Abstract
Inorganic scale deposition is one of the main challenges related to flow assurance in Oil and Gas Industry. The pre-salt oilfields has been becoming very important, especially for the Brazilian market. The operational conditions in these types of scenarios are extremely favorable for salt precipitation, leading to formation of inorganic scales during the production and transport facilities that represent a challenge for the oil industry. The main factors for inorganic scale formation includes high salinity, high temperature and considerable changes on the pH of the produced fluid. In this work, two different scenarios from Brazilian pre-salt fields were considered for evaluation of a suitable scale inhibitor that could withstand all the severity faced in pre-salt fields. The chemical previous developed which performance were here evaluated has its active content based on a polyamino polyether methylene phosphonic acid (PAPEMP) and showed great performance in avoiding scale precipitation for a long period of test with temperature up to 135 °C as well as compatibility with high calcium brines. The carried out analyses included projections on the Scale Soft Pitzer software to evaluate the thermodynamic conditions of the system, compatibility tests with brine and tests on the DSL (Dynamic Scale Loop) equipment, in order to verify the performance of inhibitors under dynamic conditions. The MIC, minimum inhibitory concentration, found for the PAPEMP-based chemical evaluated was a low one, resulting in a very economical solution for applications in Pre-Salt fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the South Atlantic area, it is possible to found huge reserves of Pre-Salt fields which are common on the coast of Brazil and West Africa. According to available data, the huge oil and natural gas reserves are located below about 2000 m thick layer of salt which covers the sedimentary basins of Campos, Santos and Espírito Santo (Beltrão et al. 2009). Below it there is a large oil reserve estimated in approximately 150,000 km2 and 300 km from the coast. This area is known as “pre-salt polygon” (Reuters 2020). The discovery of the pre-salt represented self-sufficiency in Oil for Brazil, since historically the country has always needed to import it. According to the IBP (Brazilian Petroleum Institute), by 2035, Brazil will stop importing and will become an energy exporter. The first Pre-Salt Oilfield was discovered by Petrobras in the Santos Basin in 2006 and, since then, this type of application has grown fast and become very important (Beltrão et al. 2009). This boosted the demand of oilfield production chemicals, such as scale and corrosion inhibitors, emulsion breakers, biocides, among others, in order to ensure the treatment of several operational issues in these types of scenarios (Husseini 2018).
Among the main problems on operations are the appearance of inorganic salts scale, which can be difficult to remove and cause significant production losses (Haarberg et al. 1992; Kamal et al. 2018; Li et al. 2017). In this setting, the use of chemical scale inhibitors is considered the most practical and economical method (Rosa et al. 2019). Scale inhibitors are chemical substances that can delay or retard the formation of scale when injected to the brine (Crabtree et al. 1999). The management of inorganic scale is considered a challenge for oil and gas industry and hence the importance of continuous research for the development of chemical solutions that can mitigate this problem with an efficient cost/benefit relation and considering an environmental impact. Furthermore, as some of the mature pre-salt filed reach the mid-life, water ratios increase, and so the demand for new scale inhibitors that can endure and maintain its efficiency with the increasing fraction of water over the years of oil production (Koh and Hayum 2022). Reports on literature shows the importance to develop new molecules that can be able to face all the severe conditions of pre-salt fields and the upcoming changes (Bruhn et al. 2017; Petersohn 2019; Silva et al. 2023; Neves Junior et al. 2023) and the phosphorus-containing molecules—in special the phosphonates ones—have been of great interest due to its characteristics as discussed by Liu and Zhang (2022) and Zhang (2020) in their work. Many authors have shared their studies of developing and evaluating the efficiency of new scales inhibitors as reported by Mady et al. (2017), Wang et al. (2019), Xu et al. (2019) and Zheng at al. (2022).
The development of a suitable scale inhibitor that could withstand all the severity faced in pre-salt fields needs to consider not only the operational facilities but the conditions of the filed, which are mainly high salinity, high temperature, high CO2 content and considerable changes on the pH of the produced fluid. In addition, the scale inhibitor needs to be suitable and effective for different types of scales as the deposits are mostly formed by mixed salts (Frenier and Ziauddin 2008; Mpelwa and Tang 2019).
There are different types of scale deposits in oilfields, the most common being calcium carbonate and sulfate scales (barium, strontium or calcium) mainly due to its low to moderate solubility in water, as can be seen in Table 1. The scale formation is influenced by many thermodynamics and kinetics factors (Bai and Bai 2019; Crabtree et al. 1999; Hoang 2015; Kamal et al. 2018). Carbonate deposits, for example, are formed mainly due to the decrease in pressure and the increase in temperature. The solubility of CaCO3 decreases with increasing temperature (Dyer and Graham 2002; Li et al. 2017). Through the release of carbon dioxide from oil during its production process, an increase in the pH of the medium is observed, which can promote, for example, the occurrence of carbonate scales (Atkinson and Mecik 1997; Crabtree et al. 1999; Kamal et a. 2018). On the other hand, sulfate scale is present whenever there is a mixture of incompatible waters—waters that can chemically react with each other. This mixture occurs, for example, when sea water is used in injection wells to improve the oil displacement in production wells on offshore fields (Abib et al. 2018).
Considering the severity of Pre-Salt fields conditions, to prevent problems related to scale formation, an ideal inhibitor needs to have broad capacity to prevent several scales, tolerance to high salinity brines, temperature resistance and suitable pH range of action (Bai and Bai 2019). The study of scale inhibitors with strong chelating properties, high thermal stability, huge calcium tolerance and in special the threshold effect had fundamental importance in this work. The threshold effect is a mechanism type of action of the scale inhibitor defined by adding a substoichiometric quantity of the product but enough to hinder a scale deposit by interfering in the growing crystals, delaying crystal growth, and/or absorbing onto the crystal’s dynamic sites, distorting its morphology (Abdel-Aal and Sawada 2003; Issabayev et al. 2018; Mohammad and Mazumder 2020; Liu and Zhang 2022).
The development of a high-performance solution was achieved after dynamic performance evaluation, saturation index and mass of precipitate prediction on Pre-Salt fields conditions, as well as the chemistry type impact on brine compatibility, especially when submitted to high-stress scenarios. A promising scale inhibitor with active content based on a polyamino polyether methylene phosphonic acid (PAPEMP), high chelation and dispersion effects, high calcium tolerance, and good scale inhibition effects was developed after achievement of satisfactory test results. In this work, is described the procedure used to evaluate the PAPEMP-based scale inhibitor developed considering the two of the most severe scenarios founded at pre-salt fields, choose due to its high temperature and high calcium concentration, in order to submit the chemical product developed to the adverse and severe conditions faced on pre-salt.
Methodology
The methodology for this work initially included software simulation to predict the saturation indexes of the brine as well as the theoretical deposition rates based on thermodynamic conditions. As next steps, it was performed brine compatibility tests and dynamic tests in the DSL in order to evaluate the efficiency of inhibition considering not only thermodynamic conditions of scale precipitation but also the kinetics ones. Two of the most severe scenarios of Pre-salt were selected to evaluate the performance of the scale inhibitor developed, named in this paper as “Scenario A”, Sc. A, and “Scenario B”, Sc. B which key data collected for formation brines are summarized in Table 2.
The chemical properties and specifications of the scale inhibitor developed and evaluated in this study are summarized in Table 3.
Software simulations
For all the conditions tested considering Sc. A and Sc. B, Saturation Indexes (SI) and mass of precipitate to re-establish the equilibrium (ppt), were calculated by Scale Soft Pitzer software, the software developed by the Brine Chemistry Consortium at the Rice University (M. Thomson’s and A. Khan’s group).
Dynamic scale inhibition performance test (DSL)
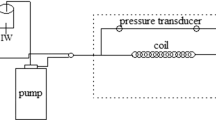
Dynamic scale inhibition tests have been performed by using a Dynamic Scale Rig (Techbox Systems H400) with automatic data recording of differential pressure through a stainless-steel coil. The instrument is equipped with two double pistons pumps (Knauer Azura P4.1 S), one used for cationic brine and one for anionic or “inhibited” anionic brine and the cleaning solutions. The oven (Memmert UF55Plus) set is suitable for temperature up to 250 °C.
In this test, anionic and cationic brines were pumped separately through two 2-m-long pre-heating coils and then combined by a union tee in a 1-meter stainless-teel coil (ID 0.5 mm). Differential pressure between the inlet and outlet of the coil was measured by a pressure transducer until it reaches the designed threshold value which in this study was 10 psi for the blank test and 0.5 psi for efficiency approval of the product. Temperature, pressure, and flow rate are set up based on field conditions. The pH of anionic brine is buffered by bubbling a mixture of CO2 and N2 while to buffer the pH of cationic brine HCl and NaOH solutions are used. After each test, 10% acetic acid solution, 5% alkaline EDTA solution and DI water are used to clean and restore the coil. In each experiment, a successful test is when the pressure increase does not achieve the target threshold after a time equal to 60 or 120 min. The experiment is designed as a single dosage test, where only cationic and inhibited anionic brine are used. The inhibited anionic brine contains 2x the inhibitor concentration to be tested in the DSL. When a concentration step is not successful—which means that the threshold differential pressure value is achieved—a new inhibited anionic brine with a different dosage must be prepared.
BaCl2 ·2H2O, CaCl2·2H2O, NaCl, KCl, SrCl2·6H2O and MgCl2·6H2O had been used for the preparation of cationic brine. NaHCO3, NaSO4, KBr, CH3COONa and NaCl had been used for the anionic brine. All these inorganic salts were ACS reagents of Sigma Aldrich commercial grades. Both cationic and anionic brines had been prepared by adding all the required number of salts (except NaHCO3) to DI water. Brines were left under stirring overnight, then filtered with a 0.45 μm pore size cellulose acetate membrane. NaHCO3 were added prior each test. After the completely dissolution of this salt, both brines had their pH adjusted and then the DSL test were started.
Compatibility tests
Compatibility tests had been performed removing scaling anions (sulfate and bicarbonate) from testing brine. The PAPEMP-based scale inhibitor was added to the brine in order to have final concentrations up to 5,000 or 300,000 mg/L. After shaking, vials were stored in oven for 24 h at testing temperature and the visual aspect were registered after 1 h, 2 and 24 h. Scale inhibitor is considered approved in compatibility test if in all concentrations as product no turbidity and/or precipitation or phase separation is observed after 24 h.
Results and discussion
In order to know the severity and types of scales predicted for the two selected Pre-salt scenarios A and B, simulations on the Scale Soft Pitzer software were performed to access the Saturation Indexes (SI) and deposit rate (ppt). In the simulations, risk of scale is described by Saturation Index (SI) and the theoretical precipitate to achieve equilibrium (ppt)—considered here as deposit rate—for the type of scale contemplated. SI > 0 means that scale formation is thermodynamically possible and higher the SI and ppt, higher the risk of scale deposition. The simulation data obtained under the conditions of Scenario A and B are summarized in Table 4. Based on this data, very high mixed carbonate and sulfate scales are predicted for Sc. A with high SI and deposit rates. Regards Sc. B, the predictions are positive SI for Calcite, Barite, and strontium sulfate, being Calcite the most concerning one. In terms of deposit rate, Calcite is by far the most critical scale but with high amount of strontium sulfate expected as well.
To assess the efficiency of the scale inhibitor, the Dynamic Scale Loop (DSL) test is one of the best ways to evaluate its performance in preventing scale formation under scenario’s temperature and pressure. In this test, the Minimum Inhibitory Concentration, MIC, i.e. the minimum dosage of the product necessary to prevent scale formation, is determined. Considering the brines composition in Table 2, calcium content varies a lot depending on the field, which can be very high, almost 10,000 mg/L, as seen in Sc. A. Furthermore, also bicarbonate content is quite high in the considered cases and especially for Sc. A, the amount of sulfate is quite high as well, what justify the prediction of high mixed carbonate and sulfate scales for this case.
The results of the DSL test at Scenario A conditions are shown in Fig. 1. DSL tests were performed without scale inhibitor, a blank test, to observe the dynamic scale potential of the scenario. The duration of the blank test less than 20 min shows the severity of the case, which agrees with what was observed in the simulations. Besides the blank test, to determine the MIC of PAPEMP-based scale inhibitor, two additional runs were performed at 50 and 75 mg/L dosages. According to the results obtained, the PAPEMP-based scale inhibitor provided excellent performance with only 75 mg/L as MIC, showing a very good threshold effect since the dosage was far from the stoichiometric required considering the amount of scaling cations in the brine. These results suggest that the PAPEMP-based scale inhibitor was able to interfere in the process of crystal formation resulting in the prevention of scale deposits.
Results of compatibility test showed that the product had full compatibility with Sc. A brine as shown in Fig. 2. It was not observed any turbidity or precipitated in the flask’s samples at 63 °C with concentrations up to 300,000 mg/L of the PAPEMP-based scale inhibitor as product, confirming its tolerance to high amount of calcium considering that this scenario has almost 10,000 mg/L of calcium.
Concerning Scenario B, which has the higher temperature that leads to severe carbonate scale formation in addition to high-stress-temperature that the product was submitted and must have resistance to—since, in general, phosphorous-containing-scale-inhibitor has less thermal stability than polymeric species (Liu and Zhang 2022)—DSL tests were also performed in a blank run (without inhibitor) and the MIC were determined with run at 150, 200 and 250 mg/L dosages. DSL tests are displayed in Fig. 3. The blank time was around five minutes, showing the high severity due to the high temperature of Sc. B. The resulted MIC was 250 mg/L yet confirming the threshold effect of the PAPEMP-based scale inhibitor developed. In addition, as at higher temperature higher dosages of phosphorous-containing-scale-inhibitor can lead to formation of phosphate salts, it was also evaluated the efficiency of the scale inhibitor at 750 mg/L, that corresponds to 3x the determined MIC, to see if the inhibitor itself could promote phosphate scale formation if overdosed. Even at 3x the MIC, the scale inhibitor had performed very well showing a flat line and no noticed changes in differential pressure, confirming the thermal resistance and system stability of the PAPEMP-based product developed. Furthermore, the product also proved to be fully compatible with Sc. B brine on the concentrations evaluated (up to 5000 mg/L) at 135 °C. As seen in Fig. 4, it was not observed turbidity or any precipitate formed in the sample flasks, once again affirming the calcium tolerance of the product.
Conclusions
Pre-Salt applications are growing fast in Brazil making this country one of the most important Oil producers. Therefore, scale inhibitors demand is growing as well. Typical conditions and brines can vary a lot depending on which field is considered. In most of the cases, Calcite is the typical scale with SI usually high and theoretical deposit rate that can reach very high values. In order to face this issue, PAPEMP-based scale inhibitor was developed. As described in the paper, it showed great efficiency in two of the most severe pre-salt scenarios and so overcoming standard chemistries in inhibiting Calcite under these conditions, also inhibiting other scales formations as sulfates, with high efficiency and calcium tolerance by delivering a great cost-benefit to the treatment. Further advantages are detectability, mild pH (> 4), which makes handling safer, and good solvent compatibility are additional benefits.
References
Abdel-Aal N, Sawada K (2003) Inhibition of adhesion and precipitation of CaCO3 by aminopolyphosphonate. J Cryst Growth 256(1–2):188–200. https://doi.org/10.1016/S0022-0248(03)01354-X
Abib GAP, Da Cruz GF, Vaz Junior ASL (2018) Study of barium sulfate dissolution by scale dissolver based on solutions of dtpa. Anais da Academia Brasileira de Ciências 90(3):3185–3196. https://doi.org/10.1590/0001-3765201820170728
Atkinson G, Mecik M (1997) The chemistry of scale prediction. J Petrol Sci Eng 17:113–121. https://doi.org/10.1016/S0920-4105(96)00060-5
Bai Y, Bai Q (2019) Subsea engineering handbook, 2nd edn. Subsea corrosion and scale. Gulf Professional Publishing. https://doi.org/10.1016/b978-0-12-812622-6.00017-8
Beltrão RLC et al (2009) SS: Pre-salt Santos basin—challenges and new technologies for the development of the pre-salt cluster, Santos Basin, Brazil. In: Paper presented at the Offshore Technology Conference, Houston, Texas. https://doi.org/10.4043/19880-MS
Bruhn CHL et al (2017) Campos and Santos Basins: 40 years of reservoir characterization and management of shallow- to Ultra-Deep Water, Post- and Pre-Salt Reservoirs —historical overview and future challenges. OTC Brasil. https://doi.org/10.4043/28159-MS
Crabtree M et al (1999) Fighting scale: removal and prevention. Oilfield Rev 11:30–45 https://www.slb.com/-/media/files/oilfield-review/fighting. Accessed 23 May 2022
da Rosa KRSA et al (2019) Improved protocol for scale inhibitor evaluation: a meaningful step on scale management. In: Paper presented at the offshore technology conference Brasil, Rio de Janeiro, Brazil, October 2019. https://doi.org/10.4043/29683-MS
Dyer SJ, Graham GM (2002) The effect of temperature and pressure on oilfield scale formation. J Petrol Sci Eng 35:95–107. https://doi.org/10.1016/S0920-4105(02)00217-6
Frenier WW, Ziauddin M (2008) Formation, removal, and inhibition of Inorganic Scale in the Oilfield environment. Society of Petroleum Engineers, Richardson
Haarberg T et al (1992) Scale formation in reservoir and production equipment during oil recovery. An equilibrium model. SPE Prod Eng 7:75–84. https://doi.org/10.2118/19449-PA
Hoang TA (2015) Mechanisms of scale formation and inhibition. In: Mineral scales and deposits: scientific and technological approaches. Elsevier B.V. https://doi.org/10.1016/B978-0-444-63228-9.00003-6
Husseini T (2018) Tracing the history of exploration in the Brazilian pre-salt oil region. Tracing the history of exploration in the Brazilian pre-salt oil region. https://www.offshore-technology.com/features/pre-salt-oil-region-brazil/. Accessed 12 Aug 2022
Issabayev YA et al (2018) Synthesis of unexplored aminophosphonic acid and evaluation as scale inhibitor for industrial water applications. J Water Process Eng 22:192–202. https://doi.org/10.1016/j.jwpe.2017.12.007
Kamal MS et al (2018) Oilfield scale formation and chemical removal: a review. J Petrol Sci Eng 171:127–139. https://doi.org/10.1016/j.petrol.2018.07.037
Koh K, Hayum L (2022) Can Brazil’s mature pre-salt oil fields continue stellar performances? Online Report: Wood Mackenzie. https://www.woodmac.com/news/opinion/can-brazils-mature-pre-salt-oil-fields-continue-stellar-performances/#form. Accessed 03 Jan 2023
Li J et al (2017) Scale formation and control in oil and gas fields: a review. J Dispers Sci Technol 38:661–670. https://doi.org/10.1080/01932691.2016.1185953
Lide DR (ed) (2005) CRC handbook of chemistry and physics, 85th edn. Boca Raton, Florida
Liu Y, Zhang P (2022) Review of phosphorus-based polymers for mineral scale and corrosion control in Oilfield. Polymers 14(13):2673–2699. https://doi.org/10.3390/polym14132673
Mady MF, Kelland MA (2017) Overview of the synthesis of salts of organophosphonic acids and their application to the management of Oilfield Scale. Energy Fuels 31(5):4603–4615. https://doi.org/10.1021/acs.energyfuels.7b00708
Mohammad A, Mazumder J (2020) A review of green scale inhibitors: process, types, mechanism and properties. Coatings 10:928–956. https://doi.org/10.3390/coatings10100928
Mpelwa M, Tang S-F (2019) State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: a review. Pet Sci 16:830–849. https://doi.org/10.1007/s12182-019-0299-5
Neves Junior A et al (2023) Assessing EOR strategies for application in brazilian pre-salt reservoirs. Geoenergy Sci Eng 223:211508. https://doi.org/10.1016/j.geoen.2023.211508
Petersohn E (2019) Pre-salt super play: leading Brazil into the World’s Top 5 oil suppliers. In: AAPG Latin America and Caribbean Region Geoscience Technology Workshop, Rio de Janeiro, Brazil, Conference Abstract. https://doi.org/10.1306/30625Petersohn2019
Reuters (2020) Produção de petróleo no Brasil tem recorde em janeiro. Brazil: Exame Magazine. https://exame.com/economia/producao-de-petroleo-no-brasil-tem-recorde-em-janeiro/ . Accessed 09 Sep 2022
Silva VO et al (2023) Building Options for the brazilian pre-salt: a technical-economic and infrastructure analysis of offshore integration between energy generation and natural gas exploration. Resour Policy 81:103305. https://doi.org/10.1016/j.resourpol.2023.103305
Wang W et al (2019) Preparation and evaluation of phosphate scale inhibitor for oilfield produced water. Key Eng Mater 814:511–516. https://doi.org/10.4028/www.scientific.net/KEM.814.511
Xu RJ et al (2019) Synthesis of new phosphate and the evaluation as scale inhibitor in oilfield. Key Eng Mater 814:493–498. https://doi.org/10.4028/www.scientific.net/KEM.814.493
Zhang P (2020) Review of synthesis and evaluation of inhibitor nanomaterials for Oilfield Mineral Scale Control. Front Chem. https://doi.org/10.3389/fchem.2020.576055
Zheng M et al (2022) Development and performance evaluation of Scale-Inhibiting Fracturing Fluid System. Processes 10:2135–2149. https://doi.org/10.3390/pr10102135
Acknowledgements
The research and development studies reported on this paper would never be possible without the support and commitment of Technical Professionals from Italmatch Chemicals as well as its board of Directors who delivered the appropriate guidance and allowed the projects workflow. The financial and laboratory resources provided by the Italmatch Chemicals organization combined with trust in its employees have enabled important discoveries for the science and technology of flow assurance in the Oil and Gas industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare their employment relationship with Italmatch Chemicals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is an invited, extended version of the manuscript presented in the 1st Flow Assurance Technology Congress and published in the FATC22 Anais.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borges, E., Lessa, J. Scale inhibitor qualification: evaluation for treatment in Brazilian pre-salt fields. Braz. J. Chem. Eng. 41, 713–720 (2024). https://doi.org/10.1007/s43153-023-00385-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00385-0