Abstract
The COVID-19 pandemic that started in 2019 and resulted in significant morbidity and mortality continues to be a significant global health challenge, characterized by inflammation, oxidative stress, and immune system dysfunction.. Developing therapies for preventing or treating COVID-19 remains an important goal for pharmacology and drug development research. Polyphenols are effective against various viral infections and can be extracted and isolated from plants without losing their therapeutic potential. Researchers have developed methods for separating and isolating polyphenols from complex matrices. Polyphenols are effective in treating common viral infections, including COVID-19, and can also boost immunity. Polyphenolic-based antiviral medications can mitigate SARS-CoV-2 enzymes vital to virus replication and infection. Individual polyphenolic triterpenoids, flavonoids, anthraquinonoids, and tannins may also inhibit the SARS-CoV-2 protease. Polyphenol pharmacophore structures identified to date can explain their action and lead to the design of novel anti-COVID-19 compounds. Polyphenol-containing mixtures offer the advantages of a well-recognized safety profile with few known severe side effects. However, studies to date are limited, and further animal studies and randomized controlled trials are needed in future studies. The purpose of this study was to review and present the latest findings on the therapeutic impact of plant-derived polyphenols on COVID-19 infection and its complications. Exploring alternative approaches to traditional therapies could aid in developing novel drugs and remedies against coronavirus infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In late 2019, a cluster of viruses identified as the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) surfaced in Wuhan, China, resulting in cases of pneumonia [1, 2]. SARS-CoV-2, a highly transmissible virus with an initially unknown identity, has sparked a global pandemic, affecting more than 500 million individuals and leading to the tragic loss of over 6 million lives by April 2022 [3]. Patients infected with COVID-19 exhibit a diverse array of non-specific symptoms, such as high body temperature, aching throat, queasiness, throwing up, muscle pain, and a feeling of lightheadedness [4]. While the majority of individuals typically recover from their illnesses within a short period, approximately five percent of patients may endure severe and life-threatening conditions, such as acute respiratory distress syndrome (ARDS) and multiple organ dysfunctions [5]. The elderly and individuals with chronic diseases are at a higher risk of severe COVID-19 [6]. Despite the development of vaccines for COVID-19, new viral variants, such as Delta, Lambda, and Omicron, continue to emerge [7]. The increased spread of COVID-19 is attributed to the rapid genetic recombination of the S protein in the receptor-binding domain (RBD) [8]. The virus manifests itself in different organs such as the brain, kidneys, lungs, and gastrointestinal tract, with angiotensin-converting enzyme-2 (ACE2) being a key player in the development of the disease [9,10,11].
COVID-19 follows three stages, including primary viral infection, the pulmonary stage, and the inflammatory stage [12, 13]. Cytokine storms of upregulated interleukins tumor necrosis factor and excessive coagulation contribute to further disease severity and mortality [14]. Critically ill COVID-19 patients show higher levels of neutrophils, proinflammatory cytokines, and reactive oxygen species (ROS) [15]. Endothelial cell dysfunction and platelet aggregation can also worsen the severity of COVID-19 [15]. Polyphenols, which are abundant antioxidants found in the diet, can have anti-inflammatory and antiviral effects [16]. Polyphenols have one or more phenolic rings containing hydroxyl groups and are classified into several active types, such as flavonoids, isoflavones, and phenols [17,18,19,20]. The immune system, inflammatory cytokine storm, and oxidative stress play significant roles in the pathogenesis of COVID-19 [21]. Therefore, therapeutic agents with anti-viral, anti-inflammatory, antioxidant, and immunomodulatory activities may be beneficial in preventing and treating COVID-19 [22]. This review article aims to discuss the potential beneficial effects of various polyphenols in treating COVID-19.
Methods
For this research, we conducted a thorough search across various online databases like Web of Science, MEDLINE (PubMed), Scopus, and Embase, utilizing specific keywords such as "Polyphenols," "viral diseases," "virus-related diseases," "COVID-19," "SARS-CoV-2," and "coronaviruses." Our focus was on selecting peer-reviewed studies written in English that were pertinent to our study. Any articles lacking sufficient data on polyphenols and COVID-19 were eliminated from consideration. The features of the studies that met our criteria are summarized in Table 1.
Clinical manifestations of COVID-19
COVID-19 presents a range of symptoms in the general population. This infectious disease is primarily spread through air droplets, with pulmonary symptoms being the most common. Clinically, COVID-19 can appear as either a symptomless infection or a severe illness with complications such as severe pneumonia, acute respiratory distress syndrome, respiratory failure, or organ failure [39, 40]. The available data suggests that COVID-19 can impact organs beyond the lungs, including the neurological, olfactory, cardiovascular, digestive, liver, kidney, endocrine, and skin systems. A significant number of patients experience a range of neurological symptoms related to COVID-19. Gastrointestinal symptoms are often linked to a longer duration of illness. Liver function impairment is associated with the severity of the disease. Critically ill COVID-19 patients are more prone to experiencing acute kidney injury. Additionally, diabetes ketoacidosis and high blood sugar levels are among the endocrine issues observed in COVID-19 cases. The most common skin symptoms seen were skin bumps that looked like frostbite or cold sores, then red spots with bumps, blister-like sores, purple or dead skin patches, widespread rashes, and tiny bruises [41]. Additionally, a thorough examination revealed that six symptoms were prevalent in over 25% of cases, which included fever, cough, shortness of breath, fatigue, malaise, and sputum/secretions. Alongside these common symptoms were neurological manifestations, skin issues, loss of appetite, muscle pain, sneezing, sore throat, runny nose, goosebumps, headaches, chest discomfort, and diarrhea. Hemoptysis was the least commonly reported sign/symptom. In studies that involved over 100 patients, fevers, coughs, and shortness of breath were the most frequently encountered symptoms [42].
SARS-CoV-2 entry and reproduction
The infection process of SARS-CoV-2 involves several important steps for the spike protein to attach and penetrate the host cell. The spike protein interacts with ACE2 receptors on the cell surface, facilitating the entry of the virus [43, 44]. The spike protein is then split by the activation of the S1 subunit in its receptor-binding domain, which releases the S1 and enables the S2 to merge with the cell membrane [45, 46]. The spike protein needs cellular proteases like Transmembrane serine protease 2 (TMPRSS2) to prepare for fusion with the host cell. The S protein also triggers the entry of SARS-CoV into the cell by endocytosis, which can be dependent or independent of the virus [47, 48]. Endocytosis is the process of cells taking in substances from the outside. SARS-CoV can use different ways of endocytosis to get into cells, such as clathrin, caveolae, macropinocytosis, or other new methods that do not involve clathrin or caveolae. Endocytic pathways that rely on the virus are called SARS-CoV-dependent endocytosis, while those that do not are called SARS-CoV-independent endocytosis [49, 50].
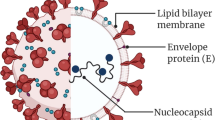
The membranes of the virus and the host cell join together, forming a fusion pore that lets the viral genome enter the cell cytoplasm [51]. This is how the virus gets to the host cell’s DNA and makes copies of itself. The viral RNA genome that gets into the cytoplasm is replicated, and the new genome is turned into two structural proteins and polyproteins [52]. The enveloped glycoproteins then go to the Golgi or the endoplasmic reticulum [52]. The nucleocapsid, which is made of nucleocapsid proteins and genomic RNA, is also created [53]. The virus particles are then found in the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), and the virus-filled vesicles fuse with the plasma membrane to let the virus out [53, 54].
The role of coronavirus infection in the presentation of antigens
When the virus infiltrates the host cells, its antigens are transferred to the antigen-presenting cells (APCs), which play a crucial role in the body's immune response against the virus [55]. The identification of antigenic peptides occurs through the interaction with the major histocompatibility complex (MHC) of the human leukocyte antigen (HLA), ultimately resulting in the activation of specific cytotoxic T lymphocytes (CTLs) targeting viral infections [55, 56]. By focusing on the STAT1-IRF1-NLRC5 axis, SARS-CoV-2 has been able to lower the levels of MHC-I expression by hindering the activation of the MHC class I pathway responsible for introducing antigens to T cells. This decrease in MHC-I activation is a result of the virus's spike glycoprotein interfering with peptide binding to MHC molecules [57, 58]. Furthermore, variations in the mannose-binding lectin (MBL) gene, which influences antigen presentation, have been correlated with susceptibility to SARS-CoV infection [59].
Humoral and cellular immunity
The humoral and cellular immune antigen response is induced by both B and T cells [60]. As with other acute viral infections, IgM and IgG antibody profiles are produced against the SARS-CoV virus [60, 61]. IgM antibodies are significantly reduced by the end of week 12, while IgG antibodies can persist for a long time, suggesting that IgG antibodies may primarily play a protective role [62, 63]. Patients with SARS-CoV-2 have been observed to have significantly fewer CD8+ and CD4+ T cells in their peripheral blood, although they are still active [64].
Immune response
COVID-19 pneumonia is characterized by hypoxemia, which can progress to acute respiratory distress syndrome and alveolar edema in severe cases [65]. The over-expression of inflammatory cytokines is a hallmark of critical COVID-19, which can be detected in both the bloodstream and the lungs [65, 66]. Various immune system responses, such as from neutrophils and macrophages, sense damaged structures and pathogens and trigger inflammatory reactions, which are supported by endothelial and epithelial cells that are susceptible to infection and inflammation-induced cell death [67, 68].
Cytokines are induced downstream of pattern recognition receptors (PRRs) that sense danger-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) related to the disorder of homeostasis or viral molecules, respectively [69, 70]. This leads to the production of specific cytokines and increases the likelihood of developing "viral sepsis" [61]. SARS-CoV-2-induced viral sepsis is characterized by high levels of pro-inflammatory cytokines, including TNFα, IL-8, IL-6, interferon-γ (IFNγ), and IL-17, which are closely associated with disease severity [62, 71]. In contrast, the essential antiviral response, dominated by type III IFN (IFNγ) and type I IFN (IFNα/β) responses, is delayed and reduced in COVID-19 patients [71, 72].
Coronavirus immune evasion
There have been sophisticated mechanisms developed by SARS-CoV-2 to evade the host immune response [73, 74]. Antagonizing dsRNA sensors [75], inhibiting IFN synthesis, and targeting viral sensing processes [76] are some strategies for evasion. Furthermore, the virus utilizes several evasion mechanisms, including spike protein camouflage, MHC-I inhibition, and interferon inhibition [77]. There are several ways in which the spike protein of SARS-CoV-2 contributes to immune evasion, including mutations that alter neutralizing antibodies and immunity to antibody-mediated immunity [78]. RBMs of RBDs are primary targets for neutralizing antibodies, and most vaccine developments for COVID-19 target the spike protein [79]. Antiviral responses are slowed by viral proteins that act on viral sensing processes so that the virus can block the immune response [76]. Additionally, SARS-CoV can create double-membrane vesicles that lack PRRs, allowing the virus to replicate within them undetected by the host cells [74, 80].
Food sources, bioavailability, and pharmacological characteristics of polyphenols
Several molecules with polyphenol structures (many hydroxyl groups on aromatic rings) have been recognized in plants and fungi [81]. These compounds are classified into diverse groups based on the structural elements that connect the rings or the number of phenolic rings they have in their structure. The flavonoids have two aromatic rings in their structure, which are connected by 3 carbon atoms, forming an oxygen heterocycle (ring C) that includes anthocyanidin flavones, flavonols, flavanones, and isoflavones [82]. Polyphenols can also be associated with various organic acids and carbohydrates, as well as with each other. Drinks and fruits such as red wine and tea are essential sources of polyphenols. Some polyphenols, such as quercetin, are found in the vast majority of plant products (cereals, vegetables, fruits, juices, legumes, tea, wine, etc.), while others are found in certain foods (isoflavones in soy, flavonoids in citrus, fluoride in apple) [83, 84].
Although polyphenols are prevalent in human food, they may not initially be effective in the body because they may be poorly absorbed in the intestine, metabolized or substrates for transporters, and are often rapidly eliminated [84]. Many polyphenols are metabolized similarly with the aglycone (without sugar) form absorbed from the small intestine [85]. The vast majority of polyphenols exist in food in the form of polymers, esters, or glycosides that cannot be readily absorbed in their native form [85]. The small intestine and the liver are the two main sites where polyphenols are conjugated during the absorption process [86]. This proceeding phase-II metabolism chiefly involves glucuronidation, methylation, and sulfation. In this process, many xenobiotics undergo metabolic detoxification that serves to limit any potential toxic effects and enhances their hydrophilicity, thus enabling the polar metabolites to be effectively eliminated through bile and urine [86].
The conjugation mechanisms are very efficient, so aglycones are commonly present in low concentrations in blood after the use of nutritional doses. Circulating polyphenols are extensively bound to albumin. Lactase-phlorizin hydrolase (LPH), which is particularly abundant in small intestinal epithelial cells, is substrate-specific for flavonoid-O-beta glycosides, and the released aglycone may enter epithelial cells as a result of inactive release [87]. On the other hand, there is also another digestive enzyme called cytosolic beta-glucosidase (CBG) in epithelial cells, which intermittently hydrolyzes many phenolic glycosides after transfer through the epithelium [88]. For CBG glycosylation to occur, (polar) glycosides must first be transferred to epithelial cells [87, 89].
Numerous polyphenols, such as luteolin, curcumin, quercetin, and resveratrol, play an important role in various physiological conditions of the body, such as regulating the body's metabolism, including pain, digestion, depression, and detoxification [90,91,92,93,94,95]. Polyphenols are also effective in biochemical pathways such as inhibiting NF-κB, TLR-4, and MAPK pathways, reducing the expression of lipid peroxidation, increasing the expression of Nrf-2, and modulating the activity of the immune system [96]. Furthermore, polyphenols have been shown to play a significant role in the prevention and management of various diseases, including cancer, infectious diseases, cardiometabolic disorders, and weight loss [84, 97,98,99,100,101,102,103,104,105,106,107,108,109], though controversial reports also exist [110,111,112,113]. Ongoing research is focused on determining the exact mechanisms by which these compounds exert their effects on disease processes. As such, incorporating polyphenol-rich foods into our diets may have potential therapeutic benefits in the prevention and treatment of various diseases. Figure 1 shows the structure of some selected flavonoids.
Anti-inflammatory and antioxidant effects of polyphenols
Polyphenols have been shown to possess anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines and enzymes [114]. These substances can disrupt the communication pathways associated with inflammation, ultimately decreasing the release of substances that cause inflammation. By inhibiting the creation of signaling molecules like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), polyphenols have the potential to alleviate the extreme inflammatory reaction observed in severe cases of COVID-19 [115]. Curcumin and resveratrol exert antioxidant effects by activating Nrf2 and inhibiting the NFκB pathway [93, 94, 116,117,118]. The synergistic blend of curcumin, vitamin C, and glycyrrhizinic acid enhances interferon production and modulates the inflammatory response, thereby bolstering the immune defense against SARS-CoV-2 infection. Additionally, curcumin and quercetin are pivotal in regulating iron levels through their iron-chelating properties. Essentially, they function as iron chelators, resulting in hepatocyte degradation, a reduction in the iron storage protein ferritin, an elevation in TfR1, and stimulation of iron regulatory proteins [119, 120]. Research has shown that hydroxytyrosol possesses strong antioxidant and anti-inflammatory properties, making it a potential treatment for pulmonary fibrosis in rats. Additionally, it has been found to reduce oxidative stress caused by respiratory neutrophil bursts. Another polyphenol, Epigallocatechin gallate (EGCG), commonly found in teas, has been proven to inhibit pro-inflammatory agents including TNF, IL-1, COX-2, iNOS, IL-1β, and IL-6 signaling pathways [121] and suppress the gene or protein expression of inflammatory cytokines and inflammation-related enzymes in green tea [28]. EGCG can also play a role in suppressing inflammation by reducing the expression of nuclear factor-kB and other inflammatory factors such as MAPKs and AKT/PI3K/mTOR [29, 121]. Kaempferol has been recognized as a promising candidate for treating both COVID-19 and pulmonary fibrosis (PF) conditions. It works by targeting key proteins such as epidermal growth factor receptor (EGFR), proto-oncogene tyrosine-protein kinase SRC (SRC), mitogen-activated protein kinase 3 (MAPK3), MAPK1, MAPK8, RAC-alpha serine/threonine-protein kinase (AKT1), transcription factor p65 (RELA), and phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) [122]. Furthermore, studies have revealed that EGFR, interleukin 17 (IL-17), tumor necrosis factor (TNF), hypoxia-inducible factor 1 (HIF-1), phosphoinositide 3-kinase/AKT serine/threonine kinase (PI3K/AKT), and Toll-like receptor signaling pathways play a critical role in combatting the co-occurrence of COVID-19 and pulmonary fibrosis [122]. In a study conducted by Tang et al. [123], it was found that administering quercetin to rats with sepsis led to a notable decrease in the expression of TNF-α and HMGB 1, while also resulting in an increase in AMPK expression. Additionally, quercetin was observed to hinder the production of inflammatory mediators like leukotrienes and prostaglandins in septic mice by suppressing inflammatory enzymes such as COX-2.
Moreover, quercetin was found to be effective in promoting the M2 polarization of macrophages through the activation of AMPK-activating transcription factor 3 (ATF3) pathways, which are calcium-dependent. This natural compound also prevents the excessive production of inflammatory factors [124]. Not only do polyphenols in green tea possess antioxidant qualities, but they have also been found to exhibit pro-oxidant properties, though this impact is not necessarily detrimental. Research has indicated that the consumption of green tea can help lower oxidative stress and protect cells from damage. In certain illnesses, the polyphenols found in green tea may contribute to maintaining redox balance [125]. Due to their properties, polyphenols have been discovered to have anti-inflammatory and health-promoting effects that play a key role in preventing disease. Moreover, polyphenols help in reducing the production of inflammatory cytokines and genes related to inflammation by modulating the immune system and employing anti-inflammatory mechanisms [126]. Studies have indicated that Resveratrol can offset the negative impacts of the angiotensin II (Ang II)/angiotensin II type 1 receptor (AT1R) pathway, as well as boost the AT2R/Angiotensin 1–7 (Ang 1–7)/Mas receptor pathway. This helps protect the aging kidney from damage [127]. This particular impact of resveratrol on the two opposing pathways within the renin-angiotensin system (RAS) may also prove advantageous for individuals with SARS-CoV-2 infection [128]. Angiotensin-converting enzyme 2 (ACE-2) governs the classical RAS, which regulates blood pressure and fluid balance, but unfortunately, this enzyme is exploited by SARS-CoV-2 to infiltrate cells [129]. Resveratrol has a significant impact on the essential pathways related to the development of SARS-CoV-2. These pathways include the regulation of the RAS system, the expression of ACE-2 receptors, the activation of the immune system, and the inhibition of the release of inflammatory cytokines [130]. Significantly, resveratrol supplementation has been observed to decrease ACE-2 levels along with leptin, a pro-inflammatory adipokine, in adipose tissue where ACE-2 is abundantly present. This positive impact could potentially influence the course of COVID-19 [131]. More research is needed to fully grasp and explain the exact process by which resveratrol hinders the entry of the SARS-CoV-2 virus or reduces the severity of COVID-19. Current studies are looking into different combinations and preparations containing resveratrol. It has been observed that resveratrol effectively inhibits the replication of the SARS-CoV-2 virus without causing significant damage to cells [132, 133]. Another study conducted on human primary bronchial epithelial cell cultures infected with SARS-CoV-2 yielded similar results [134]. Xu, Li [135] showed in a study that resveratrol and polydatin, compounds extracted from the Chinese herb Polygonum cuspidatum, have a targeted inhibition on certain proteases of SARS-CoV-2 when tested in a lab setting. Furthermore, Marinella proposed that resveratrol (combined with indomethacin) may serve as a potential supplements in managing or preventing SARS-CoV-2 infection, as evidenced by promising results in a canine coronavirus model [136]. In a recent study that was randomized, double-blind, and placebo-controlled, it was demonstrated that resveratrol had a significant impact on reducing the number of hospitalizations, emergency room visits related to COVID, and cases of pneumonia in outpatients with mild COVID-19 when compared to those in the placebo group [137]. The research emphasizes the potential healing benefits of resveratrol and its ability to combat respiratory tract infections. This was explored in a review article by Filardo and colleagues in a review article [138]. Resveratrol's potential to suppress hypoxia-inducible factors indicates promise in mitigating severe symptoms of COVID-19, especially in individuals who are obese [139, 140]. In addition, resveratrol has been shown to have anti-thrombotic and anti-inflammatory effects that may help lessen the severity and mortality of COVID-19. There is potential for further research to investigate its ability to alleviate blood clotting complications linked to DNA Adenovirus vector vaccines [141]. Additional research is needed to investigate the role of resveratrol specifically in pediatric patients and elderly COVID-19 patients experiencing excessive oxidative stress [141]. To evaluate the positive impact of curcumin on patients with COVID-19, Hassaniazad et al. [142] conducted a randomized, triple-blind clinical trial. Their study focused on examining the effects of nano micelles containing curcumin on various types of immune responses at a cellular level and the overall clinical outcomes. The researchers found that curcumin was able to significantly reduce the activity of the T-Box Transcription Factor (TBX1) gene, which is associated with Th1 responses. At the same time, curcumin was shown to increase the expression of the forkhead box P3 (FOXP3) gene, which is linked to the regulatory T-cell population in COVID-19 patients. Th1 responses are known to promote inflammatory reactions by activating CD8 T lymphocytes and macrophages through the release of cytokines such as IFN-γ and IL-12 [143]. In individuals infected with COVID-19, the heightened cellular immune reactions targeting virus-infected lung cells frequently result in a cytokine storm, which plays a role in the damage to lung tissue [144]. One way to potentially reduce the impact of COVID-19 is by adjusting how the body's immune system responds. Curcumin has been shown to effectively adjust the body's immune response by decreasing the activity of the TBX1 gene, which is linked to a type of immune response known as Th1. Furthermore, curcumin also affects the activity of the RORC gene associated with Th17 responses and the GATA3 gene related to Th2 response. This suggests that curcumin not only helps regulate inflammatory immune responses through Th1 and regulatory T cells but also through Th17 and Th2 responses [145]. Curcumin has shown promise in regulating T cell-mediated immune responses in individuals with COVID-19. Research indicates that curcumin can reduce the expression of the RORC gene, which is linked to the Th17 response seen in COVID-19 patients. This suggests that curcumin may have an immunomodulatory effect on inflammatory responses driven by neutrophils, as well as the inflammation that occurs following Th17-related responses [145]. Multiple proposed mechanisms of how polyphenols can act on COVID-19 are illustrated in Fig. 2.
Proposed multiple mechanisms of action of polyphenols on COVID-19. AMPK, AMP-activated protein kinase; ERK, extracellular signal-regulated kinase; IKKα, inhibitory κB kinase α; IL-1β, interleukin-1β; iNOS, inducible nitric oxide synthase; IκBα, an inhibitor of κBα; JNK/SAPK, c-jun N-terminal or stress-activated protein kinases; LPSs, lipopolysaccharides; NF-κB, nuclear factor kappa B; NO, nitric oxide; NK, natural killer; Nrf-2, nuclear factor erythroid 2-related factor 2; p-IκBα, phosphorylated-IκBα; RNS, reactive nitrogen species; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α
Immunomodulatory effects of polyphenols
Effects of polyphenols on polymorphonuclear macrophages and monocytes
Monocytes, myeloid cells, and macrophages are crucial players in the progression of severe COVID-19. This includes various types such as monocytes, monocyte-derived macrophages (MDMs), resident alveolar macrophages (AMs), and transmissible MDMs. Their significant role cannot be understated in the severity of critical COVID-19 outcomes [146]. Research indicates that in cases of severe disease, Anti-inflammatory Macrophages (AMs) are depleted from bronchoalveolar lavage (BAL) fluids, whereas proinflammatory Monocyte-Derived Macrophages (MDMs) are plentiful in the BAL fluids of critically ill COVID-19 patients. This imbalance leads to an overproduction of inflammatory cytokines by macrophages in critically ill patients, exacerbating the severity of COVID-19 [147, 148]. Medical treatments that influence the body's immune system can be key in managing diseases. These treatments include non-steroidal anti-inflammatory drugs (NSAIDs) and small non-peptide molecules like polyphenols. Research on animals with conditions like sepsis, SARS-COVID, dynamic simulation, and molecular docking has shown that polyphenols found in plants, such as resveratrol, quercetin, epigallocatechin gallate, and curcumin, may have the ability to inhibit the impact of SARS-CoV-2 infection by regulating the immune response [149,150,151,152,153].
Research conducted on animals and in controlled laboratory environments has shown that quercetin can enhance the immune system by promoting various immune functions. These include increasing the movement of neutrophils toward infection sites, enhancing the ability of macrophages to engulf pathogens, boosting the cytotoxic activity of natural killer cells, and stimulating the proliferation of lymphocytes when exposed to certain compounds. Furthermore, data is suggesting that quercetin plays a role in regulating specific genes involved in the production of cytokines [154]. Moreover, studies have shown that curcumin can boost the differentiation of T-reg cells and promote the polarization of M2 macrophages in laboratory settings. This increase in M2 macrophages and T-reg cells could potentially aid in the reduction of pulmonary inflammation and facilitate tissue repair following curcumin administration [149, 155]. Patients suffering from COVID-19 may experience myelosuppression, a condition marked by a reduction in the amount of neutrophils and monocytes, thereby heightening their susceptibility to secondary infections. Studies in mice have shown that curcumin enhances myelopoiesis, although the exact mechanisms behind this effect remain unknown [149]. Curcumin, quercetin, and epigallocatechin gallate can influence the function of cells in bronchoalveolar lavage (BAL) fluid, which consists of five distinct subpopulations of monocytes/macrophages in the lungs. These cells play a crucial role in producing cytokines like TNF-α, IL-8, IL-6, IL-1β, and C-X-C motif chemokine 10 (CXCL10) [149,150,151,152, 156, 157]. These compounds can control a specific group of macrophages known as Macro C2-CCL3L1 and monocytes known as Mono C1-CD14-CCL3. These cells are known to produce inflammatory molecules like CCL8, CXCL10/11, CCL20, CX3CL2, CX3, and IL-6 [158,159,160]. Furthermore, substances like curcumin and quercetin have been found to suppress the generation of IL-8, a type of inflammatory protein produced by macrophages. Additionally, these compounds can decrease the production of IL-12, MIP-1α (MCP-1), by interfering with the activation of the NF-κB signaling pathway in monocytes and macrophages. Research trials have indicated that curcumin and quercetin have the potential to elevate IL-12 levels in the nearby lymph nodes by enhancing the population of antigen-presenting cells (APC) [161,162,163]. IL-12 plays a crucial role in enhancing the activity of T cells, particularly T helper 1 (Th1) cells, by boosting the production of IFN-γ. Various research studies have suggested that polyphenols such as quercetin and curcumin can inhibit the secretion of MIP-1α, IL-1β, MCP-1, IL-8, and TNF-α by alveolar macrophages and monocytes that have been stimulated by LPS [161, 164, 165]. Resveratrol has been identified as a safe immunomodulatory substance in SARS-CoV-2 viral infection. Previous research has shown that it can block various mediators, leading to the irreversible inhibition of IFNγ and IL-2 production in splenic lymphocytes. In addition, it can also suppress the release of GM-CSF and CXCL8 chemokines, decrease IL-6 and TNFα secretion, and downregulate NF-κB in lung inflammatory cells. Furthermore, resveratrol has been found to reduce the levels of cytokines such as IL-1β, IL-8, and IL-12. In summary, resveratrol has a calming effect on the cytokine storm and helps alleviate hyper-inflammatory responses in the body [166]. Moreover, Chen et al. [167] conducted a study to examine the impact of resveratrol at a concentration of 100 mg/mL on stimulating macrophages during cases of acute kidney injury. Their findings indicated that resveratrol reduced the negative impacts of LPS on macrophages by significantly inhibiting the release of cytokines and preventing TLR-4 activation. Furthermore, Lovelace et al. [168] noted a significant reduction in pro-inflammatory cytokines and immune response in HIV patients due to the inhibitory effects of silymarin, a polyphenol, on T-cell stimulation.
Effect of polyphenols on polymorphonuclear neutrophil cells
In the human body, polymorphonuclear neutrophils (PMNs) are the most abundant type of white blood cells found in circulation. These cells play a crucial role in the body's natural immune defenses [169]. Chemokines released from virus-infected lungs attract neutrophils, causing them to infiltrate the lungs and release inflammatory cytokines like IL-8 [170]. Curcumin, luteolin, resveratrol, and quercetin have been found to diminish the migration of neutrophils into inflamed tissues by interacting with receptors on the neutrophils. These polyphenols also hinder the movement of neutrophils by suppressing the production of superoxide, AKT phosphorylation, PI3K activity, and IL-8, which acts as a mediator for neutrophil movement. Moreover, quercetin, luteolin, and curcumin influence the chemotactic behavior of neutrophils to decrease the formation of neutrophil extracellular traps (NETs), a major complication in viral sepsis that can lead to organ failure [161].
Kaneider et al. [171] demonstrated that resveratrol and quercetin interfere with the proinflammatory signaling of thrombin, leading to the prevention of decreased neutrophil function and adenosine nucleotide discharge from activated platelets. In another study, Tsai and Chen [172] indicated that resveratrol reduces integrin expression, degranulation, respiratory burst, and cell adhesion in activated neutrophils in a dose-dependent manner. Furthermore, Kaneider et al. [173] indicated that EGCG blocked neutrophil infiltration via direct action on neutrophils.
Immunomodulatory effect of polyphenols on polymorphonuclear adaptive and innate lymphocytes
Extensive research has been conducted regarding the involvement of both adaptive and innate lymphocytes in the progression of COVID-19. Specifically, Natural killer (NK) cells play a crucial role in combating the SARS-CoV-2 virus through their antiviral activity [173]. In individuals suffering from COVID-19, there is a heightened activity of blood NK cells, leading to the increased presence of CD56 bright cells. These CD56 bright cells are characterized by the expression of the cytotoxic molecules granzyme B and perforin [174]. Polyphenolic plant compounds like quercetin, curcumin, and resveratrol can decrease the release of inflammatory substances such as IL-15 and IFN/α&β by regulating the function of natural killer (NK) cells [175,176,177]. The decrease in the production of inflammatory substances leads to a decrease in the production of antigens and IFN/gamma by NK cells, ultimately decreasing the Th1 activity [177, 178].
Meroni et al. [179] investigated how different amounts of silybin (0.5, 10, and 25 mg/mL) affected the activation of human T lymphocytes. They found that silybin had a significant dose-dependent effect on decreasing the proliferation response to the monoclonal anti-CD3 antibody. Additionally, they noted that silybin led to an increase in the proliferation of alloantigen or mitogen-stimulated lymphocytes in a dose-dependent manner, as well as an enhancement in the secretion of IL-4, IFN-γ, and IL-10 [179, 180]. The immune system of COVID-19 patients responds by activating more lymphocytes, which are white blood cells that include CD8+ and CD4+ T cells. These T cells also multiply and diversify their receptors to fight the virus. CD4+ Th lymphocytes are divided into several subunits, including T regulatory cells, Th17, Th1, and Th2 [181, 182].
Multiple research studies have demonstrated that certain polyphenols like luteolin, resveratrol, quercetin, green tea, and curcumin can hinder the growth and specialization of Th17 cells. This is achieved by inhibiting the production of related orphan receptor gamma t (RORγt) IL‐17, IL‐21, and IL‐6, as well as the phosphorylation of signal transducers and activators of signal transducer and activator of transcription 3 (STAT3) [183,184,185]. Polyphenols such as curcumin, resveratrol, quercetin, and luteolin also help reduce the expression of CD8+ T cells by reducing macrophage-derived inflammatory factors such as IL-1β, CXCL10, and IL-6 [124, 186,187,188]. Furthermore, research has demonstrated that EGCG is capable of hindering the replication of HIV-1 at multiple points in the process. Specifically, the compound found in tea disrupts the interaction between gp120 and CD4 by interfering with the activity of reverse transcriptase [189]. A study suggested that EGCG prevents the expression of p24 antigen on macrophages, isolated CD4+ T cells, and CD4 receptor cells, relative to its dose [190].
Innate and adaptive immunity responses can be regulated by CD4+ CD25+ Tregs. Tregs produce cytokines such as IL-4 and IL-10, which can significantly impact the immune response to COVID-19 [191]. The activity and development of CD25+ CD4+ Tregs are dependent on FOXP3, a transcription factor protein expressed mostly by these cells. Studies have shown that resveratrol and curcumin can increase the expression of CD25+ CD4+ Foxp3+ Tregs, leading to upregulation of IL‐10 and TGF‐β production, which can reduce the proliferation activity of CD25− CD4+ T cells in the spleen of septic mice [192,193,194].
DCs play a crucial role in initiating both adaptive and innate immune responses and are part of the proprietary antigenic prescribing cells [195]. Curcumin has been found to prevent the maturation of DCs and block the up-regulation of CD 80, MHC II, CD86, and CD40 on the surfaces of DCs, while also inhibiting LPS-induced TNF-α, IL-1β, IL-12, and IL-6 production, as well as MAPK phosphorylation and NF-κB nuclear translocation [195]. Additionally, treatment with quercetin has been shown to reduce IL-12 production and increase the expression of ILT4, ILT5, immunoglobulin-like transcript (ILT)-3, CD39, and CD73 in septic rats [194].
Effects of polyphenols on reduced mucin hypersecretion
Early symptoms of COVID-19 mediated by SARS-CoV-2-induced cytokine storm include excessive mucin secretion, which activates various inflammatory pathways and contributes to the pathogenesis of the disease [196]. Mucins play a crucial role in defending the respiratory tract's front line against invading microbes and viruses in response to physiological stimuli [197, 198]. A high viral load can cause overproduction of mucins, leading to obstruction of the respiratory tract lumen and limited airflow [197]. Additionally, hypersecretion of mucins leads to ciliary dysfunction, which further exacerbates COVID-19 symptoms by compromising respiratory clearance [198]. The initial signs are connected to the excessive production of mucins caused by SARS-CoV-2, leading to an inflammatory storm that has the potential to trigger various inflammatory pathways [197, 199].
Several studies have shown that natural compounds can alleviate COVID-19 symptoms related to excessive mucin production. Liang et al. [200] demonstrated that epigallocatechin-3-gallate (50 mg/kg) reduced inflammation, neutrophils, airway mucus production, and collagen deposition in rats. In other studies, Li et al. showed that quercetin can prevent the induction of MUC5AC by human neutrophil elastase in a dose-dependent manner, by utilizing the PKC/MAPK/EGFR/ERK1/2 signal transduction pathways [201]. Additionally, a further study demonstrated that curcumin at a dose of 10 mg in NCI-H292 cells inhibits LPS-induced inflammation and airway mucus hypersecretion via the upregulation of Nrf2 gene expression [202]. Curcumin also attenuated mucus hypersecretion and inflammation in mice, blocked IL-4-induced expression of MCP-1 and MUC5AC both in vivo and in vitro through a peroxisome proliferator-activated receptor gamma (PPARγ) dependent NF-κB signaling pathway [203].
Polyphenols preventing SARS-CoV-2 entry/fusion
Polyphenols attaching to S protein
Protein S is one of the major membrane glycoproteins, which is part of the class I viral fusion glycoproteins [204]. The circumferential amino subunit (S1) attaches independently to cellular receptors, while the carboxy terminus (S2) is located in the viral coat and is critical to aid in the integration of cell membranes and viruses [38, 205, 206]. Coronaviruses are only capable of entering cells via their S protein [206]. Luteolin, quercetin, emodin, and curcumin in different doses prevented SARS-CoV infection by hindering the virus from entering Vero-E6 cells [207, 208]. Luteolin's strong affinity for the SARS-CoV S protein suggests a potent antiviral mechanism that disrupts the protein's function [207, 209]. In addition, one study discovered that emodin, an anthraquinone polyphenol found in rhubarb roots (Rheum officinale), interfered with the S-ACE2 protein [210]. Research using computer simulation techniques has revealed that fifteen specific plant compounds found in A. paniculata can bind and engage with the S-protein, as well as the C-terminal and N-terminal cleavage sites on the viral polyprotein of SARS-CoV-2 [211]. According to dynamic simulation and molecular docking analyses, it is predicted that various plant polyphenols like neohesperidin, apigenin 6,8-diC-glucoside, hesperidin from C. medical, curcumin, and citrus can interact with the protein S RBD. As a result, these polyphenols can potentially inhibit the effects of SARS-CoV-2 [212,213,214]. Hesperidin is believed to interact with the binding site between ACE2 and the S protein by binding to the central shallow pit on the surface of the S protein RBD [215]. A temporary treatment for COVID-19 approved by the FDA involves naringenin, a polyphenol present in various fruits and herbs such as grapefruit. This compound has shown strong binding energy with the spike glycoprotein associated with the virus [215, 216]. Epigallocatechin gallate, a compound plentiful in tea and Rhodiola spp. (Golden Root), has been shown to interact with the S RBD protein [217].
Polyphenols that target ACE2
ACE2 is a metalloproteinase enzyme found on the cell membrane in various tissues including the kidneys, heart, lungs, epithelial cells, blood vessels, and liver [218]. ACE2 plays a crucial role as an enzyme within the physiological renin-angiotensin system. It has been hypothesized that ACE2 could serve as a key gateway for SARS-CoV-2 to infect host cells, making it a potential target for therapeutic interventions against foreign substances [219, 220]. Research suggests that eriodictyol, a compound present in Yerba santa (Eriodictyon californicum), shows a strong attraction towards human ACE2 receptors. Through a molecular analysis utilizing a computational model of the SARS-CoV-2 protein binding to the human ACE2 receptor, it was established that eriodictyol exhibits the greatest affinity for this receptor [37, 219]. Several other research studies have shown that compounds like catechin and curcumin, which are flavonoids, may have the ability to interact with ACE2 through the formation of hydrogen bonds, carbon-hydrogen bonds, and π-σ interactions [221]. It is becoming increasingly clear that regulatory ACE2 expression may contribute to modulating COVID-19 symptoms. SARS-CoV infection decreased ACE2 receptor expression, which may exacerbate the severity of the disease [36, 222]. New research indicates that polyphenols like curcumin and resveratrol, which are present in red wine grapes, have the potential to influence the impact of SARS-CoV-2 by controlling the function and expression of ACE2 [222, 223]. Therefore, polyphenols have the potential to reduce SARS-CoV-2 viral infection by attaching to the ACE2 receptor, preventing the virus from entering cells, and regulating the severity of lung damage associated with COVID-19 by altering ACE2 expression [223, 224].
Relationship between polyphenols and SARS-CoV-2 proliferation
Polyphenols preventing SARS-CoV-2 viral proteases
Coronaviruses rely on replicase polyproteins that undergo processing by viral proteases [225, 226]. Out of all the proteases identified, 3CLpro stands out for its crucial function in facilitating the replication and transcription processes of SARS-CoV-2, establishing it as a primary target for drug development against coronavirus infections [226,227,228]. Natural compounds that have been found to inhibit SARS-CoV proteases include terpenoids [229, 230], flavonoids [231,232,233], cinnamic amides [234], diarylheptanoids [235, 236], and coumarins [237]. In vitro, inhibition of 3CLpro has been demonstrated with gallocatechin gallate, epigallocatechin gallate, isoquercetin, and quercetin [233, 238, 239]. Flavonoids lacking the OH group at the 5'B-ring position have been shown to reduce the hindering activity of 3CLpro [233]. Flavonoids such as isoliquiritigenin, kaempferol, naringenin, margarine, and curcumin have been found to interfere severely with the SARS-CoV-2 3CLpro and PLpro substrate binding domains [232, 236]. Quercetin not only inhibits the H+-ATPase of lysosomal membranes and prevents virus coat removal [240], but also inhibits the ATPase of proteins related to drug resistance, which increases the bioavailability of some antiviral drugs [34, 241]. Hence, quercetin shows great potential as a candidate for antiviral treatments [32, 242].
The role of polyphenols in inhibiting SARS-CoV-2 RdRp
Focusing on inhibiting the SARS-CoV-2 RdRp enzyme has emerged as a key approach in developing treatments for COVID-19 [243]. Resveratrol has been found to notably inhibit MERS-CoV proliferation in vitro by preventing nucleocapsid protein and RNA expression [243]. Other studies have reported that myricetin, epigallocatechin gallate, baicalin from Scutellaria baicalensis, (2S)-eriodictyol 7-O-(6″-O-galloyl)-β-d-glucopyranoside (EBDGp) from P. emblica, a β2-adrenoceptor polyphenolic receptor agonist, and quercetin showed high affinity for RdRp in both SARS-CoV and SARS-CoV-2 [33, 215, 244, 245]. Furthermore, a research study investigating the efficacy of P. amarus against SARS-CoV-2 infection revealed that computer-generated docking models of polyphenol flavonoid compounds (such as astragalin, kaempferol, quercetin, quercetin-3-O-glucoside, and quercetin) along with tannins (such as corilagin and geraniin) exhibited a strong attraction towards binding to RdRp (RNA-dependent RNA polymerase) and papain-like protease on SARS-CoV-2 in comparison to remdesivir [211].
Side effects and toxicity associated with polyphenols
Finding compounds with minimal cytotoxicity is one of the biggest challenges in the development of new therapeutic agents [31, 32, 246]. Polyphenols have an advantage in this regard, as they have a high safety profile without major side effects [224]. Studies have shown that when polyphenols are consumed in the form of fruits and vegetables, their toxic effects are highly unlikely [35, 247]. Additionally, most polyphenols do not exhibit cytotoxicity at high concentrations [25,26,27, 30, 246]. However, some polyphenols may have toxic effects in high doses. For instance, quercetin, despite being generally safe, may induce side effects such as headache and paresthesias in the hands and legs at doses of up to 1 g daily. High doses of quercetin may also pose a potential risk of renal toxicity, and it could interact with antibiotics such as quinolones and other drugs such as diclofenac, celecoxib, and immunosuppressants, leading to an increased side effect profile [24, 241, 248]. Similarly, under high-dose conditions, some studies have shown that EGCG may have a significant prooxidant effect, which can be both beneficial and potentially harmful. High doses of EGCG have been reported to cause cytotoxicity in vitro and may result in liver damage, kidney damage, and gastrointestinal disturbances (vomiting and diarrhea) [23, 249, 250]. Furthermore, emodin may have irreversible pathological effects on organs and disrupt glutathione and fatty acid metabolism in very high doses (4000 mg/kg). Emodin is also genotoxic and reproductively toxic [208]. To mitigate drug toxicity, it is crucial to avoid the long-term administration of high doses. Enhancing bioavailability is fundamental to reducing the administered dose.
Conclusion
The present review discussed the efficacy of various polyphenols in suppressing COVID-19. It has been shown that polyphenols can significantly reduce COVID-19 severity and the damage induced by viral infection by acting as immunomodulators, anti-inflammatory, and antioxidants. There are multiple molecular and cellular pathways involved in their mechanism of action against COVID-19, including disrupting viruses' life cycle by binding to their essential proteins, inhibiting viral enzymes, and disrupting viral structural proteins from interacting with host cells which ultimately leads to inhibition of cytokine storm, reduction of oxidative damage, and increase in virus resistance. To confirm such findings, further studies on patients are required to demonstrate the efficacy and safety of the specific polyphenols. Moreover, polyphenols tend to be poorly bioavailable, which requires additional research into pharmaceutical technology leading to the right formulation.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme-2
- AMPK:
-
AMP-activated protein kinase
- AMs:
-
Alveolar macrophages
- APCs:
-
Antigen-presenting cells
- ARDS:
-
Acute respiratory distress syndrome
- ATF3:
-
Activating transcription factor 3
- BAL:
-
Bronchoalveolar lavage
- CBG:
-
Cytosolic β-glucosidase
- CCL:
-
C–C motif chemokine ligand
- CD:
-
Cluster of differentiation
- COX:
-
Cyclooxygenase
- CTLs:
-
Cytotoxic T lymphocytes
- DAMPs:
-
Danger-associated molecular patterns
- dsRNA:
-
Double-stranded RNA
- EGCG:
-
Epigallocatechin gallate
- EGFR:
-
Epidermal growth factor receptor
- ERGIC:
-
Endoplasmic reticulum-Golgi intermediate compartment
- ERK:
-
Extracellular signal-regulated kinase
- FOXP3:
-
Forkhead box P3
- HIF-1:
-
Hypoxia-inducible factor 1
- HLA:
-
Human leukocyte antigen
- HRSV:
-
Human respiratory syncytial virus
- IFNγ:
-
Interferon-γ
- Ig:
-
Immunoglobulin
- IκBα:
-
Inhibitor of κBα
- IKKα:
-
Inhibitory κB kinase α
- IL:
-
Interleukin
- ILT:
-
Immunoglobulin-like transcript
- iNOS:
-
Inducible nitric oxide synthase
- JNK/SAPK:
-
C-jun N-terminal or stress-activated protein kinases
- LPH:
-
Lactase-phlorizin hydrolase
- LPSs:
-
Lipopolysaccharides
- MAPK:
-
Mitogen-activated protein kinase
- MBL:
-
Mannose-bound lectin
- MDMs:
-
Monocyte-derived macrophages
- MHC:
-
Major histocompatibility complex
- MIP:
-
Macrophage inflammatory protein
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NETs:
-
Neutrophil extracellular traps
- NF-Κb:
-
Nuclear factor kappa B
- NK:
-
Natural killer
- NLRP-3:
-
Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- NO:
-
Nitric oxide
- Nrf-2:
-
Nuclear factor erythroid 2-related factor 2
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PAMPs:
-
Pathogen-associated molecular patterns
- PF:
-
Pulmonary fibrosis
- PIK3CA:
-
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha
- PMNs:
-
Polymorphonuclear neutrophils
- RORγt:
-
Related orphan receptor gamma t
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- PRRs:
-
Pattern recognition receptors
- RBD:
-
Receptor-binding domain
- RBM:
-
Receptor-binding motif
- ROS:
-
Reactive oxygen species
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SeV:
-
Sendai virus
- STAT:
-
Signal transducers and activators of transcription
- TBX1:
-
T-box transcription factor
- TfR1:
-
Transferrin receptor protein 1
- TLR-4:
-
Toll like receptor 4
- TMPRSS2:
-
Transmembrane serine protease 2
- TNFα:
-
Tumour necrosis factor α
- Th1:
-
T helper 1
References
Karimi Shahri M, Niazkar HR, Rad F. COVID-19 and hematology findings based on the current evidences: a puzzle with many missing pieces. Int J Lab Hematol. 2021;43:160–8.
Salavati E, Hajirezaee H, Niazkar HR, Ramezani MS, Sargazi A. COVID-19 patients may present with myocarditis: a case report emphasizing the cardiac involvement of SARS-CoV-2. Med J Islam Repub Iran. 2021;35:104.
COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–36.
Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31:1–10.
Montenegro F, Unigarro L, Paredes G, Moya T, Romero A, Torres L, et al. Acute respiratory distress syndrome (ARDS) caused by the novel coronavirus disease (COVID-19): a practical comprehensive literature review. Expert Rev Respir Med. 2021;15:183–95.
Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID-19: a systematic review and meta-analysis. PLoS ONE. 2021;16: e0250602.
Wang C, Han J. Will the COVID-19 pandemic end with the Delta and Omicron variants? Environ Chem Lett. 2022;20:2215–25.
Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou JJ, et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–9.
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–40.
Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–9.
Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;27:041.
Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–42.
Calabretta E, Moraleda JM, Iacobelli M, Jara R, Vlodavsky I, O’Gorman P, et al. COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;193:43–51.
Tomerak S, Khan S, Almasri M, Hussein R, Abdelati A, Aly A, et al. Systemic inflammation in COVID-19 patients may induce various types of venous and arterial thrombosis: a systematic review. Scand J Immunol. 2021;94: e13097.
Badawy MA, Yasseen BA, El-Messiery RM, Abdel-Rahman EA, Elkhodiry AA, Kamel AG, et al. Neutrophil-mediated oxidative stress and albumin structural damage predict COVID-19-associated mortality. Elife. 2021;10: e69417.
Leri M, Scuto M, Ontario ML, Calabrese V, Calabrese EJ, Bucciantini M, et al. Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci. 2020;21:1250.
Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1618.
Vajdi M, Farhangi MA. Citrus peel derived’poly-methoxylated flavones (PMF). Int J Vitam Nutr Res. 2021;93:252–67.
Zhou H, Zheng B, McClements DJ. Encapsulation of lipophilic polyphenols in plant-based nanoemulsions: Impact of carrier oil on lipid digestion and curcumin, resveratrol and quercetin bioaccessibility. Food Funct. 2021;12:3420–32.
Prabhu S, Molath A, Choksi H, Kumar S, Mehra R. Classifications of polyphenols and their potential application in human health and diseases. Int J Physiol Nutr Phys Educ. 2021;6:293–301.
Melenotte C, Silvin A, Goubet A-G, Lahmar I, Dubuisson A, Zumla A, et al. Immune responses during COVID-19 infection. Oncoimmunology. 2020;9:1807836.
Chen G-L, Munyao Mutie F, Xu Y-B, Saleri FD, Hu G-W, Guo M-Q. Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals. 2020;13:55.
Jo S, Kim S, Shin DH, Kim M-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35:145–51.
Schettig R, Sears T, Klein M, Tan-Lim R, Matthias R Jr, Aussems C, et al. COVID-19 patient with multifocal pneumonia and respiratory difficulty resolved quickly: possible antiviral and anti-inflammatory benefits of quercinex (nebulized quercetin-NAC) as adjuvant. Adv Infect Dis. 2020;10:45–55.
Ryu YB, Jeong HJ, Kim JH, Kim YM, Park J-Y, Kim D, et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg Med Chem. 2010;18:7940–7.
Verma D, Mitra D, Paul M, Chaudhary P, Kamboj A, Thatoi H, et al. Potential inhibitors of SARS-CoV-2 (COVID 19) proteases PLpro and Mpro/3CLpro: molecular docking and simulation studies of three pertinent medicinal plant natural components. Curr Res Pharmacol Drug Discov. 2021;2: 100038.
Schwarz S, Sauter D, Wang K, Zhang R, Sun B, Karioti A, et al. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80:177–82.
Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27–32.
Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS, et al. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-κB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–82.
Villagomez J. In vitro antiviral activity of black tea polyphenols on sindbis virus in vero cells. Theses Dissert Culminating Proj. 2017;19:1–33.
Ge M, Xiao Y, Chen H, Luo F, Du G, Zeng F. Multiple antiviral approaches of (–)-epigallocatechin-3-gallate (EGCG) against porcine reproductive and respiratory syndrome virus infection in vitro. Antivir Res. 2018;158:52–62.
Chiow KH, Phoon MC, Putti T, Tan BK, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9:1–7.
Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–29.
Di Pierro F, Derosa G, Maffioli P, Bertuccioli A, Togni S, Riva A, et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med. 2021;14:2359–66.
Huang H, Liao D, Zhou G, Zhu Z, Cui Y, Pu R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine. 2020;77: 153230.
Liu S, Lu H, Zhao Q, He Y, Niu J, Debnath AK, et al. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim Biophys Acta Gen Subj. 2005;1723:270–81.
Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947–55.
Pasquereau S, Nehme Z, Haidar Ahmad S, Daouad F, Van Assche J, Wallet C, et al. Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro. Viruses. 2021;13:354.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Astuti I. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr Clin Res Rev. 2020;14:407–12.
Tsai P-H, Lai W-Y, Lin Y-Y, Luo Y-H, Lin Y-T, Chen H-K, et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. 2021;84:3–8.
da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, Farias de Oliveira T, Campos Alcântara R, Monteiro Arnozo G, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133:377–82.
Letarov AV, Babenko VV, Kulikov EE. Free SARS-CoV-2 spike protein S1 particles may play a role in the pathogenesis of COVID-19 infection. Biochem (Mosc). 2021;86:257–61.
Yi C, Sun X, Ye J, Ding L, Liu M, Yang Z, et al. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17:621–30.
Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci USA. 2004;101:4240–5.
Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA. 2009;106:5871–6.
Yang B, Jia Y, Meng Y, Xue Y, Liu K, Li Y, et al. SNX27 suppresses SARS-CoV-2 infection by inhibiting viral lysosome/late endosome entry. Proc Natl Acad Sci USA. 2022;119: e2117576119.
Koch J, Uckeley ZM, Doldan P, Stanifer M, Boulant S, Lozach PY. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 2021;40: e107821.
Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301.
Bayati A, Kumar R, Francis V, McPherson PS. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021;296: 100306.
Li X, Yuan H, Li X, Wang H. Spike protein mediated membrane fusion during SARS-CoV-2 infection. J Med Virol. 2023;95: e28212.
Vkovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–70.
Hopfer H, Herzig MC, Gosert R, Menter T, Hench J, Tzankov A, et al. Hunting coronavirus by transmission electron microscopy–a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues. Histopathology. 2021;78:358–70.
Okalang U, Mualem Bar-Ner B, Rajan KS, Friedman N, Aryal S, Egarmina K, et al. The spliced leader RNA silencing (SLS) pathway in Trypanosoma brucei is induced by perturbations of endoplasmic reticulum, golgi complex, or mitochondrial protein factors: functional analysis of SLS-inducing kinase PK3. MBio. 2021;12:e02602-e2621.
Cun Y, Li C, Shi L, Sun M, Dai S, Sun L, et al. COVID-19 coronavirus vaccine T cell epitope prediction analysis based on distributions of HLA class I loci (HLA-A,-B,-C) across global populations. Hum Vaccin Immunother. 2021;17:1097–108.
Hernández-Doño S, Sánchez-González RA, Trujillo-Vizuet MG, Zamudio-Castellanos FY, García-Silva R, Bulos-Rodríguez P, et al. Protective HLA alleles against severe COVID-19: HLA-A* 68 as an ancestral protection allele in Tapachula-Chiapas, Mexico. Clin Immunol. 2022:108990.
Yoo J-S, Sasaki M, Cho SX, Kasuga Y, Zhu B, Ouda R, et al. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat Commun. 2021;12:6602.
Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2020;11:1949.
Dos Santos ACM, Dos Santos BRC, Dos Santos BB, de Moura EL, Ferreira JM, Dos Santos LKC, et al. Genetic polymorphisms as multi-biomarkers in severe acute respiratory syndrome (SARS) by coronavirus infection: a systematic review of candidate gene association studies. Infect Genet Evol. 2021;93: 104846.
Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:2255–8.
Jiang H-W, Li Y, Zhang H-N, Wang W, Yang X, Qi H, et al. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat Commun. 2020;11:1–11.
Shang Z, Chan SY, Liu WJ, Li P, Huang W. Recent insights into emerging coronavirus: SARS-CoV-2. ACS Infect Dis. 2020;7:1369–88.
De Wit E, Van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–34.
Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55: 102763.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Toor SM, Saleh R, Sasidharan Nair V, Taha RZ, Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162:30–43.
Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–80.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–73.
Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43.
Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22:32–40.
Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80:5927–40.
Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–93.
Yao T, Foo C, Zheng G, Huang R, Li Q, Shen J, et al. Insight into the mechanisms of coronaviruses evading host innate immunity. Biochim Biophys Acta Mol Basis Dis. 2023;1869: 166671.
Minkoff JM, tenOever B. Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol. 2023;21:178–94.
Rubio-Casillas A, Redwan EM, Uversky VN. SARS-CoV-2: a master of immune evasion. Biomedicines. 2022;10:1339.
Chakraborty C, Sharma AR, Bhattacharya M, Lee S-S. A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and their emerging variants with escape mutations. Front immunol. 2022;13: 801522.
Mittal A, Khattri A, Verma V. Structural and antigenic variations in the spike protein of emerging SARS-CoV-2 variants. PLoS Pathog. 2022;18: e1010260.
Zhang S, Wang L, Cheng G. The battle between host and SARS-CoV-2: innate immunity and viral evasion strategies. Mol Ther. 2022;30:1869–84.
El Gharras H. Polyphenols: food sources, properties and applications—a review. Food Sci Technol Int. 2009;44:2512–8.
Mrduljaš N, Krešic G, Bilušic T. Polyphenols: food sources and health benefits in functional food-improve health through adequate food. In: Hueda MC, editor. London: IntechOpen; 2017. https://www.intechopen.com/
Guyot S, Marnet N, Laraba D, Sanoner P, Drilleau J-F. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien). J Agric Food Chem. 1998;46:1698–705.
Tresserra-Rimbau A, Lamuela-Raventos RM, Moreno JJ. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem Pharmacol. 2018;156:186–95.
Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S-S2085.
D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–42.
Tapiero H, Tew K, Ba GN, Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56:200–7.
Sesink AL, Arts IC, Faassen-Peters M, Hollman PC. Intestinal uptake of quercetin-3-glucoside in rats involves hydrolysis by lactase phlorizin hydrolase. J Nutr. 2003;133:773–6.
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, Inflammation and Immunity. Nutrients. 2016;8:167.
Mitsunari K, Miyata Y, Matsuo T, Mukae Y, Otsubo A, Harada J, et al. Pharmacological effects and potential clinical usefulness of polyphenols in benign prostatic hyperplasia. Molecules. 2021;26:450.
Naeini F, Tutunchi H, Razmi H, Mahmoodpoor A, Vajdi M, Sefidmooye Azar P, et al. Does nano-curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial. J Food Biochem. 2022;46: e14093.
Vajdi M, Sefidmooye Azar P, Mahmoodpoor A, Dashti F, Sanaie S, Kiani Chalmardi F, et al. A comprehensive insight into the molecular and cellular mechanisms of action of resveratrol on complications of sepsis a systematic review. Phytother Res. 2023;37:3780–808.
Vajdi M, Karimi A, Karimi M, Farhangi MA, Askari G. Effects of luteolin on sepsis: a comprehensive systematic review. Phytomedicine. 2023:154734.
Karimi A, Jazani AM, Darzi M, Azgomi RND, Vajdi M. Effects of curcumin on blood pressure: a systematic review and dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33(11):2089–101.
Sahebkar A. Curcuminoids for the management of hypertriglyceridaemia. Nat Rev Cardiol. 2014;11:123.
González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104 (Suppl 3):S15–27. https://doi.org/10.1017/S0007114510003910.
Gazzerro P, Proto MC, Gangemi G, Malfitano AM, Ciaglia E, Pisanti S, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–46.
Coustan DR. Pharmacological management of gestational diabetes: an overview. Diabetes Care. 2007;30:S206–8.
Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–50.
Van Gaal L, Dirinck E. Pharmacological approaches in the treatment and maintenance of weight loss. Diabetes Care. 2016;39:S260–7.
Palinski W. Immunomodulation: a new role for statins? Nat Med. 2000;6:1311–2.
Ahmadi A, Jamialahmadi T, Sahebkar A. Polyphenols and atherosclerosis: a critical review of clinical effects on LDL oxidation. Pharmacol Res. 2022;184: 106414.
Cicero AFG, Sahebkar A, Fogacci F, Bove M, Giovannini M, Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. Eur J Nutr. 2020;59:477–83.
Keihanian F, Saeidinia A, Khameneh Bagheri R, Johnston ThP, Sahebkar A. Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol. 2018;233:4497–511.
Vajdi M, Hassanizadeh S, Hassanizadeh R, Bagherniya M. Curcumin supplementation effect on liver enzymes in patients with nonalcoholic fatty liver disease: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Rev. 2024;12:nuad166.
Marjaneh RM, Rahmani F, Hassanian SM, Rezaei N, Hashemzehi M, Bahrami A, et al. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. J Cell Physiol. 2018;233:6785–98.
Mohajeri M, Sahebkar A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: a review. Crit Rev Oncol Hematol. 2018;122:30–51.
Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A. The protective role of curcumin in myocardial ischemia–reperfusion injury. J Cell Physiol. 2018;234:214–22.
Panahi Y, Fazlolahzadeh O, Atkin SL, Majeed M, Butler AE, Johnston TP, et al. Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. J Cell Physiol. 2019;234:1165–78.
Sahebkar A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:822–35.
Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors - Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55.
Sahebkar A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin Nutr. 2014;33(3):406–14.
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017;1:35.
Chojnacka K, Lewandowska U. Inhibition of pro-inflammatory cytokine secretion by polyphenol-rich extracts in macrophages via NF-κB pathway. Food Rev Int. 2023;39:5459–78.
Murata T, Jamsransuren D, Matsuda S, Ogawa H, Takeda Y. Rapid virucidal activity of japanese saxifraga species-derived condensed tannins against SARS-CoV-2, influenza A virus, and human norovirus surrogate viruses. Appl Environ Microbiol. 2023;89: e0023723.
Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143: 110102.
Panieri E, Buha A, Telkoparan-Akillilar P, Cevik D, Kouretas D, Veskoukis A, et al. Potential applications of NRF2 modulators in cancer therapy. Antioxidants. 2020;9:193.
Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL, et al. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2012;108:1272–9.
Lammi C, Arnoldi A. Food-derived antioxidants and COVID-19. J Food Biochem. 2021;45: e13557.
Chourasia M, Koppula PR, Battu A, Ouseph MM, Singh AK. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules. 2021;26:1200.
Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-inflammatory action of green tea. Antiinflamm Antiallergy Agents Med Chem. 2016;15:74–90.
Li X, Yao X, Wang Y, Hu F, Wang F, Jiang L, et al. MLH1 promoter methylation frequency in colorectal cancer patients and related clinicopathological and molecular features. PLoS ONE. 2013;8: e59064.
Tang D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, et al. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41:651–60.
Karimi A, Naeini F, Azar VA, Hasanzadeh M, Ostadrahimi A, Niazkar HR, et al. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine. 2021;86: 153567.
Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res. 2011;55:844–54.
Truong V-L, Jeong W-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci Hum Wellness. 2022;11:502–11.
Jang I-A, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, et al. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients. 2018;10:1741.
Aksoy H, Karadag AS, Wollina U. Angiotensin II receptors: Impact for COVID-19 severity. Dermatol Ther. 2020;33: e13989.
Warner FJ, Rajapaksha H, Shackel N, Herath CB. ACE2: from protection of liver disease to propagation of COVID-19. Clin Sci. 2020;134:3137–58.
Ramdani L, Bachari K. Potential therapeutic effects of resveratrol against SARS-CoV-2. Acta Virol. 2020;64.
de Ligt M, Hesselink MK, Jorgensen J, Hoebers N, Blaak EE, Goossens GH. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte. 2021;10:408–11.
Yang M, Wei J, Huang T, Lei L, Shen C, Lai J, et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother Res. 2021;35:1127.
Rossi GA, Sacco O, Capizzi A, Mastromarino P. Can resveratrol-inhaled formulations be considered potential adjunct treatments for COVID-19? Front immunol. 2021;12: 670955.
Ter Ellen BM, Dinesh Kumar N, Bouma EM, Troost B, van de Pol DP, Van der Ende-Metselaar HH, et al. Resveratrol and pterostilbene inhibit SARS-CoV-2 replication in air–liquid interface cultured human primary bronchial epithelial cells. Viruses. 2021;13:1335.
Xu H, Li J, Song S, Xiao Z, Chen X, Huang B, et al. Effective inhibition of coronavirus replication by Polygonum cuspidatum. Front Biosci (Landmark Ed). 2021;26:789–98.
Marinella MA. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/COVID-19. Int J Clin Pract Suppl. 2020;74: e13535.
McCreary MR, Schnell PM, Rhoda DA. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci Rep. 2022;12:10978.
Filardo S, Di Pietro M, Mastromarino P, Sessa R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol Ther. 2020;214: 107613.
AbdelMassih A, Yacoub E, Husseiny RJ, Kamel A, Hozaien R, El Shershaby M, et al. Hypoxia-inducible factor (HIF): the link between obesity and COVID-19. Obes Med. 2021;22: 100317.
Hoang T. An approach of fatty acids and resveratrol in the prevention of COVID-19 severity. Phytother Res. 2021;35:2269.
Angeli F, Spanevello A, Reboldi G, Visca D, Verdecchia P. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1–8.
Hassaniazad M, Eftekhar E, Inchehsablagh BR, Kamali H, Tousi A, Jaafari MR, et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. 2021;35:6417–27.
Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–55.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34: 101623.
Djalali M, Abdolahi M, Hosseini R, Miraghajani M, Mohammadi H, Djalali M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: a randomized controlled clinical trial. Complement Ther Clin Pract. 2020;41: 101256.
Meidaninikjeh S, Sabouni N, Marzouni HZ, Bengar S, Khalili A, Jafari R. Monocytes and macrophages in COVID-19: friends and foes. Life Sci. 2021;269: 119010.
MacDonald L, Alivernini S, Tolusso B, Elmesmari A, Somma D, Perniola S, et al. COVID-19 and RA share an SPP1 myeloid pathway that drives PD-L1+ neutrophils and CD14+ monocytes. JCI Insight. 2021;6(13): e147413.
Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA, Tirupathi R, et al. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9:436.
Thimmulappa RK, Mudnakudu-Nagaraju KK, Shivamallu C, Subramaniam KT, Radhakrishnan A, Bhojraj S, et al. Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19. Heliyon. 2021;7: e06350.
Yang M, Wei J, Huang T, Lei L, Shen C, Lai J, et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured vero cells. Phytother Res. 2021;35(3):1127–9.
Ungarala R, Munikumar M, Sinha SN, Kumar D, Sunder RS, Challa S. Assessment of antioxidant, immunomodulatory activity of oxidised Epigallocatechin-3-Gallate (green tea polyphenol) and its action on the main protease of SARS-CoV-2—an in vitro and in silico approach. Antioxidants. 2022;11:294.
Aucoin M, Cooley K, Saunders PR, Cardozo V, Remy D, Cramer H, et al. The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv Integr Med. 2020;7:247–51.
Mohammadi A, Blesso ChN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019;66:1–19.
Manjunath SH, Thimmulappa RK. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: potential role in prevention and management of COVID-19. J Pharm Anal. 2022;12:29–34.
Chai Y-S, Chen Y-Q, Lin S-H, Xie K, Wang C-J, Yang Y-Z, et al. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946.
Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1–M2 macrophages in inflammation. Oncotarget. 2018;9:17937.
Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J Nutr Biochem. 2019;69:19–30.
Momtazi-Borojeni AA, Abdollahi E, Nikfar B, Chaichian S, Ekhlasi-Hundrieser M. Curcumin as a potential modulator of M1 and M2 macrophages: new insights in atherosclerosis therapy. Heart Fail Rev. 2019;24:399–409.
Zingg JM, Hasan ST, Cowan D, Ricciarelli R, Azzi A, Meydani M. Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J Cell Biochem. 2012;113:833–40.
Lee S-M, Moon J, Cho Y, Chung JH, Shin M-J. Quercetin up-regulates expressions of peroxisome proliferator-activated receptor γ, liver X receptor α, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr Res. 2013;33:136–43.
Karimi A, Ghodsi R, Kooshki F, Karimi M, Asghariazar V, Tarighat-Esfanjani A. Therapeutic effects of curcumin on sepsis and mechanisms of action: a systematic review of preclinical studies. Phytother Res. 2019;33:2798–820.
Bhaskar S, Sudhakaran P, Helen A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 2016;310:131–40.
Zhong Y, Liu C, Feng J, Li JF, Fan ZC. Curcumin affects ox-LDL-induced IL-6, TNF-α, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-κB/miR33a signaling pathway. Exp Ther Med. 2020;20:1856–70.
Abe Y, Hashimoto SHU, Horie T, Abe Y, Hashimoto SH, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–7.
Naeini F, Tutunchi H, Razmi H, Mahmoodpoor A, Vajdi M, Sefidmooye Azar P, et al. Does nano‐curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial. J Food Biochem. 2022:e14093.
Domi E, Hoxha M, Kolovani E, Tricarico D, Zappacosta B. The importance of nutraceuticals in COVID-19: What’s the role of resveratrol? Molecules. 2022;27:2376.
Chen L, Yang S, Zumbrun EE, Guan H, Nagarkatti PS, Nagarkatti M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol Nutr Food Res. 2015;59:853–64.
Lovelace ES, Maurice NJ, Miller HW, Slichter CK, Harrington R, Magaret A, et al. Silymarin suppresses basal and stimulus-induced activation, exhaustion, differentiation, and inflammatory markers in primary human immune cells. PLoS ONE. 2017;12: e0171139.
Alarcon P, Espinosa G, Millan C, Saravia J, Quinteros V, Enriquez R, et al. Piscirickettsia salmonis-triggered extracellular traps formation as an innate immune response of Atlantic salmon-derived polymorphonuclear neutrophils. Biology. 2021;10:206.
Cui S-N, Tan H-Y, Fan G-C. Immunopathological roles of neutrophils in virus infection and COVID-19. Shock (Augusta, GA). 2021;56:345.
Kaneider NC, Mosheimer B, Reinisch N, Patsch JR, Wiedermann CJ. Inhibition of thrombin-induced signaling by resveratrol and quercetin: effects on adenosine nucleotide metabolism in endothelial cells and platelet–neutrophil interactions. Thromb Res. 2004;114:185–94.
Tsai YF, Chen CY, Chang WY, Syu YT, Hwang TL. Resveratrol suppresses neutrophil activation via inhibition of Src family kinases to attenuate lung injury. Free Radic Biol Med. 2019;145:67–77.
Littera R, Chessa L, Deidda S, Angioni G, Campagna M, Lai S, et al. Natural killer-cell immunoglobulin-like receptors trigger differences in immune response to SARS-CoV-2 infection. PLoS ONE. 2021;16: e0255608.
Masselli E, Vaccarezza M, Carubbi C, Pozzi G, Presta V, Mirandola P, et al. NK cells: a double edge sword against SARS-CoV-2. Adv Biol Regul. 2020;77: 100737.
Bernini R, Velotti F. Natural polyphenols as immunomodulators to rescue immune response homeostasis: Quercetin as a research model against severe COVID-19. Molecules. 2021;26:5803.
Bill MA, Bakan C, Benson DM, Fuchs J, Young G, Lesinski GB. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol Cancer Ther. 2009;8:2726–35.
Liao M-T, Wu C-C, Wu S-FV, Lee M-C, Hu W-C, Tsai K-W, et al. Resveratrol as an adjunctive therapy for excessive oxidative stress in aging COVID-19 patients. Antioxidants. 2021;10:1440.
Ha Y, Kim O-K, Nam D-E, Kim Y, Kim E, Jun W, et al. Effects of Curcuma longa L. extracts on natural killer cells and T cells. J Korean Soc Food Sci Nutr. 2015;44:307–13.
Meroni P, Barcellini W, Borghi M, Vismara A, Ferraro G, Ciani D, et al. Silybin inhibition of human T-lymphocyte activation. Int J Tissue React. 1988;10:177–81.
Valková V, Ďúranová H, Bilčíková J. Milk thistle (Silybum marianum): a valuable medicinal plant with several therapeutic purposes. J Microbiol Biotechnol Food Sci. 2020;9:836.
Urra J, Cabrera C, Porras L, Ródenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217: 108486.
Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85:36–42.
Yang Y, Zhang X, Xu M, Wu X, Zhao F, Zhao C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int Immunopharmacol. 2018;54:153–62.
Bastaminejad S, Bakhtiyari S. Quercetin and its relative therapeutic potential against COVID-19: a retrospective review and prospective overview. Curr Mol Med. 2021;21:385–91.
Imler TJ Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+ IL-10+ T cells, CD4− IFN-γ+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–43.
Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H. Targeting regulatory T cells by curcumin: a potential for cancer immunotherapy. Pharmacol Res. 2019;147: 104353.
Khazdair MR, Anaeigoudari A, Agbor GA. Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: a scoping review. Asian Pac J Trop Biomed. 2021;11:327–34.
Kim S-H, Saba E, Kim B-K, Yang W-K, Park Y-C, Shin HJ, et al. Luteolin attenuates airway inflammation by inducing the transition of CD4+ CD25–to CD4+ CD25+ regulatory T cells. Eur J Pharmacol. 2018;820:53–64.
Sodagari HR, Bahramsoltani R, Farzaei MH, Abdolghaffari AH, Rezaei N, Taylor-Robinson AW. Tea polyphenols as natural products for potential future management of HIV infection-an overview. J Nat Remed. 2016;16:60–72.
Nance CL, Siwak EB, Shearer WT. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J Allergy Clin Immunol. 2009;123:459–65.
Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-β-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904.
Yang Y, Paik JH, Cho D, Cho J-A, Kim C-W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–7.
Chai Y-S, Chen Y-Q, Lin S-H, Xie K, Wang C-J, Yang Y-Z, et al. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946.
Michalski J, Deinzer A, Stich L, Zinser E, Steinkasserer A, Knippertz I. Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology. 2020;225: 151929.
Kim G-Y, Kim K-H, Lee S-H, Yoon M-S, Lee H-J, Moon D-O, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol. 2005;174:8116–24.
VasanthiDharmalingam P, Karuppagounder V, Watanabe K, Karmouty-Quintana H, Palaniyandi SS, Guha A, et al. SARS–CoV-2 mediated hyperferritinemia and cardiac arrest: preliminary insights. Drug Discov Today. 2021;26:1265.
Prasher P, Sharma M. Targeting mucin hypersecretion in COVID-19 therapy. Future Med Chem. 2022;14(10):681–4.
Khan MA, Khan ZA, Charles M, Pratap P, Naeem A, Siddiqui Z, et al. Cytokine storm and mucus hypersecretion in COVID-19: review of mechanisms. J Inflamm Res. 2021;14:175.
Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. MBio. 2020;11:e00398-e420.
Liang Y, Liu KWK, Yeung SC, Li X, Ip MSM, Mak JCW. (−)-Epigallocatechin-3-gallate reduces cigarette smoke-induced airway neutrophilic inflammation and mucin hypersecretion in rats. Front Pharmacol. 2017;8:618.
Li N, Li Q, Zhou XD, Kolosov VP, Perelman JM. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human airway epithelial cells. Int Immunopharmacol. 2012;14:195–201.
Lin X-P, Xue C, Zhang J-M, Wu W-J, Chen X-Y, Zeng Y-M, et al. Curcumin inhibits lipopolysaccharide-induced mucin 5AC hypersecretion and airway inflammation via nuclear factor erythroid 2-related factor 2. Chin Med J. 2018;131:1686–93.
Zhu T, Chen Z, Chen G, Wang D, Tang S, Deng H, et al. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPAR<i>γ</i>-dependent NF-<i>κ</i>B signaling pathway in vivo and in vitro. Mediat Inflamm. 2019;2019:4927430.
Du L, He Y, Zhou Y, Liu S, Zheng B-J, Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–36.
Gallagher TM, Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–4.
Bosch BJ, Van der Zee R, De Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–11.
Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–9.
Shao Q, Liu T, Wang W, Liu T, Jin X, Chen Z. Promising role of emodin as therapeutics to against viral infections. Front pharmacol. 2022;13: 902626.
Omolo CA, Soni N, Fasiku VO, Mackraj I, Govender T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol. 2020;883: 173348.
Ho T-Y, Wu S-L, Chen J-C, Li C-C, Hsiang C-Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74:92–101.
Hiremath S, Kumar H, Nandan M, Mantesh M, Shankarappa K, Venkataravanappa V, et al. In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2. 3 Biotech. 2021;11:1–8.
Bellavite P, Donzelli A. Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits. Antioxidants. 2020;9(8):742.
Davella R, Gurrapu S, Mamidala E. Phenolic compounds as promising drug candidates against COVID-19-an integrated molecular docking and dynamics simulation study. Mater Today Proc. 2022;51:522–7.
Haridas M, Sasidhar V, Nath P, Abhithaj J, Sabu A, Rammanohar P. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: in silico evidence for cues from Ayurveda. Future J Pharm Sci. 2021;7:1–9.
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–88.
Ubani A, Agwom F, Morenikeji OR, Shehu NY, Umera EA, Umar U, et al. Molecular docking analysis of selected phytochemicals on two SARS-CoV-2 targets. F1000Research. 2020;9:1157.
Tallei TE, Tumilaar SG, Niode NJ, Kepel BJ, Idroes R, Effendi Y, et al. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: a molecular docking study. Scientifica. 2020;2020:6307457.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4.
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:e1–9.
Parvathaneni V, Gupta V. Utilizing drug repurposing against COVID-19—efficacy, limitations, and challenges. Life Sci. 2020;259: 118275.
Jena AB, Kanungo N, Nayak V, Chainy GB, Dandapat J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci Rep. 2021;11:2043.
Kim EN, Kim MY, Lim JH, Kim Y, Shin SJ, Park CW, et al. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis. 2018;270:123–31.
Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–20.
Mehany T, Khalifa I, Barakat H, Althwab SA, Alharbi YM, El-Sohaimy S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: a review with research evidence and underlying mechanisms. Food Biosci. 2021;40: 100891.
Ren Z, Yan L, Zhang N, Guo Y, Yang C, Lou Z, et al. The newly emerged SARS-like coronavirus HCoV-EMC also has an" Achilles’ heel": current effective inhibitor targeting a 3C-like protease. Protein Cell. 2013;4:248–50.
Dai W, Zhang B, Jiang X-M, Su H, Li J, Zhao Y, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–5.
Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–93.
Paraiso IL, Revel JS, Stevens JF. Potential use of polyphenols in the battle against COVID-19. Curr Opin Food Sci. 2020;32:149–55.
Park J-Y, Kim JH, Kim YM, Jeong HJ, Kim DW, Park KH, et al. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg Med Chem. 2012;20:5928–35.
Murugan NA, Pandian CJ, Jeyakanthan J. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J Biomol Struct Dyn. 2021;39:4415–26.
Kim DW, Seo KH, Curtis-Long MJ, Oh KY, Oh J-W, Cho JK, et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J Enzyme Inhib Med Chem. 2014;29:59–63.
Park J-Y, Yuk HJ, Ryu HW, Lim SH, Kim KS, Park KH, et al. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J Enzyme Inhib Med Chem. 2017;32:504–12.
Nguyen TTH, Woo H-J, Kang H-K, Nguyen VD, Kim Y-M, Kim D-W, et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34:831–8.
Song YH, Kim DW, Curtis-Long MJ, Yuk HJ, Wang Y, Zhuang N, et al. Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol Pharm Bull. 2014;37:1021–8.
Park J-Y, Jeong HJ, Kim JH, Kim YM, Park S-J, Kim D, et al. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol Pharm Bull. 2012;35:2036–42.
Das S, Sarmah S, Lyndem S, Singha RA. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J Biomol Struct Dyn. 2021;39:3347–57.
Park J-Y, Ko J-A, Kim DW, Kim YM, Kwon H-J, Jeong HJ, et al. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J Enzyme Inhib Med Chem. 2016;31:23–30.
Ghosh R, Chakraborty A, Biswas A, Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors—an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. 2021;39:4362–74.
Mbikay M, Chrétien M. Isoquercetin as an anti-covid-19 medication: a potential to realize. Front pharmacol. 2022;13: 830205.
Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–8.
Soto ME, Guarner-Lans V, Soria-Castro E, Manzano Pech L, Pérez-Torres I. Is antioxidant therapy a useful complementary measure for covid-19 treatment? An algorithm for its application. Medicina. 2020;56:386.
Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005;272:4725–40.
Chien M, Anderson TK, Jockusch S, Tao C, Li X, Kumar S, et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res. 2020;19:4690–7.
Singh S, Sk MF, Sonawane A, Kar P, Sadhukhan S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: an in-silico analysis. J Biomol Struct Dyn. 2021;39:6249–64.
Pandey K, Lokhande KB, Swamy KV, Nagar S, Dake M. In silico exploration of phytoconstituents from Phyllanthus emblica and Aegle marmelos as potential therapeutics against SARS-CoV-2 RdRp. Bioinform Biol Insights. 2021;15:11779322211027404.
Morán-Santibañez K, Peña-Hernández MA, Cruz-Suárez LE, Ricque-Marie D, Skouta R, Vasquez AH, et al. Virucidal and synergistic activity of polyphenol-rich extracts of seaweeds against measles virus. Viruses. 2018;10:465.
Brugh M Jr. Butylated hydroxytoluene protects chickens exposed to Newcastle disease virus. Science. 1977;197:1291–2.
Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, et al. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. 2018;62:1700447.
Ouyang J, Zhu K, Liu Z, Huang J. Prooxidant effects of epigallocatechin-3-gallate in health benefits and potential adverse effect. Oxid Med Cell Longev. 2020;2020:9723686.
Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–73.
Funding
This article has not received any funding.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript; AS, BDR, MV, and MB conceptualized the review paper. MV, AK, SH, MAF, and MB prepared the original draft. GA, BDR, NMD, and AS were involved in the critical revision of the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vajdi, M., Karimi, A., Hassanizadeh, S. et al. Effect of polyphenols against complications of COVID-19: current evidence and potential efficacy. Pharmacol. Rep 76, 307–327 (2024). https://doi.org/10.1007/s43440-024-00585-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-024-00585-6