Abstract
Controlling inorganic-salt precipitation during oil production at various stages of crude-oil processing is a complex task, especially with the increase in criticality of produced-water compositions and working conditions (e.g., high calcium content, high salinity, and temperatures). Using reliable models to predict the saturation index and choosing the ideal conditions for laboratory tests are critical to the effective selection of scale inhibitors capable of ensuring production flow. The dynamic tube blocking test (TBT) is commonly used to assess the performance of scale inhibitors. In some Brazilian presalt scenarios, it has been determined that this test is very sensitive to certain factors, such as the brine pH, the inner diameter (ID) of the capillary, and other operating conditions. The objective of this work was to evaluate the influence of the variation of these factors on scale inhibitor efficiency. Performance evaluations of scale inhibitors in Brazilian presalt conditions, varying only the coil size and the brine pH within specified narrow ranges, and the impact on product efficiency. Based on the studies performed it could be concluded that the pH range considered for the tests should be narrow enough to not lead to drastic variation of the saturation index. With regards to the coil ID, narrower ones could lead to higher minimum inhibition concentrations (MICs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The formation of inorganic scale during oil production is a significant challenge in flow assurance. Different types of scale, primarily insoluble sulphate and carbonate salts, can be formed. Sulfate scales are primarily formed when seawater is injected into the reservoir for oil recovery, as a result of the mixing of incompatible waters. Seawater is rich in sulphate, while formation water is rich in metals such as calcium, barium, and strontium, and the interaction between these ions promotes the formation of sulfates. Carbonate scales are formed primarily as a result of thermodynamic variations, such as changes in pH, pressure, and temperature, in the system during the oil production process, and these variables directly affect the solubility of the salts.
Scale precipitation occurs by means of a four-stage process: supersaturation, nucleation, crystal growth around the nucleus, and the deposition of salts on surfaces. Supersaturation is achieved in a solution when the concentration of solutes is greater than the equilibrium concentration. Nucleation is the process of obtaining the smallest aggregate of a crystalline phase. During this stage, the ions start to gather in groups and stabilize after reaching a certain critical size. After a stable nucleus has been formed in the system, small aggregates tend to grow around the nucleus, generating larger particles, and the agglomeration of these crystals on surfaces results in the formation of scale layers (Da Rosa et al. 2019).
Scale inhibitors have served as excellent tools to mitigate problems caused by the formation of these insoluble salts. Scale inhibitors can act by means of three mechanisms: prevent nucleation, prevent crystal growth, or as dispersants. Inhibition during nucleation occurs through endothermic adsorption of the inhibitor, causing the dissolution of the embryonic precipitate nucleus. The second inhibition mechanism is that the adsorption of the inhibitor increases the free energy of the precipitate nucleus, resulting in an increase in the energetic barrier to crystal growth. Another potential theory for this phenomenon is that the primary force governing adsorption is simply the hydrophobic repulsion of the inhibitor molecule by the solution. This adsorption prevents crystal growth. The inhibitor’s anionic functional groups are responsible for the initial approach to the crystal surface, and the presence of several groups provides a high density of negative charge, which results in a strong electrostatic interaction with the surface. Finally, dispersants act by destabilizing the aggregates already formed through crystal distortion effects, making them less stable and consequently disaggregating them. (Tomson et al. 2003; Frenier and Ziauddin 2008).
Laboratory tests are required to develop the scale inhibitors that will be applied during oil production, and they aim to simulate the conditions in the field when a product will be applied. Thus, important tests are performed during product development or screening: compatibility tests, which aim to evaluate the tolerance of an inhibitor to the calcium ion present in brine; a static efficiency test, which evaluates the efficiency of a product in preventing the formation of sulfate scales under static conditions; and a TBT, which is the focus of this work.
In a TBT, synthetic brines based on the composition of the produced water from a field are prepared and pumped into a dynamic scale loop (DSL) as separate non-scaling anionic and cationic brines. Scale formation is assessed by monitoring the pressure differential over the inlet and outlet of a stainless-steel microbore capillary contained within a heating cabinet. The DSL is conditioned to the pressure and temperature of the field, and the anionic and cationic brines are mixed immediately before they enter the microbore coil.
A test, referred to as a blank test, is initially performed without the presence of inhibitors. It is important to perform this test because it determines the duration of the pass time for tests undertaken using scale inhibitors.
The result of a TBT is highly dependent on the pH of the brine used when carbonate scales are of concern and on the ID of the capillary. Both conditions are typically predetermined by the operator. However, depending on the range of tolerable variation for the pH, as well as the diameter chosen for the capillary, these conditions could significantly influence the performance of the product being evaluated—a slight increase in pH that is still within the established range or a reduction of the capillary ID could be responsible for a significant increase in the dosage of product required to prevent scale formation in laboratory tests. Assessing these variations is important to reflect on the extent to which the laboratory is reproducing what will occur in the field or whether too-conservative conditions are being considered.
Methodology
Dynamic tests using DSL equipment were performed to understand how variations in brine pH and capillary ID affect product efficiency. A polyamino polyether methylene phosphonate (PAPEMP)-based product was evaluated in two Brazilian presalt scenarios: a topside application and a subsea application. The dosages reported are the commercial product concentration. This type of chemistry was selected due to the good performance in the scale inhibition under the studied conditions. This confirms the expectation as the PAPEMP presents good inhibition properties for calcium carbonate precipitation.
For the performance tests, synthetic brines were prepared using the brine compositions listed in Table 1. Test conditions are provided in Table 2.
Where Saturation Ratio SR and Saturation Index are:
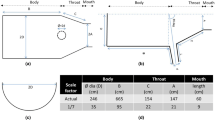
During the evaluation of the influence of the brine pH variation on the product efficiency, a variation of ±0.20 from the stated pH was permitted. This pH range is commonly used by operators for the requirements of DSL lab tests. For the study of the influence of the capillary ID, capillaries with a 0.5-, 0.75-, and 1-mm ID were evaluated at a fixed length of 1 m. For any DSL experiment, a range of microbore capillary sizes, varying in both length andID, can be used:
-
Length: typically either 1 or 3 m
-
ID: 0.5, 0.75, or 1 mm
Results and discussion
Theoretical calculations
The coil dimensions result in variations in pressure drop, residence time, shear rate, and turbulence. Figs. 1 and 2 show the theoretical calculations for a 1-m-long coil at the various typically used IDs. The following equations were used to determine the parameters:
Shear
Reynolds Number (Re)
Velocity (v)
Based on a temperature of T = 135 °C and a theoretical viscosity of µ approximately 0.5 cp (for Topside conditions), as the coil ID decreases, there is an exponential increase in the pressure drop and not unexpectedly. Lengthening the coil increases the pressure drop and the residence time proportionally. In addition to increasing the pressure drop, an exponential increase in the shear rate is also calculated when decreasing the ID, in addition to a reduction in the residence time. However, it should be noted that the Reynolds number (Re) is calculated to be in laminar flow at all conditions (Re < 2000). In relation to the TBT pass criteria shown in Table 2, the 0.5-psi threshold represents a 100% increase above the theoretical baseline for a 1-mm-ID coil, a 25% increase for a 0.75-mm-ID coil, and only a 6% increase for a 0.5-mm-ID coil, further highlighting the increasing test severity as the coil ID is reduced.
pH Sensitivity
The subsea scenario was used to evaluate the influence of brine pH on the scale inhibitor efficiency. For the TBT, the produced water in Table 1 was divided into cationic and anionic brines so that when they were mixed inside the equipment, the desired composition was obtained. pH adjustment of the cationic brine was performed with hydrochloric acid (HCl) and/or sodium hydroxide (NaOH) and of the anionic brine with carbon dioxide (CO2) such that when the brines were mixed, the pH was between 6.90 and 7.30 (7.1 ± 0.20).
The theoretical sensitivity resulting fromthe pH variation was observed through simulations using scale modeling software considering a pH range of 6.8 to 7.5 for the mixture. Fig. 3 demonstrates that when the pH is greater than 7.20, the saturation ratio increases rapidly for calcium carbonate (CaCO3).
It can also be observed that a ±0.20 variation in the pH results in different efficiencies for the same product. Fig. 4 provides the test results, and it is possible to conclude that at a pH greater than 6.90, the minimum inhibitory concentration (MIC) of the product increases because of the increase in the saturation ratio in the subsea scenario.
Coil ID influence
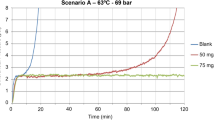
The topside scenario was used for this TBT evaluation. Capillaries with IDs of 0.5, 0.75, and 1 mm were used. The tests were performed at a fixed dosage of 75 ppm with a PAPEMP-based product, and it was observed that the capillary ID significantly influenced the scale inhibitor efficiency test results (Fig. 5). At the 75-ppm dosage using the capillaries with IDs of 0.75 and 1 mm, the product passed the inhibition efficiency tests, but for the capillary with a 0.5-mm ID, 75 ppm of the same product was not sufficient to prevent the increase in differential pressure above the criteria for 2 hours. Although not a perfect match, the measured baseline ∆P value aligned with the calculated values on the basis of the test conditions (Fig. 1).
Because mineral deposition kinetics are not fully understood, even in less-complex laboratory conditions, few kinetics models have been successfully applied in laboratory or field conditions. Furthermore, although several studies have been conducted on the impacts of scale inhibitors on mineral deposition kinetics (Barber and Heath 2019; He et al. 1994; He et al. 1995; He et al. 1996, 1999, 2002), the inhibition impacts on mineral deposition kinetics are not comprehensively understood, and most of the models developed are for mineral nucleation and crystallization in batch systems without considering flow conditions and surface deposition. Therefore, understanding the fundamental mechanisms and kinetics of mineral deposition and inhibition in laboratory conditions and applying such knowledge to field conditions remain significant challenges. Recently, researchers from Rice University published several papers Liu and Nancollas (1975); Lu et al. (2020); Lu et al. (2021) on mineral scale deposition kinetics inside flowing pipes. Using the findings from these studies, the researchers developed a new mineral surface deposition and inhibition platform and integrated the model into scale modeling software, to predict mineral scale deposition.
Although the model they developed is based on limited experimental data of barite and calcite scales, the prediction results (Table 3) obtained from using their model with different coil IDs could increase understanding of the influence of coil ID in TBT evaluations. In the deposition simulation, the brine composition and system T&P conditions for the topside scenario were used. Because the scale modeling results predicted that primarily calcite scale would form in the topside scenario and the deposition kinetic modeling results for barite were insignificant compared to the calcite deposition predictions, only calcite deposition predictions were listed in Table 4. As suggested by the simulation results, the calculated saturation ratio, the maximum quantity of precipitation, and the predicted deposition mass are exactly the same for the different coil IDs, but the deposition radii (mm/h) differ. Coil size will not affect the brine’s SR and mass of precipitation. The thermodynamic and kinetics of scale formation will not be affected by the coil size used in a DSL test.As the coil ID decreases, the predicted deposition radius at each section of the coil increases accordingly because at a given time, the same deposition mass is predicted each coil, while the capillary available inner space for deposition decreases with the decrease in capillary ID. This could explain why in the TBT evaluation, the same scale inhibitor product dosage was not sufficient to prevent an increase in the differential pressure when testing the 0.5-mm-ID coil, but the differential pressure was less than the passing criterion when either the 0.75-mm-ID coil or the 1-mm-ID coil was used. If the added scale inhibitor achieved 95% inhibition and x quantity of scale solids still formed and were deposited on the tubing wall, according to the simulation results, the deposition masses are equal for all three capillary IDs. However, the impact on the differential pressure from an equal quantity of scale deposit is quicker on the capillary coil with the smaller ID as a result of more-restricted flow compared to a capillary with a larger ID, which is also consistent with the theoretical calculation of ΔP shown in Figs. 1 and 2.
Based on these evaluations, it can be concluded that the smaller the capillary ID is, the more critical, severe, and restrictive the TBT testing conditions are, and scale inhibitor molecules with better performances and/or greater dosages are often required to pass a test under those conditions.
Conclusions
Based on these evaluations, it can be concluded that the pH tolerance range should be narrow enough to capture the field conditions and be reproduced in the laboratory without permitting exacerbated variations to the saturation index of the scenario under evaluation. This can be particularly observed in the cases of high-scaling-tendency brines. A slight increase in brine pH cansignificantly change the saturation index/ratio of scaling carbonate minerals. As a result, the MIC of a scale inhibitor substantially increases.
With regards to capillary ID, tests performed on a 0.5-mm ID could result in a scenario much worse than the field conditions. In such harsh conditions, the MIC of a selected scale inhibitor obtained through laboratory TBT testing could be significantly greater than the actual treatment dosage needed in the field. This is a problem because the MIC obtained in such tests might not represent the actual MIC required under the production conditions of the field and, as a practical consequence, could result in an increase of the cost for deposition control of inorganic scale.
Therefore, it is extremely important to always evaluate whether laboratory tests are consistent with the production conditions in the field, and the MIC obtained through laboratory TBTs should be considered an initial recommended treatment rate and the actual treatment rate of a scale inhibitor must be optimized after the selected scale inhibitor is deployed in the field, and appropriate monitoring techniques are implemented to capture the performance of the inorganic scale deposition control program.
Data availability
The data that support the findings of this study are available from the corresponding author, André Saraceno, upon reasonable request.
References
Barber M, Heath S (2019) SPE International Conference on Oilfield Chemistry. SPE-193580-MS. Galveston-TX, USA
Da Rosa K, Fonte R, Do Rosário F, Freitas T, Penna M, Castro B, Da Silva M, Da Silva G, Silva J, Figueiredo M (2019) Offshore Technology Conference Brasil. OTC-29683-MS. Rio de Janeiro
Frenier WW, Ziauddin M (2008) Formation, removal, and inhibition of inorganic scale in the oilfield environment. Society of Petroleum Engineers, Texas
He S, Oddo JE, Tomson MB (1994) The inhibition of gypsum and barite nucleation in NaCl brines at temperatures from 25 to 90 C. Appl Geochem 9(561):567. https://doi.org/10.1016/0883-2927(94)90018-3
He S, Oddo JE, Tomson MB (1995) The nucleation kinetics of strontium sulfate in NaCl solutions up to 6 m and 90 C with or without inhibitors. J Colloid Interface Sci 174(327):335
He S, Kan AT, Tomson MB (1996) Mathematical inhibitor model for barium sulfate scale control. Langmuir 12:1901–1905. https://doi.org/10.1021/La950876x
He S, Kan AT, Tomson MB (1999) Inhibition of calcium carbonate precipitation in NaCl brines from 25 to 90 °C. Appl Geochem 14:17–25
He S, Kan AT, Tomson MB (2002) Inhibition of mineral scale precipitation by polymers. Water Soluble Polym. Springer, Boston, pp 163–171
Liu S-T, Nancollas GH (1975) The crystal growth and dissolution of barium sulfate in the presence of additives. J Colloid Interface Sci 52:582–592. https://doi.org/10.1016/0021-9797(75)90284-2
Lu Y-T, Harouaka K, Paudyal S, Ko S, Dai C, Gao S, Deng G, Zhao Y, Wang X, Mateen S, Kan A, Tomson M (2020) Kinetics of barium sulfate deposition and crystallization process in the flowing tube. Ind Eng Chem Res 59:7299–7309. https://doi.org/10.1021/acs.iecr.0c00112
Lu Y-T, Kan A, Tomson M (2021) Nucleation and crystallization kinetics of barium sulfate in the hydrodynamic boundary layer: an explanation of mineral deposition. Cryst Growth Des 21(3):1443–1450. https://doi.org/10.1021/acs.cgd.0c01027
Tomson MB, Fu G, Watson MA, Kan AT (2003) Mechanisms of mineral scale inhibition. SPE Prod Oper 18(03):192–199
Acknowledgments
The authors would like to thank Schlumberger Production Chemistry for permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saraceno, A., Fan, C., Alves, D. et al. Effects of laboratory test sensitivities on the efficiency of scale inhibitors for Brazilian applications. Braz. J. Chem. Eng. 41, 729–735 (2024). https://doi.org/10.1007/s43153-023-00400-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00400-4