Abstract
The aim of the study was to optimize fermentation parameters for coproduction of protease and cellulase from Bacillus subtilis M-11 in solid state fermentation by a statistical approach and to evaluate the stability and compatibility of enzymes for detergent formulation. Eight different substrates were investigated to find the best medium supporting maximum enzyme productions. Box–Behnken design (BBD) was employed to optimize culture conditions for the coproduction of proteases and cellulose. The effect of pH and temperature, surfactants and commercial detergents on stability of the enzymes was evaluated. Oat flour was found to be the best substrate for production of both enzymes. The results indicate that BBD is an effective tool for optimizing the medium for coproduction of enzymes and the fermentation parameters that affect production of the enzymes. Furthermore, the enzymes showed excellent compatibility with detergents, as well as high stability in wide ranges of temperature (25–60 °C) and pH (7.0–9.5). In conclusion, the present study offers a cost-effective method to coproduce protease and cellulase from B. subtilis M-11. Also, it can be suggested that both the enzymes have strong potential for usage in the detergent industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Use of cellulose and protease in industrial applications such as laundry detergents, animal feed paper, and waste management are rapidly increasing due to their economic and ecological advantages (George et al. 2014; Kalaiyarasi et al. 2017). Cellulase, lipase, protease and amylase are major enzymes incorporated in detergent formulations (Hmidet et al. 2009). Microorganisms are preferred for the production of the enzymes, because of a significant yield, and low-cost (Niyonzima and More 2015a). Especially, there are many studies on the production of cellulase (Irfan et al. 2017; Sreena and Sebastian 2018; Shahid et al. 2016) and protease (Sahin et al. 2015; Singh and Bajaj 2016; Kalaiyarasi et al. 2017) from the genus Bacillus.
The detergent industry exploits about 60% of all enzymes produced but needs more amounts of enzymes. The most important challenge in meeting this need is the high cost of enzyme production. The substrates used for the fermentation medium account for 30–40% of the production cost of industrial enzymes (Niyonzima and More 2015a). In a single fermentation medium, combined production of enzymes can be an important step to reduce the cost.
Another way of reducing the cost of production is the usage of cheap substrates such as agroindustrial products (Niyonzima and More 2015a). At the same time, production of enzymes in the SSF currently gains more attention due to its advantages such as higher volumetric productivity and less water usage (Kalaiyarasi et al. 2017; Krishna 2005). Substrates used in SSF are commonly natural products such as agroindustrial wastes that render a carbon source (Pandey et al. 1999).
Microbial production of enzymes with the SSF process is influenced by various major factors such as the selection of a suitable substrate and microorganism, the moisture level of substrate, temperature, pH and incubation time (Krishna 2005). Performing this process using the conventional one-factor-at-a-time approach of optimization is both laborious and time-consuming. However, the process disregards the combined interaction of the variables and does not guarantee to attain optimal conditions. In recent years, to solve this problem model-based optimization techniques have been proposed (Zhang et al. 2018). The use of statistical approaches such as Box–Behnken Design has gained a lot of impetus to predict optimal fermentation conditions and to understand the interactions among various parameters with a minimum number of experiments. Kalaiyarasi et al. (2017) reported that protease and cellulase productions increased four-fold in the statistical design-optimized medium, compared to the non-optimized medium.
The utilization of enzymes in detergents is limited due to the high cost of production (Niyonzima and More 2015a). The coproduction of enzymes in a single-cultivation medium with inexpensive substrates can bring down the cost. The industrial demand for enzymes, which are stabile to pH, temperature, and surfactants, continues to stimulate the search for new enzyme sources. Therefore, the study aimed to coproduce protease and cellulase from Bacillus subtilis M-11 in the same SSF medium and to optimize fermentation parameters for maximal enzyme activity using Box–Behnken Design (BBD). The stability and compatibility of the obtained enzymes for detergent formulation was evaluated in the presence of commercial detergents and some detergent constituents.
Materials and methods
Materials and microorganism
Casein from Carlo Erbaa, Carboxymethylcellulose (CMC) Sodium Salt from Alfa Aesar and Folin-Ciocalteu reagent from Merck were purchased. Other chemical reagents used in experiments were analytical grade.
Bacillus subtilis M-11 strain was isolated from soil, Turkey. The bacterial isolate was identified at Adnan Menderes University, Arts and Science Faculty, Biology Department. Bacillus subtilis M-11 was grown on nutrient agar at 37 °C for 24 h for inoculum preparation. A loopful of the growth was transferred to nutrient broth. The broth culture was incubated at 37 °C at 120 rpm for 24 h. It was used as an inoculum through all the study.
Solid state fermentation and substrate selection
Eight different organic substrates, including wheat bran, corncob, barley flour, Phaseolus vulgaris L. Hull, oat stalk, oat flour, bean flour, and chickpea flour, were obtained from local market. To select a potential substrate that supported maximum enzyme production for SSF, the organic substrates were screened individually to produce both the enzymes. The SSF medium containing 3 g of the organic substrates in a 100 mL Erlenmeyer flask was autoclaved at 121 °C for 15 min. The medium was inoculated with 100 µL of 24 h grown culture broth under sterile conditions and incubated at 37 °C, after adding phosphate buffer (0.05 M, pH 7.5) to adjust the moisture level (75%) of the medium. After that, production of both the enzymes was checked every 24 h for 96 h. For activity assays, the cells were separated from the medium by centrifugation at 8000 rpm for 20 min. The clear supernatant was used as the enzyme source. The best substrate, which provided the maximal activities of protease and cellulase, was used for optimization of enzyme production by BBD.
Enzymes production and statistical optimization
In the present study, the Box–Behnken statistical experimental design method was utilized to define the effects of parameters such as incubation time, temperature, initial pH, and initial moisture level on the coproduction of the cellulase and protease (Pawar and Rathod, 2018). The fermentation parameters, including incubation time (24–72 h), incubation temperature (27–57 °C), initial pH (5.5–9.5), and initial moisture level (60–90%), were selected for BBD. BBD at three levels was described as high, medium and low (− 1, 0, and + 1) (Table 1). A total of 29 experiments were constructed in the experimental design method and the center point was repeated five times to test the reproducibility of the test results. All the experiments were carried out in duplicates and the response function coefficients were determined by regression using the experimental data and Design-Expert V7 trial version. For four variable systems, the model equation is as follows:
where Y is the predicted response, β0 is the model constant; A, B, C, and D are independent variables; β1, β2, β3 and β4 are linear coefficients and β11, β22, β33 and β44 are the quadratic coefficients.
ANOVA was used to analyze the experimental results of protease and cellulase production. The optimum values of the variables were obtained by analyzing three-dimensional plots. After optimization, experiments were conducted to validate the predicted protease and cellulase activities under the optimum fermentation conditions predicted by the statistical model.
Determination of enzyme activities
Protease activity was determined using the Cupp-Enyard methods (2008). Enzyme (25 µL) was incubated for 10 min at 37 °C after adding 0.65% (w/v) casein solution (1 mL) prepared in 50 mM phosphate buffer (pH 7.5). The reaction was stopped by addition of 1 mL of TCA (trichloroacetic acid). The reaction mixture was centrifuged at 4000 rpm for 5 min after the incubation for 10 min. After that, 500 µL of supernatant was transferred to another test tube and 2 mL of Na2CO3 solution and 1 mL of fivefold-diluted Folin–Ciocalteau reagent were added. The mixture was incubated for 10 min at 37 °C. The amount of tyrosine released was determined spectrophotometrically at 660 nm against the enzyme blank. One unit of protease activity was defined as the amount in micromoles of tyrosine equivalents released from casein per minute at 37 °C, pH 7.5. The enzyme activity was expressed as IU/gds (gram dry substrate).
Cellulase activity was determined using the DNS (3,5-dinitrosalicylic acid) method (Ghose 1987). In this assay, the amount of reducing sugars released from carboxymethylcellulose (CMC) dissolved in 50 mM citrate buffer (pH 5) was determined. The enzyme and 1% (w/v) CMC solution were incubated for 30 min at 50 °C. After the reaction was stopped by addition of DNS solution, the samples were boiled for 15 min and then cooled. The optical density was measured at 540 nm. The cellulase activity was determined using a calibration curve for glucose. One unit of enzyme activity was defined as the amount of enzyme that released 1 µmol of glucose per minute. The enzyme activity was expressed as IU/gds (gram dry substrate).
Effect of temperature and pH on activity and stability of protease and cellulase
The effect of pH on activity and stability of both enzymes was defined by incubating the enzymes (1 mL) with 50 mM phosphate buffer (1 mL) with pH of 6.5–10.0, without substrate, for 1 h at room temperature and the residual activities were then estimated.
The effect of temperature on activity and stability of both enzymes was defined by measuring the residual activity of the enzyme at 25–70 °C, without substrate, after incubation for 1 h. Also, thermostability of the enzymes at 40 °C was evaluated. The half-life time is determined as the time required to detect the loss of 50% of the protease and cellulase activity (Petlamul and Boukaew 2019).
Relative activities for all tests were determined by taking 100% as the enzyme activity at the start of the experiment and were measured by the standard assay procedure.
Detergent compatibility of the enzymes
The effect of surfactants and oxidizing agent on enzymes was tested by incubating the enzymes with anionic (SDS-sodium dodecyl sulfate) and nonionic (Tween 20, − 40 and − 80) surfactants and oxidizing agent (H2O2) at 40 °C for 1 h, and subsequently assaying for the residual enzymes activities. Activities of both the enzymes were measured to compare with the control sample without any surfactants.
To evaluate detergent compatibility of both the enzymes, the detergent brands used were Alo®, Omo®, Tursil®, Persil®, and Peros®. Detergents were diluted in distilled water to give a final concentration of 7 mg/mL to simulate washing conditions. The mixture was heated at 100 °C for 15 min to neutralize the enzymes that could be present in the detergent formulations. Later, these enzymes (2560 IU/gds of protease activity, 24.33 IU/gds of cellulase activity) were incubated in detergent solution prepared in water of 630 ppm total dissolved solids (TDS) at 40 °C for 1 h. The enzyme activities were measured compared with the control sample without any detergent (Hmidet et al. 2009; Rekik et al. 2019).
Results and discussion
Effects of different substrates on protease and cellulase activity
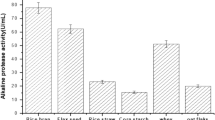
The choice of microorganisms and substrate are the most important factors in SSF (Soccol et al. 2017). In this study eight different organic materials were investigated to determine a suitable medium for production of both enzymes in SSF. The maximum production of both enzymes from Bacillus subtilis M-11 was performed at 48 h (37 °C, 75% initial moisture level, pH 7.5) under the same conditions. The results obtained from 3 substrates were very close; however, oat flour supported the highest enzyme activities (Table 2). Therefore, the coproduction of cellulase and protease was maintained with oat flour as substrate and optimized by a statistical approach.
Inexpensive and readily available substrates can be used to produce commercial enzymes by SSF. Given recent literature, proteases produced from agro-industrial sources are the third most studied group of enzymes produced by SSF (Soccol et al. 2017). In this study, it was found that the maximum activities obtained were 19.13 IU/gds for cellulase and 678.66 IU/gds for protease in SSF with oat flour. There are many studies that use various organic sources as the substrate in protease production. Singh and Bajaj (2016) found that cotton seedcake supported maximum protease production. The protease production by Bacillus spp. was carried out using agro-industrial materials (chickpea, gram black, gram husks, and wheat bran) as substrate (Prakasham et al. 2006; Govarthanan et al. 2014).
Most of the scientific studies published in the recent literature on SSF are related to cellulases and xylanases production (Soccol et al. 2017). Bacillus spp., which has excellent fermentation properties, is considered to be an important microbial source, but has been less used for cellulase production. Cellulase production has been obtained from bacterial strains such as B. subtilis K-18 (Irfan et al. 2017), B subtilis strain MU S1 (Sreena and Sebastian 2018), and B. megaterium (Shahid et al. 2016). In this study, cellulase production was carried out successfully from Bacillus subtilis M-11.
Statistical optimization of protease and cellulase coproduction
A new medium design is needed to increase enzyme production by low-cost solid substrates due to the commercial importance of enzymes. The combination of medium and fermentation conditions is an important factor that remarkably changes the yield in SSF (Govarthanan et al. 2014). Statistical design techniques were successfully used to perform cellulose, protease and uricase production (Kalaiyarasi et al. 2017; Pawar and Rathod 2018). BBD is one of the Response Surface Methodology (RSM) designs. BBD is more influential and easier to adjust and interpret the experiments. Therefore, this method is widely applied in many studies (Li et al. 2012).
In the study, BBD was employed to define the optimum level of parameters that provided maximum enzyme production and to understand the relationships between the fermentation parameters (incubation time, temperature, initial moisture, and pH) and the enzyme activities. The experimental design is presented in Table 3 with the experimental results. The variance analysis of the quadratic regression models suggested that the models are significant as summarized in Table 4. For protease and cellulase activity responses the regression equations obtained are as follows:
The importance of model terms is controlled by their respective p values. Also, “The Lack of Fit F-value” results indicate that the lack of fit is not significant relative to the pure error. Non-significant lack of fit is desirable. For protease and cellulase productions, the predicted R2 (0.96 and 0.94, respectively) and adjusted R2 (0.98 and 0.97, respectively) values were in reasonable agreement with the value of R2 (0.99 and 0.98, respectively). The values of R2 imply a correlation between the experimental results and predicted values. It shows that the experiments are very reliable (Garai and Kumar 2013).
The statistical analysis indicates that the variables tested have a significant effect on protease and cellulase production. As shown in Table 5, A, B, D, AB, AD, BD, A2, B2, C2, D2 are significant model terms for protease production. Also, A, B, D, AB, A2, B2 are significant model terms for cellulase production.
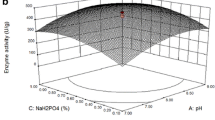
The three-dimensional (3-D) response surface graphs were plotted to illustrate the interaction of two variables and the optimum level of each variable for maximum protease and cellulase activities. The 3-D plot graphical representations are shown in Figs. 1 and 2.
Figure 1 presents the interaction between the two variables for protease production. Temperature affects the microbial growth, spore and product formation. Figure 1a shows that protease activity increases between 48 h and 60 h, as well as that an incubation temperature higher than 42 °C causes a sharp decrease in the protease production. This could be because of the denaturing of the protein structure (Niyonzima and More 2015b). Pouryafar et al. (2015) observed the highest protease production from B. licheniformis at an optimum incubation time of 48 h. The optimal values of protease production from B. mojavensis A21 are 67.68 h and 30.26 °C (Mhamdi et al. 2014). The incubation time and temperature required for maximum production of the enzyme vary among Bacillus species and depend upon environmental and cultural conditions (Singh and Bajaj 2015).
Figure 1b shows that the increase in incubation time with a corresponding increase in pH resulted in an increase in protease activity. In the model, protease production was found to be significantly affected by pH (p < 0.0001). An increase in protease activity was observed with an increase in pH from 7.5–8.5. In previous studies, pH 9.0 was the optimum for the production of protease from Bacillus subtilis K-1 and Bacillus sp. SKK11 (Singh and Bajaj 2016; Govarthanan et al. 2014), while Bacillus sp. NB34 was optimum at pH 10 for protease production (Kumar et al. 2014). Pawar and Rathod (2018) found that the production of protease from Bacillus licheniformis increased in range of pH 8–9.5. Moreover, the interaction between pH and incubation time (A*D) was significant (p < 0.0001) for interactive terms (Table 5).
The lower or higher moisture level in SSF affects microbial activity. High moisture level prevents oxygen penetration and leads to decreased substrate porosity. However, low moisture level may cause poor microbial growth (Pandey et al. 1999). Figure 1c, d, f show that protease activity was affected by altering pH, time, and temperature with initial moisture levels. For enzyme production, the effect of initial moisture level was limited (p > 0.05) compared with pH, incubation temperature and time. The effect of temperature and pH on the protease activity is presented in Fig. 1e and there is an increase in protease activity at approximately pH 8.5 and incubation temperature between 35 and 42 °C. The interaction between pH and temperature (A*B) was found to be significant (p < 0.0001) as seen in Table 5. Based on the results, the model predicted the maximum protease activity of 2808.13 IU/gds under the optimal condition (pH 8.8, 29.06 °C, 70.7 h and initial moisture level of 78%). The predicted activity was close to the experimental protease activity of 2819.15 IU/gds.
Figure 2 indicates the response surface plots for values of the variable, while other variables tested are fixed at the zero (0) level for analysis of cellulase production except incubation time (+ 1). In this work, as shown in Fig. 2a, cellulase activity increases at 42 °C and 72 h. As the incubation temperature increases, the cellulase activity also increased and reached a maximum at the optimum temperature. After the optimal temperature, a decrease in cellulase activity was observed. Also, pH and temperature have synergistic effects on cellulase production (Fig. 2c). An increase in cellulase production was observed with an increase in the range of pH 7.5–9 and at 35–42 °C. Accordingly, the combined effects of incubation time and initial pH on cellulase production are displayed in Fig. 2f. Figure 2b, d, e show that cellulase activity is affected by altering pH, time, and temperature (p < 0.0001), whereas there is no significant effect of initial moisture level (p > 0.05). As a result, the optimal condition for maximum cellulase production was predicted to be 39.5 °C, pH 8.5, 60.3% of moisture level and 71.7 h with 26.26 IU/gds of cellulase activity, which was close to experimental cellulase activity of 25.45 IU/gds.
There is a strong influence of initial pH, incubation temperature and time on enzyme production. The alkalophilic Bacillus strains produce alkaline cellulase in the pH range of 8.5–9.5, used as a detergent constituent (Sadhu et al. 2013). The maximum cellulase production from Bacillus spp. was found at 45 °C (Verma et al. 2012). The previous study reported that cellulase production diminished at pH 4.5 and pH 9.5. The maximum cellulase production was obtained at a temperature of 42 °C (Shajahan et al. 2017). Also, detergent compatible enzymes are mostly reported to be secreted in the temperature range of 30–50 °C (Niyonzima and More 2015a). These results were consistent with our findings. In this work, both enzymes were successfully co-produced from B. subtilis M-11 in SSF under optimal conditions determined by BBD. Also, the fermentation conditions, including incubation time, temperature and initial pH, were found to have a significant effect on the productions of these enzymes.
Lastly, the statistical model developed for the coproduction of cellulose and protease was validated. For this, the predicted set of optimized conditions which gave maximum activities for both enzymes was obtained using the point prediction feature of the Design Expert software and the model validity was tested. The optimum coproduction conditions for both the enzymes were predicted to be pH 8.6, 36.2 °C, 72.6% moisture level and 72 h. The maximal protease and cellulase activities were 2501.46 IU/gds and 25.06 IU/gds, respectively. These predictions were validated by experiments in which 2560.13 IU/gds of protease activity and 24.33 IU/gds of cellulase activity were obtained. All experimental activity values corresponded well with the predicted values. This implies confirmation of the validity of the statistical model. These results indicate that the BBD is an effective and reliable tool for maximizing protease and cellulase coproduction.
Effects of pH and temperature on protease and cellulase activities
The pH and the temperature stability of the enzyme are very important parameters for their industrial use. For use in detergents, enzymes are anticipated to remain stable at alkaline pH values, at relatively high temperatures such as 40–50 °C, and with detergent constituents (Sellami-Kamoun et al. 2008). In this study, the effect of pH on enzyme activities was performed in the pH range between 6.0 and 10.0. Protease and cellulase showed optimum activity at pH 8.5 and 8, respectively. Similarly, the cellulases from Bacilullus spp. were found to show optimum activity at pH 7 and 8.0 (Lee et al. 2008; Ladeira et al. 2015). The proteases used in detergent formulations are reported to show maximum activity at pH values of 8.0–10.0 (Sellami-Kamoun et al. 2008).
As seen in Fig. 3a, b, the effect of the temperature on the activity and stability of enzymes was evaluated. Both enzymes retained 100% activity at 40 °C while 83.28% of the cellulase activity and 62.3% of protease activity was retained at 60 °C for 1 h (Fig. 3a). Cellulases from Bacillus species are thermostable in the temperature range of 0–50 °C (Shahid et al. 2016).
In this work, thermostability of the enzymes at 40 °C was evaluated; the half-life (t1/2) times of protease and cellulase were 7 and 10 days, respectively (Fig. 3b). The results show that the enzymes were able to maintain their activities for a long time, although the enzyme activities decreased at prolonged time. Furthermore, the cellulose produced was resistant to proteolysis, as seen in Fig. 3b. The stability of cellulase may be affected by proteolytic activity of protease when enzymes are used together in industrial process. Therefore, cellulases should be resistant to proteolysis. It has been suggested that coproduced enzymes have better resistance to each other (Niyonzima and More 2015a). The results indicate that the enzymes were highly active and stable over wide ranges of temperature (25–60 °C) and pH (7.0–9.5).
Detergent compatibility
Enzymes are utilized in a very small amount in detergent formulations to enhance the cleaning ability of detergents. A good detergent enzyme must be stable and compatible with all detergent constituents such as oxidizing agents, surfactants and other additives (Niyonzima and More 2015a).
In this study, the effect of the surfactants and the oxidizing agent on stability of the enzyme was evaluated after 1 h of incubation at 40 °C (Table 6). The protease activity increased in the presence of various surfactants and oxidizing agents. Similarly, protease activity was reported to be increased in the presence of surfactants in previous studies (Beg and Gupta 2003; Rekik et al. 2019). However, there are studies reporting a decrease in activity of protease in the presence of various surfactants (Ghorbel et al. 2014; Lam et al. 2018). Also, we found that the protease activity was retained: 64.91% and 68.63% of its initial activity in the presence of 0.5% and 0.25% SDS, respectively. The stability towards SDS is favored for usage of enzymes in detergent (Hmidet et al. 2009).
The bacterial cellulases in detergent formulations must be stable in the presence of surfactants (Niyonzima 2019). We found that the cellulase activity increased in the presence of H2O2, Tween, 40, Tween 80 and SDS. Similar stimulation was reported in the presence of SDS, H2O2, Tween 20, 60, and 80 for cellulases of Bacillus species (Gaur and Tiwari 2015). On the contrary, Sadhu et al. (2013) found that the cellulase activity was reduced by SDS and Tween-80. As a result, in addition to their stability in the presence of other surfactants, cellulase activity increased with SDS in the study. Our findings are very important because the loss of enzymes activity with SDS is a limiting factor in their utilization in detergents (Imran et al. 2018).
In this study, both the enzymes were relatively stable after 1 h of incubation at 40 °C in the presence of five commercial detergent tested (Table 6). It was observed that the protease in the presence of detergents showed very good stability, ranging from 84 to 98%. Different results were reported by other studies. For example, protease from B. licheniformis NH1 strain retained 83–100% of its initial activity in the presence of all the tested commercial detergents after incubation for 1 h at 40 °C (Hmidet et al. 2009). In another study, the activity of protease from Virgibacillus sp. CD6 strain ranged from 63 to 165% in the presence of commercial detergents (Lam et al. 2018).
On the other hand, cellulase was less stable than protease in the presence of all detergents tested, except for Tursil®. Highest detergent compatibility for cellulase was attained with Tursil® 90.85% then, Alo® 81.71%, Persil® 79.91%, Omo® 78.57%, Peros® 77.5%. The findings are in accordance with the results of other studies (Sadhu et al. 2013, Imran et al. 2018). In this study, the cellulase and protease activities displayed significant stability and compatibility with the commercial detergents tested. The property of these enzymes may make them a good candidate for laundry detergent formulations.
In conclusion, this study can function to develop a more economical industrial process for the production of these enzymes. Also, both of the enzymes coproduced from B. subtilis M-11 showed excellent thermostabity, surfactant and detergent compability. Therefore, these detergent-compatible enzymes, which can be coproduced on an inexpensive substrate, may be cost-effective suitable candidates to meet the detergent enzyme requirement.
Change history
20 June 2020
The authors found a mistake in Figs. 1 and 2 of this published paper.
References
Beg QK, Gupta R (2003) Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb Technol 32(2):294–304
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay-casein as a substrate. J Vis Exp 19:899. https://doi.org/10.3791/899
Garai D, Kumar VA (2013) Box–Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L.. Ind Crops Prod 44:352–363
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15(19):1–12
George N, Sondhi S, Soni SK, Gupta N (2014) Lime and sulphide-free dehairing of animal skin using collagenase-free alkaline protease from Vibrio metschnikovii NG155. Indian J Microbiol 54(2):139–142
Ghorbel S, Kammoun M, Soltana H, Nasri M, Hmidet N (2014) Streptomyces flavogriseus HS1: isolation and characterization of extracellular proteases and their compatibility with laundry detergents. BioMed Res Int. https://doi.org/10.1155/2014/345980
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Govarthanan M, Park SH, Kim JW, Lee KJ, Cho M, Kamala-Kannan S, Oh BT (2014) Statistical optimization of alkaline protease production from brackish environment Bacillus sp. SKK11 by SSF using horse gram husk. Prep Biochem Biotechnol 44(2):119–131
Hmidet N, Ali NEH, Haddar A, Kanoun S, Alya SK, Nasri M (2009) Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: characterization and potential application as detergent additive. Biochem Eng J 47(1–3):71–79
Imran M, Zahid Anwar M, Zafar M, Ali A, Arif M (2018) Production and characterization of commercial cellulase produced through Aspergillus niger IMMIS1 after screening fungal species. Pak J Bot 50(4):1563–1570
Irfan M, Mushtaq Q, Tabssum F, Shakir HA, Qazi JI (2017) Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express 7(29):1–9
Kalaiyarasi M, Vijayaraghavan P, Raj SRF, Vincent SGP (2017) Statistical approach for the production of protease and cellulase from Bacillus cereus KA3. Bioprocess Eng 1(4):93–103
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25(1–2):1–30
Kumar D, Chauhan PS, Puri N, Gupta N (2014) Production of alkaline thermostable protease by immobilized cells of alkalophilic Bacillus sp. NB 34. Int J Curr Microbiol Appl Sci 3(10):1063–1080
Ladeira SA, Cruz E, Delatorre AB, Barbosa JB, Leal Martins ML (2015) Cellulase production by thermophilic Bacillus sp.: SMIA-2 and its detergent compatibility. Electron J Biotechnol 18(2):110–115
Lam MQ, Mut NNN, Thevarajoo S, Chen SJ, Selvaratnam C (2018) Characterization of detergent compatible protease from halophilic Virgibacillus sp. CD6. 3 Biotech 8(2):104
Lee YJ, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH et al (2008) Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Biores Technol 99(2):378–386
Li W, Zhao LC, Wang Z, Zheng YN, Liang J, Wang H (2012) Response surface methodology to optimize enzymatic preparation of deapio-platycodin D and platycodin D from radix platycodi. Int J Mol Sci 13(4):4089–4100
Mhamdi S, Haddar A, Mnif IH, Frikha F, Nasri M, Kamoun AS (2014) Optimization of protease production by Bacillus mojavensis A21 on chickpea and faba bean. Adv Biosci Biotechnol 5(14):1049–1059
Niyonzima FN (2019) Detergent-compatible bacterial cellulases. J Basic Microbiol 59(2):134–147
Niyonzima FN, More SS (2015a) Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J Basic Microbiol 55(10):1149–1158
Niyonzima FN, More S (2015b) Detergent-compatible proteases: microbial production, properties, and stain removal analysis. Prep Biochem Biotechnol 45(3):233–258
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77(1):149–162
Pawar SV, Rathod VK (2018) Optimization of novel and greener approach for the coproduction of uricase and alkaline protease in Bacillus licheniformis by Box–Behnken model. Prep Biochem Biotechnol 48(1):24–33
Petlamul W, Boukaew S (2019) Optimisation and stabilisation of cellulase and xylanase production by Beauveria bassiana. Environ Asia 12(1):11–19
Pouryafar F, Najafpour GD, Noshadi N, Jahanshahi M (2015) Thermostable alkaline protease production via solid state fermentation in a tray bioreactor using Bacillus licheniformis ATCC 21424. Int J Environ Res 9(4):1127–1134
Prakasham RS, Rao CS, Sarma PN (2006) Green gram husk-an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour Technol 97(13):1449–1454
Rekik H, Jaouadi NZ, Gargouri F, Bejar W, Frikha F, Jmal N (2019) Production, purification and biochemical characterization of a novel detergent-stable serine alkaline protease from Bacillus safensis strain RH12. Int J Biol Macromol 121:1227–1239
Sadhu S, Saha P, Sen SK, Mayilraj S, Maiti TK (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from Bacillus strain isolated from cow dung. Springer Plus 2:10–20
Sahin S, Ozmen I, Kir E (2015) Purification immobilization and characterization of protease from local Bacillus subtilis M-11. Asia Pac J Chem Eng 10(2):241–247
Sellami-Kamoun A, Haddar A, Ali NEH, Ghorbel-Frikha B, Kanoun S, Nasri M (2008) Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol Res 163(3):299–306
Shahid ZH, Irfan M, Nadeem M, Syed Q, Qazi JI (2016) Production purification and characterization of carboxymethyl cellulase from novel strain Bacillus megaterium. Environ Prog Sustain Energy 35:1741–1749
Shajahan S, Moorthy IG, Sivakumar N, Selvakumar G (2017) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring Maharashtra. India J King Saud Univ Sci 29(3):302–310
Singh S, Bajaj BK (2015) Medium optimization for enhanced production of protease with industrially desirable attributes from Bacillus subtilis K-1. Chem Eng Commun 202(8):1051–1060
Singh S, Bajaj BK (2016) Bioprocess optimization for production of thermoalkali-stable protease from Bacillus subtilis K-1 under solid-state fermentation. Prep Biochem Biotechnol 46(7):717–724
Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, de Souza Vandenberghe LP (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Inn 1(1):52–71
Sreena CP, Sebastian D (2018) Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J Genet Eng Biotechnol 16(1):9–16
Verma V, Verma A, Kushwaha A (2012) Isolation and production of cellulase enzyme from bacteria isolated from agricultural fields in district Hardoi, Uttar Pradesh, India. Adv Appl Sci Res 3:171–174
Zhang Y, Zhang X, Qi W, Xu J, Yuan Z, Wang Z (2018) ANN and RSM based optimization of cellulase production by Hypocrea sp. Z28 by submerged fermentation. Cell Chem Technol 52(3–4):259–264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özbek Yazıcı, S., Özmen, I. Optimization for coproduction of protease and cellulase from Bacillus subtilis M-11 by the Box–Behnken design and their detergent compatibility. Braz. J. Chem. Eng. 37, 49–59 (2020). https://doi.org/10.1007/s43153-020-00025-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00025-x