Abstract
Purpose of Review
This short review updates an exhaustive one written by Correa et al. in 2019 about haptic training simulation on needle insertion in the medical field.
Recent Findings
Latest works refine well-known models and enhance setups and methods to facilitate generically getting experimental data.
Summary
We provide a complementary focus on device specifications and recent models to render this specific haptic feedback on computer-based simulators. Assessment approaches and the issues encountered when introducing such simulators into curricula are also discussed. FEM-based approaches still do not permit real-time computation but hybrid approaches as proposed by Wittek et al. in 2020 may become a good compromise. Nonetheless, psychophysical studies should be performed to determine the haptic fidelity of the various approaches found in the literature, and embed them efficiently in medical curricula. This would permit to delay the necessary final hands-on training on patients that raises ethical issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many medical procedures (blood sampling, biopsy, puncture, catheterization ... in anesthesia, brachytherapy, neurosurgery, ...) require needle insertion but this common and important gesture differs a lot according to the goal, the concerned areas of the body, and the visibility in the area. By nature, the part of the needle already inside the body is not directly visible, which makes this gesture performed almost blindly. Practitioners then require another source of information to determine if the tip of the needle has reached the target location, knowing that the needle may deflect from its initial trajectory (notably for beveled ones), may cross various layers of anatomic tissues with different mechanical behaviors (skin, fat, tendons, nerves, ...) requiring mastered insertion forces and penetration velocities from the practitioner.
One important source is the hapticFootnote 1 feedback: the needle-patient body interaction forces felt by the practitioners in their hand(s) while inserting the needle. In this way, they feel whether the needle penetrates or slips around a blood vessel wall, or enters in contact with a bone, for instance. But in some cases, this force feedback is not sufficient, such as in epidural anesthesia or intraarticular injections. Complementary information must be provided to the practitioners to help them in their gestures. For instance, in the case of epidural anesthesia, a syringe filled with a neutral solution is mounted on the needle; the way it empties through the needle provides to the practitioner crucial information about the reach of the epidural area. In other cases (brachytherapy, intraarticular injection, some epidural anesthesia, ...), real-time medical imaging provides this complementary information. In all these cases, the practitioners must learn how to manipulate and coordinate these tools taking into account these sensations and complementary information, during a long apprenticeship. Some of them require much practice before being efficient. For instance, 90 epidural insertions are necessary to obtain an 80% success rate [1].
However, this training is, in general, performed first on manikins and next on real patients that may suffer from unsuccessful first attempts. This widespread ethical issue in the medical discipline has encouraged the use of more realistic simulators that could permit safely and efficiently acquire the technical skills and delay the necessary final training on patients [2]. Computer-based simulation (CBS) has been the first response to this general requirement. However, they lack the force feedback rendering dimension. Haptic training simulators (HTS) add this feedback with the help of haptic devices, raising the fidelity of CBS [3]. For instance, a recent review of haptic training for laparoscopy is proposed in [4]. Such simulators can render the aforementioned haptic feedback to help practitioners train themselves on these sensations as many times as necessary without any risk for patients.
This short review deals with hands-on training for needle insertion with haptic training simulators. It updates a more exhaustive one written by Correa et al. in 2019 [5••] with a complementary focus on devices and models used to render this haptic feedback, and assessment approaches recently developed to provide trainees an objective evaluation of their gestures and information on how to improve them. Therefore, the following section introduces the haptic devices that could be used for such a purpose, while the “Needle Insertion Simulation Models” section details the various models that permit the control of the aforementioned haptic devices to render realistic force feedback. The “Gesture Assessment” section deals with gesture assessment.
Haptic Devices Used for Needle Insertion Simulators
Virtual reality simulators are most of the time insufficient because the haptic part is missing [6•]. The haptic part can be passively reproduced by basic mannequins. Nevertheless, if they can be sufficient for tactile feedback, they are, in general, not realistic enough in terms of force feedback. Since the democratization of additive manufacturing, some researchers have been developing multi-material components to provide haptic feedback. For example, the combination of thermoplastic polyurethane (TPU) and acrylonitrile butadiene styrene (ABS) can be used to make a phantom allowing needle insertion training [7]. Models based on gelatin can also be used [8]. However, these solutions require manufacturing new products to reproduce different behaviors. To solve this issue, the authors of [9] propose using a specific cartridge to reproduce the penetration of the needle into different layers.
However, using passive materials to provide feedback has one main issue: they will wear out over time and can be damaged by piercing. One solution is to use active haptic interfaces. These interfaces can offer configurable simulators without any damage to materials. They also permit embedding sensors to record data for gesture assessment purposes.
Commercial Haptic Devices
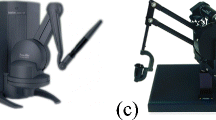
Usual haptic interfaces are based on electric actuators such as DC motors embedded in robots with serial (such as Touch by 3D System or Virtuose 6D by Haption) or parallel (such as Falcon by Novint Technolgies Inc. or Omega 6 by Force Dimensions) architectures. This not exhaustive list gathers the products usually found in the literature. Figure 1 includes photos of these interfaces. Their main difference lies in their numbers of degrees of freedom (DoF) and of degrees of force feedback (DoFF), the maximum force they can produce, their workspace, and their cost. Table 1 gathers these characteristics.
Examples of electric haptic interface. (a) Touch by 3D Systems, North Carolina (Image courtesy of 3D Systems, North Carolina), (b) Virtuose 6D by Haption GmbH. (From Dombrowski U et al., with permission from Elsevier) [10], (c) Falcon by Novint Tech. Inc. (From Yang C et al, with permission from Springer) [11], (d) Omega 6 byForce Dimension, Switzerland. (Image courtesy of Force Dimension, Switzerland)
Specifications for Needle Insertion
Needle insertion simulators require at least one DoFF to feel the axial tissue resistance during the penetration. It can be useful in terms of fidelity with real cases and pedagogical requirements also to enable the orientation of the needle around the insertion hole, which then requires 5 DoF. It could be also interesting to reproduce the lateral tissue forces while the operator changes the orientation of the needle during the penetration. It is therefore recommended to reproduce 5 DoFF to get a realistic simulator.
Examples
To increase trainees’ immersion into the simulation, it is advised to provide a mock needle on the interface. With the development of additive manufacturing, it is quite common to insert it on the haptic interface terminal tool. For instance, in [12], where authors used a Novint Falcon for their epidural simulator, they have developed a custom end effector to substitute the Novint Falcon one (see Fig. 2). It allows increasing the realism of the simulator and thus improve skills transfers to real-life situations.
Epidural simulator interface to connect to a Novint Falcon [12]
In [13], authors used two Touch X interfaces (improved version of the Touch interface by 3D Systems) to reproduce forces during ophthalmic surgical procedures. They also developed a specific end effector to allow practitioners to use similar tools as in real procedures. To improve immersion, a virtual world is added to the simulator. In [14], authors simulated a central venous vatheterization (CVC) with a virtual ultrasound probe featuring a 3D tracker and a Touch interface to simulate the CVC needle. The trainee handles the mock US probe with one hand and the needle with the other hand. The real-time position and orientation of the probe permit providing synchronized fake US images integrating the virtual needle when visible. Li et al. enhanced this setup by simulating the fake probe with a second Touch interface to render probe-patient interaction forces (see Fig. 3) [15]. The originality of this study mainly concerns the methods to compute in real-time the force to be reproduced by the haptic interfaces, taking into account the respiration of the virtual patient.
Simulator for renal biopsy based on Touch interfaces [15]
Custom Haptic Interfaces
The aforementioned commercially available interfaces can be used for many medical applications. However, some procedures require to develop dedicated haptic interfaces to be more realistic.
In [16], the authors developed a custom interface based on electric actuators and a hexapod design (see Fig. 4). This original structure, which allows obtaining 6 DoFF, lies on the unbound tool handled by the trainees. They can thus move their tool without any constraints when they are outside the virtual body. They can also change their tool and once they touch the virtual object, the tip of the hexapod is linked to the tooltip.
Simulator using a custom haptic device based on an electric actuatedhexapod [16]
In epidural procedures, practitioners handle a needle mounted on a syringe. Epidural anesthesia is a blind procedure as practitioners cannot see through the human body and do not use any ultrasound probes on daily use. To bypass this issue, practitioners connect a syringe filled with a neutral solution and push on the piston while introducing the needle. The piston resistance provides them haptic information about the localization of the tip of the needle. This resistance quickly decreases as soon as the tip reaches the area of interest. In [17], the authors used a pneumatic cylinder coupled with an artificial needle mounted on a commercial haptic interface (Virtuose6D) (see Fig. 5). Using this simulator, trainees are provided with force feedback not only from the needle (through the electric haptic device) but also from the syringe (through the pneumatic cylinder).
Conclusion
Training simulators are becoming more and more popular to learn and assess medical gestures [18]. To be efficient in terms of hands-on training, configurable, repetitive, and providing objective assessment feedback, needle insertion simulators should feature an active haptic interface. These haptic interfaces allow producing forces to allow trainees to become familiar with real procedures. However, these forces need to be realistic. For that purpose, it is necessary to compute them using biomechanical models. Next section deals with this aspect.
Needle Insertion Simulation Models
Extensive work has focused on force modeling for needle insertion into soft tissues. Some comprehensive reviews have been written either considering haptic simulation applications [5••, 19], or robot-assisted procedures [20]. In this review, we focus on works related to haptic simulations, either already in use or ought to be integrated into haptic training simulators in the coming years.
According to Azar et al., models used to render needle insertion forces in simulations belong to two categories: deformation- and fracture mechanics–based models. Deformation-based models come from the observation of forces due to the penetration of the needle into the tissues without considering underlying physics. In the second category, the needle insertion is modeled as a crack that propagates with the help of an energetic approach [21].
Deformation-Based Models
Forces exerted on the shaft of the needle during insertion into soft tissues are commonly considered as the sum of “cutting, sliding, stick-slip, tissue deformation, and displacement and peeling” [22].
In the early 2000s, Simone and Okamura [23] proposed a method to measure these different forces and to gather them into 3 components corresponding to (a) cutting forces, applied on the tip of the needle, (b) friction forces, applied along the shaft of the needle, and (c) stiffness forces, due to elasticity of the tissue before puncture, when the needle pushes against the organ causing visco-elastic deformations. They distinguished three phases with different force patterns, corresponding to the I/ prepuncture, II/ penetration, and IV/ extraction motions (in phase III, the needle is immobile). These models were obtained through ex vivo experiments with computation of values a posteriori, but later, Barbé et al. performed online estimation on in vivo specimen [24]. Figure 6 describes these phases and the total axial force pattern due to the penetration of the needle. These models have still been used in recent works such as [6•, 12, 14, 14, 15, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]. Recently, works focused on the ability to render multiple layers of tissues, using piece-wise exponential models [14] or nested boxes [25] rendering each one its stiffness and cutting forces according to [36]. To benefit from the use of active haptic feedback, these works also proposed to render different patient morphologies by adapting the size of the tissue layers [14] or box depths [25]. Extrapolation of these models to other patient types was validated with experts.
To improve rendering of cutting forces, Daniel et al. proposed the “tracking wall” algorithm as an enhancement of traditional proxy-based algorithms [26]. It allows rendering constant cutting force without chatter and capture small rejections forces occurring when the needle stops inside tissues as stated by [37]. In recent works, lateral forces or clamping forces applied on the sidewalls of the needle were modeled using proportional approaches, mimicking the elasticity of the tissue [25, 27]. In some cases, elasticity was pondered by the depth of insertion, meaning that diverging from the insertion path would become harder as the needle penetrates the tissue. In these approaches, deflection of the needle was not considered.
However, all these models consider forces as a function of penetration depth only, without considering the velocity that affects the needle insertion into viscoelastic tissues [38, 39]. Therefore, Wu et al. proposed a non-linear model rendering the force feedback as a function of the needle’s instantaneous position and velocity during the insertion phase into soft tissue. This model uses a piece-wise split into two areas separated by the moment when the tissue stops deforming while the needle proceeds. In the first area, an exponential-fitting of the penetration depth, and in the second one, the force is proportional to it. In both cases, velocity impacts the magnitude proportionally to the penetration depth. This model takes also the needle diameter as a parameter which influence is demonstrated (doubling the needle diameter, nearly doubles the force magnitude). Unfortunately, the model parameter values for porcine and bovine livers were not provided. Sadeghnejad et al. proposed, on the same approach a 3-phase piece-wise model (tissue loading deformation, fracture (point separating pre-puncture and puncture phases) and cutting) taking into account the tissue fracture event and cutting forces as a function of penetration and velocity [29]. Note that they do not model the fracture physics, only consider the change of behavior at this point. Nonetheless, these both studies do not consider the retractation/withdrawal phase.
Note that, instead of stiffness-like functions, Castro et al. use non-holonomic constraints to render forces due to contacts. This approach will be integrated into surgical simulators in future works [40]. Table 2 sums up the recent approaches to render forces using deformation-based models.
To provide realistic force feedback behavior and overcome computing limitations of finite element methods (FEM)–based approaches (see next subsection) at the same time, Wittek et al. use Meshless Total Lagrangian Explicit Dynamics to simulate tissue deformations. This model has the advantage of requiring only patient-specific geometry and two parameters (being easy to identify from intra-operative images) to render patient-specific simulations [44]. As the model computes needle-tissue interaction forces, it may be used to compute real-time force feedback in the future but this still requires some validation.
Fracture-Based Models
Two main approaches consider the modeling of the tissue fracture performed by the insertion of the needle: energy-based modeling (EBM) and finite-element modeling (FEM). This modeling should provide more precision around the fracture point as, according to [39] for porcine skin, 61% of the total insertion force comes from the fracture, 21% from the friction, and 18% from the tissue deformation. Note that these models take into account the needle velocity. However, as far as we know, no study compared the relative force rendering quality of this family of approaches in an end-user physiological study.
In EBM, the cutting is considered as the consequence of exchanges of energy between the needle and the tissue, causing crack propagation. Early works demonstrated the relevance of this approach [21, 38] but only Barnett et al. exploited this approach to predict insertion forces. Yet, this has not been embedded in a training simulator.
Extensive research on finite element method (FEM)–based needle insertion modeling has been done and synthesized in [5••]. Recent progress is reported in [45] where the modeling of the tissue fracture enables realistic FEM simulations of needle trajectories and estimation of the interaction forces matching the experimental data for deep insertion cases. In [46], multilayer tissues are considered, but this kind of approach is reserved for design validation or preplanning purposes as such simulations last several hours: they are still computationally complex to provide real-time realistic force feedback in haptic simulators. To enable this kind of modeling for real-time simulation, Bui et al. proposed, in [47], a method to minimize the complexity of mesh generation and an algorithm named CutFEM solving equations with constraints twice faster as classical FEM on a liver model (around 450 ms per iteration). They validated it on an electrode implantation simulation in deep brain stimulation. This computation velocity enables 2D or 3D rendering in simulations but is still a little slow to render directly force feedback in a haptic application where 1 kHz is the minimum sampling period to reproduce hard contacts.
Needle deflection during insertion is a function of the diameter and shape of the needle [46] and insertion forces [39]. Despite early works to investigate the force-needle deflection relationship [48], Correa et al. confirm that the “deformation of [...] tissues or organs received more attention than the deflection of needles” [5••]. In recent FEM-based methods, needles were either considered rigid [45] or deformable, using for instance the Euler-Bernoulli beam theory in [47].
Constitutive Models Representing Specific Tissues and Parameter Identification
Forces occurring during needle insertion are tied to specific properties of both needles and crossed tissues. If general assumptions are made on force distributions during needle insertion (see Fig. 6), intrinsic parameters depend on the mechanical properties of the simulated tissues. Therefore, parameters of aforementioned models need to be determined to realistically render needle insertion in specific tissues. Table 3 sums up the various recent studies that permitted to determine these parameters for various kinds of needle insertion applications.
In deformation-based models, a method to obtain parameters is fitting functions to experimental data obtained during needle insertions [28, 29, 33, 34] or traction/compression experiments [46]. Usually, these experiments are performed on cadaveric (ex vivo) or animal tissues (in vivo or ex vivo). However, in vivo data are difficult to obtain and ex vivo tissues cannot reliably replicate life-like conditions as in in vivo tissues [49, 50]. Moreover, the accuracy of mechanical properties from cadaveric samples would be hindered by freezing and thawing [33]. Therefore, it is complicated to get accurate parameters only using these methods. Several authors thus preferred to rely on literature or the experience of expert surgeons to evaluate the accuracy of force feedback through iterative try-and-test experiments. [25, 26, 27] Tissue phantoms can also be used to test behaviors of needle insertion in various conditions and verify models in early stages but were not used to identify parameters. [44, 51]
Efforts are still made to propose measurement schemes to acquire reliable force-displacement data to help in the design of needle insertion haptic simulators. Measuring devices usually rely on 6 DoF force sensors and means to track positions during insertions. The measurement apparatus can either be handheld by an expert surgeon [14, 15, 50] or be robotically inserted through tissues [15, 52]. To improve the reliability of insertion tests into ex vivo tissues, Li et al. developed a test platform that would record forces during needle insertion while simulating movements due to respiration [15].
Conclusion
Various approaches have been proposed to render forces during needle insertion into tissues. They vary in computation complexity and their ability to accurately render forces and deformations. The majority of recent works still rely on displacement-force models that heavily depend on experimental data and/or expert feedback to obtain realistic force feedback. These approaches are still being improved to grasp all the complexity of needle insertion into soft tissues and motivate the prototyping of measurement apparatus.
Gesture Assessment
To integrate haptic simulators into a medical training routine, it is essential to define two validation criteria for its use. Indeed, the simulator must allow the evaluation of the performance to qualify the gesture and quantify the progress of a trainee [53•]. On the other hand, it is necessary to study the impact of the use of new technology in the learning process compared to the classical training method [54•].
Gesture Evaluation
Whatever the practice and the application, it is necessary to objectively evaluate the mastery of the gesture and the progress of the trainee during his/her curriculum. There are many evaluation methods specific to each type of skill. In the medical field, many evaluation methods have been developed to study surgical procedures. We can classify them into two categories [55•]:
-
Subjective tests based on responses to targeted questionnaires [56, 57];
-
Objective tests from the study of skills based on measurable metrics [58, 59].
The first method to evaluate and improve the learning of a gesture is the use of subjective tests. These are usually carried out in the form of questionnaires. In the medical field, there are three well-known questionnaires:
The main issue with the subjective tests is that they do not allow for the quantification of learning because they are based on the learner’s feelings. However, they allow us to measure the degree of confidence of a learner concerning the action he/she is performing. The other problem with these tests is that they focus exclusively on a specific application (e.g. NASA TLX for robot-assisted surgery). As far as we know, for the moment, there are no subjective tests that can be used for needle insertion. This gesture focuses, most of the time, exclusively on the haptic perception of the learner. This sense, although described and studied mechanically in the literature, is only rarely present in psychomotor studies.
Concerning the objective tests, there are several methods to analyze the gesture allowing to evaluate its mastery such as the OSATS method [63, 64, 65] (for classical surgery) or the GOALS method [66, 67] (for minimally invasive surgery and in particular laparoscopy). Other metrics for surgical skill evaluation have been developed based on the orientation of surgical instruments [59]. These methods make it possible to score a specific gesture of a practitioner. This rating is based on the analysis of predefined metrics and can be compared with a reference score from an expert procedure [68]. It is thus possible to evaluate the performance of a learner and to use the results of these algorithms for training purposes to evaluate the mastery of each user [69].
Although each application has its properties, standard and commonly used metrics for the evaluation of medical procedures have been isolated. These metrics are thus highlighted in various review studies [70, 71]. The main metrics used to qualify a gesture are:
-
the TCT [72, 73] (task completion time) which corresponds to the time necessary to complete a task;
-
the economy of movement [71];
Most of the metrics presented above are applied to gestures performed in a three-dimensional environment and are particularly relevant to surgical gestures. These gestures require dexterity and navigation in space, hence the relevance of metrics such as affine velocity, curvature, regularity of movement, etc. Gesture evaluation methods provide good results for classical surgical gestures [78]. However, they have two main problems. The first is that they are difficult to generalize because they use predefined metrics and require the notation of reference gestures. In general, specific metrics are found in many medical applications. Indeed, it is important to note that the metrics are likely to change according to each application. This is for example the case for epidural anesthesia [79] or ventricular puncture [80] where the main metric of gesture mastery focuses on the interpretation of haptic sensations called “overshoot” [81, 82, 83]. Another example is prostate or uterine biopsy, where the main metric is the reached position [84, 85]. It is important to note that new automatic metric extraction methods are increasingly developed to address this issue [86].
The other recurring problem is the creation and use of a database to classify the different gestures and extract the metrics needed for evaluation. One of the possible ways to improve the creation of a usable database is to introduce gesture simulators. These simulators aim to reproduce clinical contexts to allow the learner to train for a specific task. They can be purely virtual [87], augmented reality [88, 89] or haptic [79, 90]. The main advantage of using simulators comes from their ability to extract data from learners’ gestures. This data is derived from the many sensors that the simulator may contain and can then be used to evaluate the gesture and provide accurate feedback to the learner to improve their learning. However, the use of simulators is still very little implemented in practice because they require a modification of the clinical learning routine and their impact on the learner’s curriculum is not yet detailed in the literature.
Impact of Robotics in Clinical Routine
There are several works in the field of human-machine interfaces (HMI) that have focused on the impact of robotic interfaces in surgery. For now, most of its work focuses on soft skills [91] (e.g. workflow, communication, situational awareness, teamwork [54•, 92, 93, 94]) and it uses mostly subjective metrics. In addition, a 2015 neuroscience study by Heuer et al. [95] on robotic assistance for surgery, showed that it was difficult to show that a surgeon keeps the same level of practice before and after the use of robotic systems in their clinical routine [95]. He demonstrated the risk of dependence of the learner on the specific robotic system in the context of teleoperation. However, a more recent study based on objective metrics also concluded that the use of robotic systems in the learning process achieved the desired level of competence faster than with the conventional learning routine [96].
In general, the addition of a haptic simulator in the learning process of a gesture has beneficial aspects such as the creation and use of databases allowing objective or subjective feedback to the learner or the saving of learning time [96]. It should be noted, however, that such devices are still not widely used in practice because they often require too great a change in the learning routine.
Conclusions
In this paper, we provided an updated short review about haptic training simulation on needle insertion. As an extensive overview was provided by Correa et al. in 2019 [5••], we focused here on three important and complementary aspects of such simulators. We summed up specifications concerning the haptic devices for this hands-on training application and exposed current commercial devices and recent custom realizations. We reviewed recent force modeling approaches, and detailed the issues of assessment with simulators and their integration into learning routine. We could determine that recent results refine well-known models and enhance experimental setups and methods to facilitate generically getting experimental data. FEM-based approaches still do not permit real-time computation but hybrid approaches as proposed by [44] may become a good compromise. Despite these progress, the variety of case studies make it still difficult comparing the various aforementioned modeling approaches as no comparative work has yet been performed, as far as we know. As exposed in [2], training simulators should be evaluated in terms of rendered haptic fidelity. Each complete chain (from experimental data gathering to haptic rendering evaluated using psychophysical studies) should be evaluated to determine the most efficient ones in each family of medical cases. Classifying these simulators in low/medium/high haptic fidelity families would help embed them efficiently into medical curricula.
Notes
The word “haptic” “haptomai” (ăπτoμαι) which means “touch”, gathering kinaesthetic (force) and tactile senses.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vaughan N, Dubey N, Venketesh MY, Wee K, Saacs R. A review of epidural simulators : where are we today. Med Eng Phys 2013;35(9):1235–1250.
Favier V, Subsol G, Duraes M, Captier G, Gallet P. Haptic fidelity: the game changer in surgical simulators for the next decade? Frontiers Oncology 2021;11:3110. https://doi.org/10.3389/fonc.2021.713343.
Lelevé A, McDaniel T, Rossa C. Haptic training simulation. Frontiers Virtual Real 2020; 1:3. https://doi.org/10.3389/frvir.2020.00003.
Overtoom EM, Horeman T, Jansen F-W, Dankelman J, Schreuder HWR. Haptic feedback, force feedback, and force-sensing in simulation training for laparoscopy: a systematic overview. J Surgical Educ 2019;76(1):242–261. https://doi.org/10.1016/j.jsurg.2018.06.008.
•• Corrêa CG, Nunes FL, Ranzini E, Nakamura R, Tori R. Haptic interaction for needle insertion training in medical applications: the state-of-the-art. Med Eng Phys 2019;63:6–25. https://doi.org/10.1016/j.medengphy.2018.11.002. This paper is an important recent and detailed review about haptic training simulators.
• Sainsbury B, et al. Evaluation of a virtual reality percutaneous nephrolithotomy (pcnl) surgical simulator. Frontiers Robot AI 2020;6:145. https://doi.org/10.3389/frobt.2019.00145. In this paper, the authors present a full description of a simulator that couples virtual reality and haptic interface. Their results allow to validate the face and construct validity.
Yin J, et al. 3d printed multi-material medical phantoms for needle-tissue interaction modelling of heterogeneous structures. J Bionic Eng 2021;18(2):346–360. https://doi.org/10.1007/s42235-021-0031-1.
Ng SY, Kuo Y-L, Lin C-L. Low-cost and easily fabricated ultrasound-guided breast phantom for breast biopsy training. Appl Sci 2021;11(16):7728. https://doi.org/10.3390/app11167728.
DF P, et al. Low-cost haptic simulation using material fracture. IEEE Trans Haptics 2019;12(4): 563–570. https://doi.org/10.1109/TOH.2019.2914441.
Dombrowski U, Stefanak T, Perret J. Interactive simulation of human-robot collaboration using a force feedback device. Procedia Manufact 2017;11:124–131. 27th International conference on flexible automation and intelligent manufacturing, FAIM2017, 27-30 June 2017, Modena, Italy. https://doi.org/10.1016/j.promfg.2017.07.210.
Yang C, Ma H, Fu M. Robot teleoperation technologies. Advanced technologies in modern robotic applications, pp 187–229 (Singapore: Springer Press and Springer Science+Business Media Singapore, Singapore). https://doi.org/10.1007/978-981-10-0830-6∖_6. In: Yang C, Ma H, and Fu M, editors; 2016.
Moo-Young J, Weber TM, Kapralos B, Quevedo A, Alam F. Development of unity simulator for epidural insertion training for replacing current lumbar puncture simulators. Cureus 2021; 13(2):e13409–e13409. https://pubmed.ncbi.nlm.nih.gov/33758704.
Heimann F, et al. A custom virtual reality training solution for ophthalmologic surgical clinical trials. Adv Simul 2021;6(1):12. https://doi.org/10.1186/s41077-021-00167-z.
Pepley DF, et al. Integrating cadaver needle forces into a haptic robotic simulator. J Med Devices 2018;12(1). https://doi.org/10.1115/1.4038562.
Li F, et al. Real-time needle force modeling for vr-based renal biopsy training with respiratory motion using direct clinical data. Appl Bionics Biomech 2019;2019:9756842. https://doi.org/10.1155/2019/9756842.
Aygün MM, Öğüt YC, Baysal H, Taşcioğlu Y. Visuo-haptic mixed reality simulation using unbound handheld tools. Appl Sciences 2020;10(15). https://doi.org/10.3390/app10155344.
Sénac T, et al. Simulating a syringe behavior using a pneumatic cylinder haptic interface. Control Eng Prac 2019;90:231–240. https://doi.org/10.1016/j.conengprac.2019.07.005.
Pozner CN, Eyre A. Simulation in graduate medical education. Comprehensive Healthcare Simulation: emergency Medicine pp 173–180 (Springer International Publishing, Cham). https://doi.org/10.1007/978-3-030-57367-6∖_16. In: Strother C, Okuda Y, Wong N, and McLaughlin S, editors; 2021.
Ravali G, Manivannan M. Haptic feedback in needle insertion modeling and simulation. IEEE Rev Biomed Eng 2017;10:63–77. https://doi.org/10.1109/RBME.2017.2706966.
Yang C, Xie Y, Liu S, Sun D. Force modeling, identification, and feedback control of robot-assisted needle insertion: a survey of the literature. Sensors 2018;18(2). https://doi.org/10.3390/s18020561.
Azar T, Hayward V, Bello F, Edwards PJE, (eds). Estimation of the fracture toughness of soft tissue from needle insertion. (eds Bello, F. & Edwards, P. J.E.) Lecture Notes in Computer Science, Conference on Biomedical Simulatio (ISBMS 2008). Berlin, Heidelberg: Springer Berlin Heidelberg 2008.
Brett PN, Parker T, Harrison AJ, Thomas TA, Carr A. Simulation of resistance forces acting on surgical needles. Proc Inst Mech Eng Part H: J Eng Med 1997;211(4):335–347. https://doi.org/10.1243/0954411971534467.
Okamura AM, Simone C, O’leary MD. Force modeling for needle insertion into soft tissue. IEEE Trans Biomed Eng 2004;51(10):1707–1716. https://doi.org/10.1109/TBME.2004.831542.
Barbé L, Bayle B, De Mathelin M, Gangi A. Needle insertions modeling: identifiability and limitations. Biomed Signal Process Control 2007;2(3):191–198. https://doi.org/10.1016/j.bspc.2007.06.003.
Senac T, et al (eds.) Designing an accurate and customizable epidural anesthesia haptic simulator. (ed.IEEE) Proc of the International Conference on Robotics and Automation (ICRA 2019) 2019;8353–8359 (organization IEEE, Montreal, Canada).
Alamilla Daniel MDLA, Moreau R, Tanneguy R (eds.) EMBC Development of haptic simulator for practicing the intraarticular needle injection under echography. (ed. EMBC) proc of the 42nd annual international conference of the ieee engineering in medicine biology society (EMBC 2020) 2020;4713–4716.
Di Vece C, Luciano C, De Momi E. Psychomotor skills development for veress needle placement using a virtual reality and haptics-based simulator. Int J Comput Assisted Radio Surgery 2021;16(4):639–647. https://doi.org/10.1007/s11548-021-02341-0.
Wu H, Chen C, Zhou Y, Wang J, Xie Y (eds.). IEEE/ASME VR-based haptic simulation for dynamic needle insertion. (ed. IEEE/ASME) proc of the international conference on advanced intelligent mechatronics (AIM 2019) 2019;924–929.
Sadeghnejad S, Farahmand F, Vossoughi G, Moradi H, Hosseini SMS. Phenomenological tissue fracture modeling for an endoscopic sinus and skull base surgery training system based on experimental data. Med Eng Phys 2019;68:85–93. https://doi.org/10.1016/j.medengphy.2019.02.004.
Mostafa AE, et al. Designing neurosimvr: a stereoscopic virtual reality spine surgery simulator 2017. https://prism.ucalgary.ca/handle/1880/52230.
Correa CG, Machado MADAM, Ranzini E, Tori R, Nunes FDLS. Virtual reality simulator for dental anesthesia training in the inferior alveolar nerve block. J Appl Oral Sci 2017;25(4). https://doi.org/10.1590/1678-7757-2016-0386.
Barnouin C, Zara F, Jaillet F. SciTePress (ed.) A real-time ultrasound rendering with model-based tissue deformation for needle insertion. (ed.SciTePress) proc of the 15th international conference on computer graphics theory and applications (GRAPP 2020) (Valletta, Malta) 2020.
Esterer B, et al. Characterization of tissue properties in epidural needle insertion on human specimen and synthetic materials. J Mech Behavior Biomed Materials 2020;110:103946. https://doi.org/10.1016/j.jmbbm.2020.103946.
El-Monajjed K, Driscoll M. Analysis of surgical forces required to gain access using a probe for minimally invasive spine surgery via cadaveric-based experiments towards use in training simulators. IEEE Trans Biomed Eng 2021;68(1):330–339. https://doi.org/10.1109/TBME.2020.2996980.
Ricca A, Chellali A, Otmane S. Comparing touch-based and head-tracking navigation techniques in a virtual reality biopsy simulator. Virtual Reality 2021;25(1):191–208. https://doi.org/10.1007/s10055-020-00445-7.
Kuchenbecker KJ, Fiene J, Niemeyer G. Improving contact realism through event-based haptic feedback. IEEE Trans Visualization Comput Graph 2006;12(2):219–230.
Maurin B, et al. In vivo study of forces during needle insertions (ed.Scientific W.) perspective in image-guided surgery 2004;415–422 (World Scientific).
Mahvash M, Dupont PE. Mechanics of dynamic needle insertion into a biological material. IEEE Trans Biomed Eng 2009;57(4):934–943. https://doi.org/10.1109/TBME.2009.2036856.
Barnett AC, Lee Y-S, Moore JZ. Fracture mechanics model of needle cutting tissue. J Manufac Sci Eng 2016;138(1). https://doi.org/10.1115/1.4030374.
Castro-Díaz JD, Sánchez-Sánchez P, Gutiérrez-Giles A, Arteaga-Pérez MA, Pliego-Jiménez J. Experimental results for haptic interaction with virtual holonomic and nonholonomic constraints. IEEE Access 2020;8:120959–120973. https://doi.org/10.1109/ACCESS.2020.3006715.
Asadian A, Patel RV, Kermani MR. A distributed model for needle-tissue friction in percutaneous interventions 2011;1896–1901.
Gordon A, Kim I, Barnett AC, Moore JZ. 2015. ASME (ed.) Needle insertion force model for haptic simulation. (ed.ASME) proc of the international manufacturing science and engineering conference vol 56833 V002T03A003 (organization american society of mechanical engineers).
Pepley D, et al. A virtual reality haptic robotic simulator for central venous catheterization training. J Med Devices 2016;10(3). https://doi.org/10.1115/1.4033867.
Wittek A, et al. Mathematical modeling and computer simulation of needle insertion into soft tissue. PloS one 2020;15(12):e0242704. https://doi.org/10.1371/journal.pone.0242704.
Mohammadi H, Ebrahimian A, Maftoon N. Fracture behaviour of human skin in deep needle insertion can be captured using validated cohesive zone finite-element method. Comput Biology Med 2021;139: 104982. https://doi.org/10.1016/j.compbiomed.2021.104982.
Jushiddi MG, et al. A computational multilayer model to simulate hollow needle insertion into biological porcine liver tissue. Acta Biomater. 2021;136:389–401. https://doi.org/10.1016/j.actbio.2021.09.057.
Bui HP, Tomar S, Bordas SP. Corotational cut finite element method for real-time surgical simulation: application to needle insertion simulation. Comput Methods Appl Mech Eng 2019;345:183–211. https://doi.org/10.1016/j.cma.2018.10.023.
Kataoka H, Washio T, Audette M, Mizuhara K, Heidelberg S-VB (eds.). A model for relations between needle deflection, force, and thickness on needle penetration. (ed.Heidelberg, S.-V.B.) Proc. of the international conference on medical image computing and computer-assisted intervention (MICCAI 2001) 2001;966–974 (organization Springer).
Mirza S, Athreya S. Review of simulation training in interventional radiology. Acad Radiology 2018;25(4):529–539. https://www.sciencedirect.com/science/article/pii/S107663321730435X. https://doi.org/10.1016/j.acra.2017.10.009.
Schimmoeller T, Neumann EE, Nagle TF, Erdemir A. Reference tool kinematics-kinetics and tissue surface strain data during fundamental surgical acts. Sci Data 2020;7(1):21. https://doi.org/10.1038/s41597-020-0359-0.
Jushiddi MG, et al. Simulation of biopsy bevel-tipped needle insertion into soft-gel. Comput Bio Med 2019;111:103337. https://doi.org/10.1016/j.compbiomed.2019.103337.
Brown D, Gonzalez-Vargas JM, Han D, Miller S, Moore J. ASME (ed.) Incremental needle insertion system for force and position sensing. (ed.ASME) frontiers in biomedical devices proc. of the 2020 design of medical devices conference, vol. vol 2020 design of medical devices conference (Minneapolis, Minnesota, USA, 2020). V001T06A002.
• Marvel JA, Bagchi S, Zimmerman M, Antonishek B. Towards effective interface designs for collaborative hri in manufacturing: metrics and measures. J Hum-Robot Interact 2020;9(4). https://doi.org/10.1145/3385009. This work highlights the necessity of evaluating gestures during robotic interaction and the metrics which can be used.
• Avellino I, et al. ACM (ed.) Impacts of telemanipulation in robotic assisted surgery. (ed.ACM) proc. of the chi conference on human factors in computing systems proceedings (CHI 2019), CHI conference on human factors in computing systems proceedings (CHI 2019) (Glasgow, United Kingdom) 2019. This study focuses on the impact of the robotics on the clinical routine of the surgeons.
• Close M, et al. Subjective vs computerized assessment of surgeon skill level during mastoidectomy otolaryngology–head and neck surgery 2020;1255–1257. Findings from this study discuss the evaluation of the medical gesture and explain the two different ways to evaluate it.
Charles R, Nixon J. Measuring mental workload using physiological measures: a systematic review. Appl Ergonomics 2019;74:221–232.
Adrian Cornelius M, et al. Physiological parameter response to variation of mental workload. Hum. Factors 2018;60(1):31–56. https://doi.org/10.1177/0018720817733101, pMID: 28965433.
Seung-kook J, et al. Evaluation of robotic minimally invasive surgical skills using motion studies. Proceedings of the workshop on performance metrics for intelligent systems (PerMIS ’12) 2012;198–205. https://doi.org/10.1145/2393091.2393129
Sharon Y, Jarc AM, Lendvay TS, Nisky I. Rate of orientation change as a new metric for robot-assisted and open surgical skill evaluation. IEEE Trans Med Robot Bionics 2021;3(2):414–425. https://doi.org/10.1109/tmrb.2021.3073209.
Hart SG, Staveland LE. Development of nasa-tlx (task load index): results of empirical and theoretical research. Human mental workload, vol 52 of series advances in psychology, pp 139–183 (North-Holland). https://www.sciencedirect.com/science/article/pii/S0166411508623869. In: Hancock PA and Meshkati N, editors; 1988.
Lewis JR. IBM computer usability satisfaction questionnaires: psychometric evaluation and instructions for use. Int J Human–Comput Interaction 1995;7(1):57–78. https://doi.org/10.1080/10447319509526110.
Klug B. An overview of the system usability scale in library website and system usability testing. Weave J Library User Experience 2017;1. https://doi.org/10.3998/weave.12535642.0001.602.
Martin JA, et al. Objective structured assessment of technical skill (osats) for surgical residents. Br J Surg 1997;84(2):273–8. http://www.csats.com/osats. https://doi.org/10.1046/j.1365-2168.1997.02502.x.
Niitsu H, et al. Using the objective structured assessment of technical skills (osats) global rating scale to evaluate the skills of surgical trainees in the operating room. Surgery Today 2012;43. https://doi.org/10.1007/s00595-012-0313-7.
Asif H, et al. Objective structured assessment of technical skill (osats) in the surgical skills and technology elective program (sstep): comparison of peer and expert raters. The American J Surgery, Elsevier 2021. https://doi.org/10.1016/j.amjsurg.2021.03.064.
Higuchi M, et al. Development and validation of a porcine organ model for training in essential laparoscopic surgical skills. Int J Urology 2020;27(10):929–938. https://doi.org/10.1111/iju.14315.
Vassiliou MC, et al. A global assessment tool for evaluation of intraoperative laparoscopic skills. Amer J Surgery 2005;190(1):107–113. https://doi.org/10.1016/j.amjsurg.2005.04.004.
Zia A, Essa I. Automated surgical skill assessment in rmis training. Int J Comput Assisted Radio Surgery 2018;13:731–739. https://doi.org/10.1007/s11548-018-1735-5.
Zia A, et al. Automated video-based assessment of surgical skills for training and evaluation in medical schools. Int J Comput Assisted Radio Surgery 2016;11(9):1623–1636. https://doi.org/10.1007/s11548-016-1468-2.
Cotin S, et al. “Metrics for laparoscopic skills trainers: the weakest link!”. Medical Image Comput Comput-Assisted Interven — MICCAI 2002 2002;2488:35–43.
Feng C, Rozenblit J. Conference and workshops on the engineering of computer based systems (ECBS ’08) 2008;203–209 (Belfast Ireland).
Mishra S, Ganpule A, Kurien A, Muthu V, Desai M. Task completion time: objective tool for assessment of technical skills in laparoscopic simulator for urology trainees. Indian J Urology 2008;24(1): 35–38. https://doi.org/10.4103/0970-1591.38601.
Fuerst D, Hollensteiner M, Schrempf A. ACM (ed.) A novel augmented reality simulator for minimally invasive spine surgery. (ed.ACM) Proc. of the 2014 Summer simulation multiconference, SummerSim ’14 (Society for computer simulation international, San Diego, CA USA 2014.
Fortmeier D, Mastmeyer A, Schröder J, Handels H. A virtual reality system for ptcd simulation using direct visuo-haptic rendering of partially segmented image data. IEEE J Biomed Health Inform 2016;20: 355–366.
Fard MJ, et al. Machine learning approach for skill evaluation in robotic-assisted surgery 2016. CoRR arXiv:1611.05136.
Chalasani V, et al. Development and validation of a virtual reality transrectal ultrasound guided prostatic biopsy simulator. Canadian Urological Association Jo = J de l’Association des urologues du Canada 2011;5: 19–26. https://doi.org/10.5489/cuaj.09159.
Cifuentes Quintero JA. Development of a new technique for objective assessment of gestures in mini-invasive surgery. Type theses school INSA de Lyon ; Universidad nacional de Colombia 2015. https://tel.archives-ouvertes.fr/tel-01368173.
Millan M. L’apprentissage profond pour l’évaluation et le retour d’information lors de l’apprentissage de gestes. type Theses school Sorbonne Université 2020. https://tel.archives-ouvertes.fr/tel-03191291.
Senac T, et al. Skill assessment of an epidural anesthesia using the PeriSIM simulator. IEEE Trans Med Robot Bionics 2021;3(1):106–114. https://hal.archives-ouvertes.fr/hal-03092054. https://doi.org/10.1109/TMRB.2020.3048247.
Brenke C, Fürst J, Katsigiannis S, Carolus A. High accuracy of external ventricular drainage placement using anatomical landmarks. Neurochirurgie 2020;66(6):435–441. https://doi.org/10.1016/j.neuchi.2020.09.009.
Manoharan V, Van Gerwen D, Van Den Dobbelsteen J, Dankelman J. Design and validation of an epidural needle insertion simulator with haptic feedback for training resident anaesthesiologists. 2012 IEEE Haptics Symposium (HAPTICS) 2012;341–348.
Van Adrichem L. Avoiding overshoot. DSPE Mikroniek 2009;3:36–40.
Cometa A. When millimeters count, epidural loss of resistance techniques differ : a simulator study. UFHealth: University of Florida; 2018.
Chalard R, Fazel A, Vitrani M-A. Real time estimator to perform targeted biopsies with a free-wrist robot despite large deformations of the insertion orifice. Frontiers Robot AI 2021. https://hal.archives-ouvertes.fr/hal-03385568.
Goksel O, Sapchuk K, Salcudean SE. Haptic simulator for prostate brachytherapy with simulated needle and probe interaction. IEEE Trans Haptics 2011;4(3):188–198. https://doi.org/10.1109/TOH.201134.
Guerin S, Huaulmé A, Lavoue V, Jannin P, Timoh KN. Review of automated performance metrics to assess surgical technical skills in robot-assisted laparoscopy. Surgical Endoscopy 2021. https://doi.org/10.1007/s00464-021-08792-5.
Alaker M, Wynn GR, Arulampalam T. Virtual reality training in laparoscopic surgery: a systematic review and meta-analysis. Int J Surger 2016;29:85–94. https://doi.org/10.1016/j.ijsu.2016.03.034.
Christensen NH, et al. ACM (ed.) Depth cues in augmented reality for training of robot-assisted minimally invasive surgery. (ed.ACM) proc. of the 21st international academic mindtrek conference, AcademicMindtrek’17, 120–126 (Association for computing machinery, New, York, NY USA) 2017. https://doi.org/10.1145/3131085.3131123.
Huang Y-H, et al. Catar: a novel stereoscopic augmented reality cataract surgery training system with dexterous instruments tracking technology. Proc 2018 CHI Conf Human Factors Comput Syst 2018.
Galvan A, Da Costa AK, Shields J, Kho K, Fey AM. Haptic simulator for trocar insertion training, 2021;397–402.
Yule S, Flin R, Maran N, Paterson-Brown S. Non-technical skills for surgeons in the operating room: a review of the literature. Surgery 2006;139(2):140–149. https://doi.org/10.1016/j.surg.2005.06.017.
Cheatle A, Pelikan H, Jung M, Jackson S. Sensing (co)operations: articulation and compensation in the robotic operating room. Proceedings ACM Hum-Comput Interact 3 (CSCW) 2019. https://doi.org/10.1145/3359327.
Pelikan H, Cheatle A, Jung M, Jackson S. Operating at a distance - how a teleoperated surgical robot reconfigures teamwork in the operating room. Proc ACM on Human-Comput Interaction 2018;2: 1–28. https://doi.org/10.1145/3274407.
Randell R, Honey S, Hindmarsh J, et al. A realist process evaluation of robot-assisted surgery: integration into routine practice and impacts on communication, collaboration and decision-making. Southampton (UK): health services and delivery research (NIHR) journals library 2017. https://doi.org/10.3310/hsdr05200.
Heuer H, Lüttgen J. Robot assistance of motor learning: a neuro-cognitive perspective. Neuroscience Biobehavioral Rev 2015;56:222–240. https://doi.org/10.1016/j.neubiorev.2015.07.005.
Ferrier-Barbut E, Gauthier P, Luengo V, Canlorbe G, Vitrani M-A. Measuring the quality of learning in a human-robot collaboration: a study of laparoscopic surgery. ACM Trans Human-Robot Interaction 2021. https://hal.archives-ouvertes.fr/hal-03355055.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Topical Collection on Medical and Surgical Robotics
Benjamin Delbos MSc, Dr Rémi Chalard, Dr Richard Moreau, and Dr Minh Tu Pham contributed equally to this work.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delbos, B., Chalard, R., Moreau, R. et al. Review on Needle Insertion Haptic Simulation. Curr Robot Rep 3, 259–270 (2022). https://doi.org/10.1007/s43154-022-00093-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43154-022-00093-6