Abstract
During the last years, several reports have provided evidence about adverse health effects on personal involved in Antineoplastic Drugs (ANPD) handling. ANPD has the ability to bind DNA, thus produce genotoxic damage. In this way, XRCC1 and XRCC3 proteins are necessary for efficient DNA repair and polymorphisms in this genes can be associated with an individual response to ANPD exposure. Therefore, the aim of this study was to evaluate genetic damage of occupational exposure to antineoplastic drugs and the possible effect of XRCC1 and XRCC3 polymorphisms in oncology employees from Bogotá, Colombia. Peripheral blood samples were obtained from 80 individuals, among exposed workers and healthy controls. The comet assay and Cytokinesis-block micronucleus cytome assay was performed to determinate genetic damage. From every sample DNA was isolated and genotyping for XRCC1 (Arg194Trp, Arg280His and Arg399Gln) and XRCC3 (Thr241Met) SNPs by PCR–RFLP. The exposed group showed a significant increase of comet assay results and micronucleus frequency, compared with unexposed group. It was observed a gender, exposure time and workplace effect on comet assay results. Our results showed no significant associations of comet assay results and micronucleus frequency with either genotype, allele, nor haplotype of XRCC1 and XRCC3 SNPs. The results suggest that occupational exposure to ANPD may lead to genotoxic damage and even be a risk to human health. To our knowledge, this is the first study to assess the genotoxic damage of occupational exposure to APND in South America.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antineoplastic drugs (ANPD) are substances from different chemical nature, used preferentially, but not exclusively, in the pharmacological treatment of neoplastic disease. The ANPD have the ability to inhibit tumor cells growth by disrupting cell division and killing actively growing cells [1]. These drugs can interact with nucleic acids from cell, inhibiting DNA synthesis or causing DNA damage [2]. Its potential for human health risk in occupationally exposed workers has been reported in various studies across the world [3,4,5].
Associations between polymorphisms of DNA repair genes and genotoxicity in exposed populations to ANPD and others xenobiotics, have been reported in previous studies [6,7,8,9]. The base excision repair (BER) and homologous recombination (HR) pathways are responsible for handling many different forms of DNA damage [7, 10] and essentials to maintain chromosome stability and prevent chromosomal fragmentation, translocations and deletions [9, 11]. In this way, it is well known that the X-ray repair cross complementing 1 (XRCC1) and 3 (XRCC3) proteins are necessary for efficient DNA repair by BER and HR pathways, respectively. Single nucleotide polymorphisms (SNPs) in XRCC1 and XRCC3 genes may have a functional effect, leading to genetic instability and increasing the susceptibility to cancer and other diseases [12,13,14].
Biomarkers of chromosome and DNA damage in peripheral blood lymphocytes (PBL), like micronuclei (MN) frequency and comet assay, are very helpful to identify the effects of xenobiotics on cells from occupational exposure workers. The cytokinesis-block micronucleus cytome (CBMN) assay is a method for measuring MN in human cells, indicating chromosome breaks or failures with the mitotic spindle during cell division, regarding to clastogenic or aneugenic effects, respectively [10, 14, 15]. The comet assay is sensitive and efficient to assess DNA damage in individual cells. It detects single strand breaks and alkali-labile sites and have increased its use in human biomonitoring studies during the past few decades [16,17,18].
In the present study, we evaluate the genotoxic effect of occupational exposure to antineoplastic drugs and the possible influence of XRCC1 and XRCC3 polymorphisms on individual response to exogenous insults.
Materials and methods
Subjects
The study included 40 regular employees involved in handling of ANPD from oncology units of Colombian hospitals, and 40 healthy controls without ANPD or X-rays exposition nor serious illness. Each participant completed a questionnaire about personal health, sociodemographic characteristics and ANPD exposition. Exposed and control group was matched by age and gender. Smokers and alcohol drinkers (more than 200 mL daily) were excluded from this study.
All procedures of this study were approved by the Research Ethics Committee of all institutions involved in this study. Written informed consent was obtained from all participating employee and healthy control before sampling. Three samples of peripheral blood (approximately 15 mL) was collected from exposed and control subjects. One sample was used for CBMN, other for comet assay and the other for DNA isolation.
Cytokinesis block micronucleus test (CBMN)
Briefly, a peripheral blood sample was cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C and 5% CO2 for 72 h. After this period, the culture medium was discarded and cells were rinsed with PBS and incubated with fresh culture medium containing 5 µg/mL Cytochalasin B (Sigma-Aldrich, St. Louis, MO, USA) and incubated for another 24 h. Cells were harvested, treated with cold hypotonic solution (KCl 0.075 M), and centrifuged (1500×g for 7 min) then, cells were fixed in cold methanol and acetic acid (3:1) solution. The slides were stained with 10% Giemsa solution (pH 7.4) for 10–15 min. Micronuclei in binucleated cells (MNBNCs) and nuclear division index (NDI) were analyzed as previously described [14]. MN frequencies were determined in 1000 binucleated cells (BNCs).
Comet assay
The comet assay was performed under pH alkaline conditions as previously described [17]. Firstly, lymphocytes were isolated from blood sample collected and assayed for viability using trypan blue dye exclusion. A sample of 2 × 105 cells mixed with low melting point agarose was placed on glass microscope slide, with a base layer of normal melting point agarose, and left it on ice for 10 min. The slides were immersed in lyses solution (NaCl 2.5 M, EDTA 0.1 M, Tris 10 mM, 10% DMSO and 1% Triton X 100) pH 10, overnight at 4 °C. Then, slides were placed into horizontal electrophoresis box with fresh buffer (EDTA 200 Mm, NaOH 10 N, pH > 13) to allow the DNA unwinding. Electrophoresis was conducted for 30 min at 4 °C and 25 V/300 mA. After that, the slides were washed with neutralization buffer (Tris 0.4 M, pH 7.5) for 15 min, dried at room temperature and stained with SYBER-Green (Thermo Fisher, Waltham, MO, USA). Subsequently, image analysis was performed using a fluorescence microscope (Nikon, Tokyo, Japan), equipped with the imaging system Comet Assay IVTM Software (Perceptive Instruments, St Francis House, UK). A total of 100 comets were scored for each slide. DNA damage was measured as percentage of migrated DNA in the comet tail (Tail Intensity).
Genotyping
From all exposed subjects and healthy control, genomic DNA was isolated from blood samples collected by salting-out method, using Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) and stored frozen at − 80 °C.
To analyze the XRCC1 (Arg194Trp, Arg280His and Arg399Gln) and XRCC3 (Thr241Met) SNPs, approximately 100 ng of genomic DNA was amplified in a total volume of 25 µL, consisting of 10 pmol for each primer, 2 mM of MgCl2, 0.2 mM of dNTPs and 1 U of Platinum Taq Polymerase in 1X buffer (Invitrogen, Carlsbad, CA, USA). PCR conditions for each SNP were previously reported [19, 20]. The PCR product was digested overnight at 37 °C with specific restriction endonuclease (Fermentas, Waltham, MA, USA). Finally, DNA fragments was visualized by agarose gel electrophoresis.
Statistical analysis
Statistical analysis was carried out with SPSS software version 22 for Windows (Chicago, IL, USA). Chi-square and Fisher’s exact, Pearson’s χ2 and t student tests were used to evaluate the difference in distributions for demographic variables, as well as allele and genotypes frequencies of XRCC1 and XRCC3 polymorphisms between exposed and control groups. The observed genotypes were computed and tested for Hardy–Weinberg equilibrium using Pearson’s χ2 test. The distributions of MN frequency and TI results were tested for normality by Shapiro–Wilk test; therefore, nonparametric Mann-Whitnhey U test and Kruskal–Wallis H test was performed to evaluated the significant difference between exposed and control groups, and subgroups, respectively. P values < 0.05 were considered statistically significant and all statistical tests were 2-sided.
Results
Population characteristics
General characteristics of exposed an unexposed (control group) subjects are summarized in Table 1. No significant differences were found related to age and gender in the two groups. We divided the both groups according to age mean (31.42 years old) as subjects < 32 and ≥ 32 years old. Similar distribution was observed between the groups (p = 0.501).
For the exposed group, occupational exposure time mean was 41.1 months; we divided the exposed group according this mean, 24 individuals with less than 42 months and 16 individuals with 42 or more months. Others parameters about occupational exposure (workplace and exposure hours per day to APND) were explored and presented in Table 1. The exposed group was composed of pharmacist and nurses, principally, from pharmacy (65%) and drug administration (30%) departments, respectively. Regarding ANPD used within oncology centers; Bleomycin, Carboplatin, Cyclophophamide, Cisplatin, Doxorubicin, Fluorouracil and Paclitaxel, were the most frequent APND handled. All this ANPD are classified as extremely toxic by The National Institute for Occupational Safety and Health (NIOSH).
DNA and chromosome damage
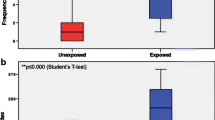
Table 2 reports TI mean and MN frequencies, in peripheral blood lymphocytes of exposed and control subjects. The statistical analysis showed a significant difference in both, DNA and chromosome damage between exposed and control group. The DNA damage, expressed by TI mean, was significant increase in personal exposed to ANPD as compared to control subjects (Fig. 1a, p < 0.0001). Indeed, to identify the principal factors involved in this results, we stratified the study group by age, gender and some occupational exposure parameters. Related to gender, we found a significant increase of DNA damage in females when compared to males in exposed individuals (Fig. 1b, p = 0.030). Occupational exposure parameters, like exposure time per day and workplace showed an effect on the DNA damage results. We observed a significant increase in TI mean of personal exposed to ANPD by ≥ 4 h per day (Fig. 1c, p = 0.011). In this way, exposed individuals from drug administration department shows a significant increase of TI mean, compared to individual from pharmacy and others departments (Fig. 1d, p = 0.034).
Regard to chromosome damage, expressed as MN frequency in 1000 binucleated cells, the results indicated a significant increase of MN frequency in personal exposed to APND as compared to unexposed individuals (p < 0.001). Age, gender and occupational parameters did not show an effect on frequency MN in both groups (Table 2). Additionally, we determinate the nuclear division index (NDI). No significant intergroup variations were observed for NDI (data not shown).
XRCC1 and XRCC3 polymorphisms
The XRCC1 Arg194Trp (rs1799782), Arg280His (rs25489) and Arg399Gln (rs25487) and XRCC3 Thr241Met (rs861539) SNPs were evaluated in exposed and control group, as shown in Table 3. The genotype frequency was computed for all four SNPs and tested for Hardy–Weinberg equilibrium, providing no evidence of population stratification. The genotypes distribution of the four SNPs was similar between exposed and control subjects (Table 3, p > 0.05).
Our results showed no significant associations of TI results and MN frequency with either genotypes, alleles, nor haplotypes of XRCC1 and XRCC3 SNPs (Table 3). However, we found a non-significant increase of MN frequency in exposed and control subjects associated to XRCC1 Trp194Trp and XRCC3 Met241Met polymorphisms. The TI results showed a non-significant decrease associated to XRCC1 Arg194Trp and Arg399Gln in exposed and control group. Others genotypes presented similar distribution of TI mean and MN frequency in exposed and control group. The complete results are presented in Table 3.
Discussion
In our study, it was explored the effect of occupational exposure to ANPD, and DNA and chromosome damage in a group of individuals handling these drugs. Identify exposure effect to specific ANPD is difficult, since exposed individuals handling different agents, and usually, their administration is in combination. Therefore, we used the comet assay and CBMN test (biomarkers of effect) to identify the genotoxic effect of APND exposure. Comet assay and CBMN test are methods to assess genotoxicity activity from different agents; since the comet assay is a sensitive method to detect DNA damage (single and double strand breaks, and alkali labile sites), such damage may have originated a short time before sampling; whereas CBMN test detect chromosome fragments from DNA breakage and chromosome loss, caused by exposure occurred long before sampling. Both, comet assay and CBMN test, are recommended to assess particular populations chronically exposed to genotoxic agents [21].
We observed a significant increase in both, TI results and MN frequency, in exposed group as compared to control group. This results indicate that, even when professionals with specific trained and compliance with standards on safe handling of ANPD, exposure and accidental contamination still presence in transportation, preparation and administration processes [22, 23]. Similar to our results, different studies from several countries reported increase of genetic damage, assess by comet assay and/or CBMN test in subjects exposed to APND [1, 6, 21, 22, 24,25,26]. Contrary to what was observed in this study, negative results for MN and/or comet assay have also reported [1, 27, 28]. These inconsistencies results could manly be explained by the variety of ANPD handled, workplace, exposure time, and protective clothing and safety measures employed.
Several of ANPD used by exposed subjects, has been classified as group 1 (Carcinogenic to human) or 2 (Probably/Possibly carcinogenic to human) by the International Agency for Research on Cancer. This compounds are only partially selective, and healthy cells may also be damaged once the ANPD can bind to DNA and induce genotoxic damage for different mechanisms as modification of bases (oxidation and/or alkylation), strand breaks and crosslinks, that can be detected by Comet assay [1, 22].
In this study, exposed group showed an effect of gender on TI results but not MN frequency.
Women exposed had higher levels of DNA damage than men exposed. This results could be explained by gender effects on DNA repair pathways, since women had less efficient double strand breaks repair and nucleotide excision repair pathway [29, 30]. Although, recent studies have identified no statistically significant gender effect on comet assay and CBMN test [22, 25, 31].
No correlation was found between DNA and chromosome damage (measure by comet assay and CBMN test, respectively) and months of exposure to ANPD; these findings are in agreement with previous investigations [22, 32, 33]. However, we found a positive association with working day. Individuals exposed by 6 or more hours per day showed an increase of TI results, but not MN frequency. Our results clear showing the genotoxic potential of ANPD on workers exposed, and it is evident that exposed subjects by more hours per day presented an increased genetic damage, this recent exposure levels can be measured by a sensitive test, like comet assay, but not CBMN test.
Here, we found significant increase of DNA damage (TI results) in personnel exposed to ANPD in drug administration department, as compared with personnel exposed in pharmacy and others departments. A plausible explanation for this results can be related with protective measures applied; once ANPD preparation is done under strict control and safer conditions; gloves, masks, safety glasses and closed gowns is required for preparation into pharmacy department. On the other hand, the use of gloves is the unique protective measure applied by personnel of drug administration department, which could be associated with highest levels of exposure to APND. In accordance with this results, some studies demonstrated in different professionals that protective measures applied on ANPD handling can significantly reduce genotoxic risk of this agents [2, 26, 34].
Specific DNA repair pathway, among them BER and HR, are activated after exposure to genotoxic agents, since DNA damage was generated. The responses to DNA damage induced by this exposure can be influenced by polymorphisms in DNA repair genes. We explored the association between DNA damage and polymorphisms in critical genes of DNA repair pathways, thus we performed an analysis of genetic damage and polymorphisms in XRCC1 and XRCC3 genes, involved in BER and HR pathways, respectively.
Similar genotypes frequencies of XRCC1 Arg194Trp, XRCC1 Arg280His, XRCC1 Arg399Gln and XRCC3 Thr241Met SNPs was found between control and exposed group. Our analysis showed non-significant associations between either genotypes of XRCC1 and XRCC3 SNPs, and genetic damage. Nevertheless, our results suggest that XRCC1 Arg194Trp and XRCC3 Thr241Met SNPs can increase MN frequency.
In concordance with our findings, few studies observed no association between genetic damage and XRCC1/XRCC3 polymorphisms [7, 8, 35]. In contrast, many works had demonstrated the XRCC1/XRCC3 polymorphisms effect in genetic damage by occupational exposure to genotoxic agents [6, 9, 10, 13, 36].
SNPs in XRCC1 and XRCC3 genes explored in this work were previously associated with colorectal cancer [37], thyroid cancer [3, 38], cervical cancer [4] and Alzheimer’s disease [4]; demonstrating the relevant role of XRCC1 and XRCC3 genes within DNA repair system. However, we found no correlation with genetic damage in our work. Our results seem to suggest that in the case of an occupational exposure to massive harmful agent, the influence of the genetic susceptibility could be difficult to be estimated as previously hypothesized by others studies [33, 39].
In conclusion, our results showed that exposed group to APND had higher TI results and MN frequencies respect to control group, provided even more proof of occupational exposure to ANPD represent a serious risk for personnel that handling these agents. XRCC1 Arg194Trp, XRCC1 Arg280His, XRCC1 Arg399Gln and XRCC3 Thr241Met polymorphisms were no associated with genetic damage in Colombian population exposed to ANPD. We found high levels of DNA damage associated with personnel exposed by 6 or more hours per day and poor safety measures. Unfortunately, we cannot compare our results with others since there are no similar studies performed in Colombian population. We recommend using comet assay and CBMN test to human biomonitoring of exposed populations to xenobiotics.
References
Buschini A, Villarini M, Feretti D, Mussi F, Dominici L, Zerbini I, Moretti M, Ceretti E, Bonfiglioli R, Carrieri M, Gelatti U, Rossi C, Monarca S, Poli P (2013) Multicentre study for the evaluation of mutagenic/carcinogenic risk in nurses exposed to antineoplastic drugs: assessment of DNA damage. Occup Eviron Med 70:789–794
Izdes S, Sardas S, Kadioglu E, Kaymak C, Ozcagli E (2009) Assessment of genotoxic damage in nurses occupationally exposed to anaesthetic gases or antineoplastic drugs by the comet assay. J Occup Health 51:283–286
King J, Alexander M, Byrne J, MacMillan K, Mollo A, Kirsa S, Green M (2016) A review of the evidence for occupational exposure risks to novel anticancer agents—a focus on monoclonal antibodies. J Oncol Pharm Pract 22:121–134
Suspiro A, Prista J (2011) Biomarkers of occupational exposure do anticancer agents: a minireview. Toxicol Lett 207:42–52
Villarini M, Gianfredi V, Levorato S, Vannini S, Salvatori T, Moretti M (2016) Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: a systematic review of the literature and meta-analysis. Mutat Res 770:35–45
Cornetta T, Padua L, Testa A, Ievoli E, Festa F, Tranfo G, Baccelliere L, Cozzi R (2008) Molecular biomonitoring of a population of nurses handling antineoplastic drugs. Mutat Res 638:75–82
Ladeira C, Viegas S, Carolino E, Gomes MC, Brito M (2013) The influence of genetic polymorphisms in XRCC3 and ADH5 genes on the frequency of genotoxicity biomarkers in workers exposed to formaldehyde. Environ Mol Mutagen 54:213–221
Sorsa M, Anderson D (1996) Monitoring of occupational exposure to cytostatic anticancer agents. Mutat Res 355:253–261
Hong SH, Szili EJ, Fenech M, Gaur N, Short RD (2017) Genotoxicity and cytotoxicity of the plasma jet-treated medium on lymphoblastoid WIL2-NS cell line using the cytokinesis block micronucleus cytome assay. Sci Rep 7:3854
Gajski G, Geric M, Orescanin V, Garaj-Vrhovac V (2018) Cytokinesis-block micronucleus cytome assay parameters in peripheral blood lymphocytes of the general population: contribution of age, sex, seasonal variations and lifestyle factors. Ecotox Environ Saf 148:561–570
Fenech M (2006) Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res 600:58–66
Jacociunas LV, de Andrade HH, Lehmann M et al (2013) Artichoke induces genetic toxicity in the cytokinesis-block micronucleus (CBMN) cytome assay. Food Chem Toxicol 55:56–59
Araujo FD, Pierce AJ, Stark JM, de Abreu BR, Ferraz AB, da Silva J, Grivicich I, Dihl RR (2002) Variant XRCC3 implicated in cancer is functional in homology-directed repair of double-strand breaks. Oncogene 21:4176–4178
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104
Lee SL, Thomas P, Hecker J, Faunt J, Fenech M (2015) Chromosomal DNA damage measured using the cytokinesis-block micronucleus cytome assay is significantly associated with cognitive impairment in South Australians. Environ Mol Mutagen 56:32–40
Villalba-Campos M, Chuaire-Noack L, Sanchez-Corredor MC, Rondon-Lagos M (2016) High chromosomal instability in workers occupationally exposed to solvents and paint removers. Mol Cytogenet 9:46
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Muñoz Aristizábal AF (2009) Assesment of DNA damage in two colombian populations of agricultirist and floriculturist. Rev UDCA Act Div Cient 1:7–16
Wang Q, Ji F, Sun Y, Qiu YL, Wang W, Wu F, Miao WB, Li Y, Brandt-Rauf PW, Xia ZL (2010) Genetic polymorphisms of XRCC1, HOGG1 and MGMT and micronucleus occurrence in Chinese vinyl chloride-exposed workers. Carcinogenesis 31:1068–1073
Mandal RK, Mittal RD (2018) Polymorphic variation in double strand break repair gene in indian population: a comparative approach with worldwide ethnic group variations. Indian J Clin Biochem 33:184–189
Maluf SW, Erdtmann B (2000) Follow-up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis-block micronuclei analysis and single cell gel electrophoresis assay. Mutat Res 471:21–27
Moretti M, Grollino MG, Pavanello S, Bonfiglioli R, Villarini M, Appolloni M, Carrieri M, Sabatini L, Dominici L, Stronati L, Mastrangelo G, Barbieri A, Fatigoni C, Bartolucci GB, Ceretti E, Mussi F, Monarca S (2015) Micronuclei and chromosome aberrations in subjects occupationally exposed to antineoplastic drugs: a multicentric approach. Arch Environ Occup Health 88:683–695
Zhang Z, Wan J, Jin X, Jin T, Shen H, Lu D, Xia Z (2005) Genetic polymorphisms in XRCC1, APE1, ADPRT, XRCC2, and XRCC3 and risk of chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol Biomark Prev 14:2614–2619
Laffon B, Teixeira JP, Silva S, Loureiro J, Torres J, Pasaro E, Mendez J, Mayan O (2005) Genotoxic effects in a population of nurses handling antineoplastic drugs, and relationship with genetic polymorphisms in DNA repair enzymes. Am J Ind Med 48:128–136
Ladeira C, Viegas S, Padua M, Gomes M, Carolino E, Gomes MC, Brito M (2014) Assessment of genotoxic effects in nurses handling cytostatic drugs. J Toxicol Environ Health A 77:879–887
El-Ebiary AA, Abuelfadl AA, Sarhan NI (2013) Evaluation of genotoxicity induced by exposure to antineoplastic drugs in lymphocytes of oncology nurses and pharmacists. J App Toxicol 33:196–201
Villarini M, Dominici L, Fatigoni C, Muzi G, Monarca S, Moretti M (2012) Biological effect monitoring in peripheral blood lymphocytes from subjects occupationally exposed to antineoplastic drugs: assessment of micronuclei frequency. J Occup Health 54:405–415
Hessel H, Radon K, Pethran A, Maisch B, Grobmair S, Sautter I, Fruhmann G (2001) The genotoxic risk of hospital, pharmacy and medical personnel occupationally exposed to cytostatic drugs—evaluation by the micronucleus assay. Mutat Res 497:101–109
Mayer PJ, Lange CS, Bradley MO, Nichols WW (1991) Gender differences in age-related decline in DNA double-strand break damage and repair in lymphocytes. Ann Hum Biol 18:405–415
Uppstad H, Osnes GH, Cole KJ, Phillips DH, Haugen A, Mollerup S (2011) Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer 71:264–270
Fenech M, Bonassi S (2011) The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 26:43–49
Kopjar N, Garaj-Vrhovac V, Kasuba V, Rozgaj R, Ramic S, Pavlica V, Zeljezic D (2009) Assessment of genotoxic risks in Croatian health care workers occupationally exposed to cytotoxic drugs: a multi-biomarker approach. Int J Hyg Environ Health 212:414–431
Cornetta T, Festa F, Testa A, Cozzi R (2006) DNA damage repair and genetic polymorphisms: assessment of individual sensitivity and repair capacity. Int J Radiat Oncol Biol Phys 66:537–545
Moretti M, Bonfiglioli R, Feretti D, Pavanello S, Mussi F, Grollino MG, Villarini M, Barbieri A, Ceretti E, Carrieri M, Buschini A, Appolloni M, Dominici L, Sabatini L, Gelatti U, Bartolucci GB, Poli P, Stronati L, Mastrangelo G, Monarca S (2011) A study protocol for the evaluation of occupational mutagenic/carcinogenic risks in subjects exposed to antineoplastic drugs: a multicentric project. BMC Public Health. 11:195
Iarmarcovai G, Sari-Minodier I, Chaspoul F, Botta C, De Meo M, Orsiere T, Berge-Lefranc JL, Gallice P, Botta A (2005) Risk assessment of welders using analysis of eight metals by ICP-MS in blood and urine and DNA damage evaluation by the comet and micronucleus assays; influence of XRCC1 and XRCC3 polymorphisms. Mutagenesis 20:425–432
Bull CF, Mayrhofer G, Zeegers D, Mun GL, Hande MP, Fenech MF (2012) Folate deficiency is associated with the formation of complex nuclear anomalies in the cytokinesis-block micronucleus cytome assay. Environ Mol Mutagen 53:311–323
Skjelbred CF, Saebø M, Wallin H, Nexø BA, Hagen PC, Lothe IM, Aase S, Johnson E, Hansteen IL, Vogel U, Kure EH (2006) Polymorphisms of the XRCC1, XRCC3 and XPD genes and risk of colorectal adenoma and carcinoma, in a Norwegian cohort: a case control study. BMC Cancer 6:67–76
Cho NY, Kim KW, Kim KK (2017) Genomic health status assessed by a cytokinesis-block micronucleus cytome assay in a healthy middle-aged Korean population. Mutat Res 814:7–13
Francois M, Hochstenbach K, Leifert W, Fenech MF (2014) Automation of the cytokinesis-block micronucleus cytome assay by laser scanning cytometry and its potential application in radiation biodosimetry. Biotechniques 57:309–312
Acknowledgements
The authors gratefully acknowledge the cooperation of all volunteers who participated in this study and support of “Asociación de Enfermería Oncológica Colombiana”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Aristizabal-Pachon, A.F., Castillo, W.O. Genotoxic evaluation of occupational exposure to antineoplastic drugs. Toxicol Res. 36, 29–36 (2020). https://doi.org/10.1007/s43188-019-00003-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-019-00003-7