference of the formulation vehicle.
The data in Fig. 7C show a 52% reduction in the amount of the chemokine CXCL1/KC (corresponding to IL-8 in humans) in the skin of irradiated hairless mice treated with the formulation added 1% of the purified fraction.
Thus, it is suggested that IL-1β, TNF-α and CXCL1/KC levels were reduced by the compounds in the purified fraction incorporated into the formulation.
The deleterious effects of UVA and UVB radiations on human skin are mainly caused by ROS. These ROS induce oxidative stress and inflammation through activation of MAPK, induction of expression of constitutive COX-2, and expression of pro-inflammatory proteins such as TNF-α, IL-6 and iNOS. Cells activated by inflammatory mediators promote the activation of NF-κβ, which dissociates from Iκβ and translocate to the nucleus, where it induces the expression of several pro-inflammatory genes (TNF-α, IL-6, IL-1β). Overexpression of these cytokines further enhances NF-κβ activation forming a feedback loop. NF-κβ and AP-1 induce expression of iNOS and COX-2 [44, 50].
Lee et al. [44] and Yin et al. [50] showed that vicenin-2 was able to reduce the levels of TNF-α, IL-6, iNOS and NO in mice renal damage induced by cecal ligation and puncture (CLP), and in the colon tissues of mice with dextran sulfate sodium (DSS)-induced colitis, respectively. The reduction of levels of inflammatory mediators was mediated by inhibition of the NF-κβ signaling pathway which is associated with increased antioxidant defenses and decreased lipid peroxidation. Myricitrin was able to inhibit the phosphorylation of kinases in vivo (mouse model) and to promote the reduction of iNOS mRNA and protein levels. Both actions resulted in the decrease of inflammatory markers such as PKC, COX-2, NOS, CXCL-1/KC, IL-6 and TNF-α in the colon tissue [29, 45].
Another research demonstrated that myricetin, the aglycon of myricitrin, downregulates the production of inflammatory factors such as TNF-α, IL-6 and IL-1β triggered by lipopolysaccharide, thereby reducing inflammation. This regulation was attributed to the inhibition of the AKT/IKK/NF-κβ and TLR4-MyD88 -NF-κβ signaling pathway. The study was performed on models of mastitis and pulmonary inflammation [51,52,53]. Myricetin also reduces the upregulation of COX-2 by inhibiting Iκβ/NFκβ, Akt and mTOR signaling, reduces the expression of cytokines and chemokines in keratinocytes inhibiting skin inflammation induced by TNF-α and ultraviolet light [53,54,55].
These data corroborate the results of the present study, which showed that the formulation containing the purified fraction of I. edulis, rich in vicenin-2 and myricitrin, may act as blockers of inflammatory mediators contributing to photoprotection and photochemoprevention of the skin.
4 Conclusion
The purified fraction of the I. edulis leaves extract presents high polyphenol and flavonoid contents, the major compounds being myricitrin (myricetin 3-rhamnoside) and vicentin-2. First, the in vitro studies showed that the purified fraction was able to protect L929 fibroblast cells against the oxidative stress induced by H2O2 and UVA radiation. Additionally, the purified fraction also showed a photoprotective effect against UVA and UVB radiation in L929 fibroblast cells.
Furthermore, the in vivo skin penetration studies have shown that myricitrin and vicentin-2 were able to penetrate the skin when incorporated into a topical formulation increasing the endogenous antioxidant potential of the skin. Finally, the formulation containing the purified fraction provided an anti-inflammatory effect on the skin of animals after exposure to UVB radiation, since it was observed a reduction in MPO activity, IL-1β and TNF-α cytokines, and CXCL1/KC chemokine. Therefore, the present study demonstrated that the purified fraction of I. edulis appears as an alternative to prevent skin damages induced by UVR, due to its antioxidant and anti-inflammatory properties, and deserves to be further investigated regarding the mechanisms involved with these effects.
Data availability
Data will be available on demand.
References
Lai-Cheong, J. E., & McGrath, J. A. (2009). Structure and function of skin, hair and nails. Medicine, 37(5), 223–226. https://doi.org/10.1016/j.mpmed.2009.03.002
Sharma, R. R., Deep, A., & Abdullah, S. T. (2021). Herbal products as skincare therapeutic agents against ultraviolet radiation-induced skin disorders. Journal of Ayurveda and Integrative Medicine, 13(1), 100500. https://doi.org/10.1016/j.jaim.2021.07.016
Holick, M. F. (2016). Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Research, 36(3), 1345–1356.
de Oca, M. K. M., Pearlman, R. L., McClees, S. F., Strickland, R., & Afaq, F. (2017). Phytochemicals for the prevention of photocarcinogenesis. Photochemistry Photobiology, 93, 956–974. https://doi.org/10.1111/php.12711
Figueiredo, S. A., de Moraes, D. C., Vilela, F. M. P., de Faria, A. N., dos Santos, M. H., & Fonseca, M. J. V. (2018). A novel research model for evaluating sunscreen protection in the UV-A1. Journal of Photochemistry and Photobiology, B: Biology, 178, 61–68. https://doi.org/10.1016/j.jphotobiol.2017.10.031
Divya, S. P., Wang, X., Pratheeshkumar, P., Son, Y.-O., Roy, R. V., Kim, D., Dai, J., Hitron, J. A., Wang, L., Asha, P., Shi, X., & Zhang, Z. (2015). Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicology and Applied Pharmacology, 284(1), 92–99. https://doi.org/10.1016/j.taap.2015.02.003
Figueiredo, S. A., Vilela, F. M. P., da Silva, C. A., Cunha, T. M., dos Santos, M. H., & Fonseca, M. J. V. (2014). In vitro and in vivo photoprotective/photochemopreventive potential of Garcinia brasiliensis epicarp extract. Journal of Photochemistry and Photobiology B: Biology, 131, 65–73. https://doi.org/10.1016/j.jphotobiol.2014.01.004
Afaq, F., & Mukhtar, H. (2006). Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Experimental Dermatology, 15, 678–684. https://doi.org/10.1111/j.1600-0625.2006.00466.x
Radhiga, T., Agilan, B., Muzaffer, U., Karthikeyan, R., Kanimozhi, G., Paul, V. I., & Prasad, N. R. (2016). Phytochemicals as modulators of ultraviolet-b radiation induced cellular and molecular events: A review. Journal of Radiation and Cancer Research, 7(1), 2–12. https://doi.org/10.4103/0973-0168.184607
Alves, G. A. D., da Silva, D. F., Teixeira, T. V., de Souza, R. O., Rogez, H., & Fonseca, M. J. V. (2020). Obtainment of an enriched fraction of Inga edulis: Identification using UPLC-DAD-MS/MS and photochemopreventive screening. Preparative Biochemistry & Biotechnology, 50(1), 28–36. https://doi.org/10.1080/10826068.2019.1658118
Souza, J. N. S., Silva, E. M., da Silva, M. N., Arruda, M. S. P., Larondelle, Y., & Rogez, H. (2007). Identification and antioxidant activity of several flavonoids of Inga edulis leaves. Journal of the Brazilian Chemical Society, 18, 1276–1280. https://doi.org/10.1590/S0103-50532007000600025
Figueiredo, S. A., Vilela, M. F. P., dos Anjos, T. N., de Pádua, A. N. F., & Fonseca, M. J. V. (2021). Evaluation of cell biomarkers as in vitro photoprotective assays for sunscreen formulations. Journal of Basic and Applied Pharmaceutical Sciences, 42, e713. https://doi.org/10.4322/2179-443X.0713
Laporte, A., Lortz, S., Schaal, C., Lenzen, S., & Elsner, M. (2020). Hydrogen peroxide permeability of cellular membranes in insulin-producing cell. BBB-Biomembranes, 1862(2), 183096. https://doi.org/10.1016/j.bbamem.2019.183096
Yu, D., Zha, Y., Zhong, Z., Ruan, Y., Li, Z., Sun, L., & Hou, S. (2021). Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation. Sensors and Actuators B: Chemical, 339, 129878. https://doi.org/10.1016/j.snb.2021.129878
Wang, H., & Joseph, J. A. (1999). Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology & Medicine, 27, 612–616. https://doi.org/10.1016/S0891-5849(99)00107-0
Silva, D. F. (2012). Extract and medium polarity fraction of Inga edulis: Physico chemical, functional, cytotoxic studies and penetration/retention evaluation of cutaneous topical formulations (p. 119). Dissertation (master). Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, Brazil
Vilela, F. M. P., Fonseca, Y. M., Jabor, J. R., Vicentini, F. T. M. C., & Fonseca, M. J. V. (2012). Effect of ultraviolet filters on skin superoxide dismutase activity in hairless mice after a single dose of ultraviolet radiation. European Journal of Pharmaceutics and Biopharmaceutics, 80, 387–392. https://doi.org/10.1016/j.ejpb.2011.10.005
Girotti, S., Fini, F., Ferri, E., Budini, R., Piazzi, S., & Cantagalli, S. (2000). Determination of superoxide dismutase in erytrocytes by a chemiluminescent assay. Talanta, 51, 685–692. https://doi.org/10.1016/S0039-9140(99)00332-X
Georgetti, S. R., Casagrande, R., Di Mambro, M. V., Azzolini, A. E. C. S., & Fonseca, M. J. V. (2003). Evaluation of the antioxidant activity of different flavonoids by the chemiluminescent method. AAPS PharmSci, 5, 210–214. https://doi.org/10.1208/ps050216
Cayrol, C., Sarraute, J., Tarroux, R., Redoules, D., Charveron, M., & Gall, Y. (1999). A mineral sunscreen affords genomic protection against ultraviolet (UV) B and UVA radiation: in vitro and in situ assays. British Journal of Dermatology, 141(2), 250–258. https://doi.org/10.1046/j.1365-2133.1999.02973.x
Chrétien, M. N., Heafey, E., & Scaiano, J. C. (2010). Reducing adverse effects from UV sunscreens by zeolite encapsulation: Comparison of oxybenzone in solution and in zeolites. Photochemistry and Photobiology, 86, 153–161. https://doi.org/10.1111/j.1751-1097.2009.00644.x
Yoshimoto, S., Kohara, N., Sato, N., Ando, H., & Ichihashi, M. (2020). Riboflavin plays a pivotal role in the UVA-induced cytotoxicity of fibroblasts as a key molecule in the production of H2O2 by UVA radiation in collaboration with amino acids and vitamins. International Journal of Molecular Sciences, 21(2), 554. https://doi.org/10.3390/ijms21020554
Vicentini, F. T. M. C., Simi, T. R. M., Del Ciampo, J. O., Wolga, N. O., Pitol, D. L., Iyomasa, M. M., Bentley, M. V. L. B., & Fonseca, M. J. V. (2008). Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. European Journal of Pharmaceutics and Biopharmaceutics, 69(3), 948–957. https://doi.org/10.1016/j.ejpb.2008.01.012
Vilela, F. M. P., Fonseca, Y. M., Vicentini, F. T. M. C., & Fonseca, M. J. V. (2013). Sunscreen protection against ultraviolet-induced oxidative stress: Evaluation of reduced glutathione levels, metalloproteinase secretion, and myeloperoxidase activity. Die Pharmazie - An International Journal of Pharmaceutical Sciences, 68(11), 872–876. https://doi.org/10.1691/ph.2013.3045
Vilela, F. M. P., Oliveira, F. M., Vicentini, F. T. M. C., Casagrande, R., Verri, W. A., Jr., Cunha, T. M., & Fonseca, M. J. V. (2016). Commercial sunscreen formulations: UVB irradiation stability and effect on UVB irradiation-induced skin oxidative stress and inflammation. Journal of Photochemistry and Photobiology, B: Biology, 163, 413–420. https://doi.org/10.1016/j.jphotobiol.2016.09.007
Bradley, P. P., Priebat, D. A., Christensen, R. D., & Rothstein, G. (1982). Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology, 78, 206–209. https://doi.org/10.1111/1523-1747.EP12506462
Casagrande, R., Georgetti, S. R., Verri, W. A., Jr., Dorta, D. J., dos Santos, A. C., & Fonseca, M. J. V. (2006). Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. Journal of Photochemistry and Photobiology B: Biology, 84(1), 21–27. https://doi.org/10.1016/j.jphotobiol.2006.01.006
Zou, B., Xiao, G., Xu, Y., Wu, J., Yu, Y., & Fu, M. (2018). Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chemistry, 255, 23–30. https://doi.org/10.1016/j.foodchem.2018.02.028
Domitrović, R., Rashed, K., Cvijanović, O., Vladimir-Knežević, S., Škoda, M., & Višnić, A. (2015). Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chemico-Biological Interactions, 230, 21–29. https://doi.org/10.1016/j.cbi.2015.01.030
Petruk, G., Illiano, A., Giudice, R. D., Raiola, A., Amoresano, A., Rigano, M. M., Piccoli, R., & Monti, D. M. (2017). Malvidin and cyanidin derivatives from açai fruit (Euterpe oleracea Mart.) counteract UV-A-induced oxidative stress in immortalized fibroblasts. Journal of Photochemistry and Photobiology B: Biology, 172, 42–51. https://doi.org/10.1016/j.jphotobiol.2017.05.013
Ryšava, A., Čižkova, K., Frankova, J., Roubalova, L., Ulrichova, J., Vostalovaa, J., Vrba, J., Zalešak, B., & Svobodova, A. R. (2020). Effect of UVA radiation on the Nrf2 signaling pathway in human skin cells. Journal of Photochemistry & Photobiology, B: Biology, 209, 111948. https://doi.org/10.1016/j.jphotobiol.2020.111948
Jiang, S. J., Chen, J. Y., Lu, Z. F., Yao, J., Che, F. D., & Zhou, X. J. (2006). Biophysical and morphological changes in the stratum corneum lipids induced by UVB irradiation. Journal of Dermatological Science, 44, 29–36. https://doi.org/10.1016/j.jdermsci.2006.05.012
Budai, M., Reynaud-Angelin, A., Szabo, Z., Tóth, S., Rontó, G., Sage, E., & Gróf, P. (2004). Effect of UVA radiation on membrane fluidity and radical decay in human fibroblasts as detected by spin labeled stearic acids. Journal of Photochemistry and Photobiology B: Biology, 77, 27–38. https://doi.org/10.1016/j.jphotobiol.2004.08.003
Moser, K., Kriwet, K., Naik, A., Kalia, Y. N., & Guy, R. H. (2001). Passive skin penetration enhancement and its quantification in vitro. European Journal of Pharmaceutics and Biopharmaceutics, 54(2), 103–112. https://doi.org/10.1016/S0939-6411(01)00166-7
Getie, M., Gebre-Mariam, T., Rietz, R., & Neubert, R. H. H. (2002). Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Pharmazie, 57, 320–322.
Wester, C. R. & Maibach, H. L. (1990). In vitro testing of topical pharmaceutical formulations. In: D. W. Osborne & A. H. Amamnn, Topical drug delivery formulations (pp. 213–220). Marcel Dekker
Harborne, J. B., & Williams, C. A. (2000). Advances in flavonoid research since 1992. Phytochemistry, 55, 481–504. https://doi.org/10.1016/S0031-9422(00)00235-1
Liakoura, V., Bornean, J. F., & Karabourniotis, G. (2003). The ability of abaxial and adaxial epiderms of sun and shade leaves to attenuate UV-A and UV-B radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology, 122, 117–125. https://doi.org/10.1034/j.1399-3054.2003.1170104.x
Merzlyak, M. N., Solovchenko, A. E., Smagin, A. I., & Gitelson, A. A. (2005). Apple flavonols during fruit adaptation to solar radiation: spectral features and technique for non-destructive assessment. Journal of Plant Physiology, 162, 151–160. https://doi.org/10.1016/j.jplph.2004.07.002
Halliday, G. M., Norval, M., Byrne, S. N., Huang, X. X., & Wolf, P. (2008). The effects of sunlight on the skin. Drug Discovery Today: Disease Mechanisms/Common skin conditions and disorders, 5(2), e201–e209. https://doi.org/10.1016/j.ddmec.2008.04.005
Svobodova, A., Walterova, D., & Vostalova, J. (2006). Ultraviolet light induced alteration to the skin. Biomedical papers of the Medical Faculty of the University Palacký, Olomouc, Czechoslovakia, 150(1), 25–38. https://doi.org/10.5507/bp.2006.003
Luster, A. D., Alon, R., & Von Adrian, U. H. (2005). Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunology, 6(12), 1182–1190.
Khan, A. A., Alsahli, M. A., & Rahmani, A. H. (2018). Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Medical Sciences (Basel, Switzerland), 6(2), 33. https://doi.org/10.3390/medsci6020033
Lee, B.-S., Yang, S., Lee, C., Ku, S.-K., & Bae, J.-S. (2020). Renal protective effects of vicenin-2 and scolymoside in a mouse model of sepsis. Brazilian Journal of Pharmaceutical Sciences, 56, e18636. https://doi.org/10.1590/s2175-97902019000418636
Zhang, X., Zhang, K., Wang, Y., & Ma, R. (2020). Effects of myricitrin and relevant mechanisms. Current Stem Cell Research & Therapy, 15, 11–17. https://doi.org/10.2174/1574888X14666181126103338
Müller, K., & Meineke, V. (2007). Radiation-induced alterations in cytokine production by skin cells. Experimental Hematology, 35, 96–104. https://doi.org/10.1016/j.exphem.2007.01.017
Baggiolini, M., Dewald, B., & Moser, B. (1994). Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Advances in Immunology, 55, 97–179. https://doi.org/10.1016/S0065-2776(08)60509-X
Murphy, P. M. (1997). Neutrophil receptors for interleukin-8 and related CXC chemokines. Seminars in Hematology, 34(4), 311–318.
Cassatella, M. A. (1999). Neutrophil-derived proteins: selling cytokines by the pound. Advances in Immunology, 73, 369–509. https://doi.org/10.1016/s0065-2776(08)60791-9
Yin, Y., Ye, L., Niu, Z., & Fang, W. (2019). Anti-inflammatory effects of vicenin-2 on dextran sulfate sodium-induced colitis in mice. Drug Development Research, 80, 546–555. https://doi.org/10.1002/ddr.21529
Kan, X., Liu, B., Guo, W., Wei, L., Lin, Y., Guo, Y., Gong, Q., Li, Y., Xu, D., Cao, Y., Huang, B., Dong, A., Ma, H., Fu, S., & Liu, J. (2019). Myricetin relieves LPS-induced mastitis by inhibiting inflammatory response and repairing the blood-milk barrier. Journal of Cellular Physiology, 234, 16252–16262. https://doi.org/10.1002/jcp.28288
Mao, M. T., & Huang, M. Y. (2017). Myricetin attenuates lung inflammation and provides protection against lipopolysaccharide-induced acute lung injury by inhibition of NF-κB pathway in rats. Tropical Journal of Pharmaceutical Research, 16(11), 2585–2593. https://doi.org/10.4314/tjpr.v16i11.3
Song, X., Tan, L., Wang, M., Ren, C., Guo, C., Yang, B., Ren, Y., Cao, Z., Li, Y., & Pei, J. (2021). Myricetin: A review of the most recente research. Biomedicine & Pharmacotherapy, 134, 111017. https://doi.org/10.1016/j.biopha.2020.111017
Lee, D., & Lee, C. S. (2016). Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κβ pathways in human keratinocytes. European Journal of Pharmacology, 784, 164–172. https://doi.org/10.1016/j.ejphar.2016.05.025
Xie, J., & Zheng, Y. (2017). Myricetin protects keratinocyte damage induced by UV through IκB/NFκb signaling pathway. Journal of Cosmetic Dermatology, 16(4), 444–449. https://doi.org/10.1111/jocd.12399
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support. The authors also thank Professor Hervé Louis Ghislain Rogez from Federal University of Pará - Brazil for kindly providing the purified fraction of Inga edulis extract.
Funding
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil [grant number 166315/2013-3] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, Brazil [grant number 001].
Author information
Authors and Affiliations
Contributions
KCC performed the experiments, collected the data and evaluated the results; FMPV and SAF performed the evaluation and interpretation of the data and the writing of the article; CHFC performed the evaluation and interpretation of the data; MJVF designed the study and performed the interpretation of the data and wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-fnancial interests to disclose.
Ethical approval
The Ethics Committee of the Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, approved the study with the use of animals under Protocol # 13.1.525.53.1.
Consent of publication
All authors agree with the publication of this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costa, K.C., Cuelho, C.H.F., Figueiredo, S.A. et al. Photochemoprevention of topical formulation containing purified fraction of Inga edulis leaves extract. Photochem Photobiol Sci 22, 2105–2120 (2023). https://doi.org/10.1007/s43630-023-00433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00433-1