Abstract
This study evaluated Streptomyces rochei strain NAM-19 solid-state fermentation of agricultural wastes to produce alkaline protease. Alkaline protease production increased with flaxseed, rice bran, and cheese whey fermentation reaching 147 U/mL at 48 h. Statistical optimization of alkaline protease production was performed using the central composite design (CDD). Results of CDD and the optimization plot showed that 4.59 g/L flaxseed, 4.31 g/L rice bran, 4.17 mL cheese whey, and a vegetative inoculum size of 7.0% increased alkaline protease production by 27.2% reaching 186 U/mL. Using the 20–70% ammonium sulfate fractionation method, the optimally produced enzyme was partially purified to fivefold. The partially purified alkaline protease was then covalently immobilized on a biopolymer carrier, glutaraldehyde-polyethylene-imine-κ-carrageenan (GA-PEI-Carr), with 90% immobilization efficiency. Characterizations revealed that immobilization improved thermostability, reusability, optimum temperature, and sensitivity towards metal ions of the free enzyme. The optimal temperature for free and immobilized enzymes was 40 and 50 °C, respectively. Both enzymes had the same optimum pH of 10. Immobilization increased Km from 19.73 to 26.52 mM and Vmax from 56.7 to 62.5 mmol min−1L−1. The immobilized enzyme retained 35% of its initial activity at 70 °C, while the free enzyme retained only 5%. The immobilized enzyme kept 80% of its initial activity at the 20th cycle. After 7 weeks of storage, the free enzyme lost all its initial activity, whereas the immobilized enzyme retained 50%. The free and immobilized enzymes were able to hydrolyze gelatin, and azo-casein demonstrating different relative activity, 85, 80, 90 and 95%, respectively, compared to casein (100%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide market for industrial enzymes was estimated at $5.7 billion in 2020 and is expected to rise 6.5% from 2021 to 2028 (VMR 2019). The market’s growth is driven by the rising demand for industrial enzymes in diverse applications, including food processing and detergent production (Nouri et al. 2024). Among the most important industrial enzymes that hydrolyze protein substances are microbial proteases (Tunga et al. 2003). Proteases are vital in cellular metabolism and have garnered attention in the industrial sector (Gupta et al. 2002). Plants and microbes produce alkaline proteases (EC 3.4.21.14), which have an optimal pH of 7–11. Bacteria and fungi produce extracellular and intracellular proteases (Zhou et al. 2009; Rizzello et al. 2007). Intracellular proteases are crucial to metabolism and cellular functions; however, extracellular proteases could hydrolyze waste substrates more efficiently (Kumar and Takagi 1999). Various cost-effective methods have been adapted to produce microbial proteases using solid-state fermentation (SSF) (Gervais and Molin 2003; El Salamony et al. 2024). Its advantages include simple fermentation equipment, high volumetric productivity, lower production costs, superior physio-chemical qualities, and less pollution. It uses less energy and produces less effluent (Adeoye et al. 2022; Yafetto 2022). This potential technique is utilized in the production of bio-products, including traditional meals, microbial cells, enzymes, and metabolites from renewable resources. This cost-effective method is popular in Africa (Bálint et al. 2005). The use of agro-industrial waste as SSF substrates has grown in recent decades (El Salamony et al. 2024). Rice and wheat bran, agro-industrial byproduct, are employed in SSF as major substrates and could be utilized as major substrates to saves energy in the dry process. It distributes moisture evenly so bacteria can grow and produce protease enzymes. Many waste substrates enhance the growth of biotechnologically-important microbial producers (Matrawy et al. 2024; Kumari Chitturi and Lakshmi 2016; Espoui et al. 2022). They also improve SSF bioprocess protease production due to its availability and inexpensive cost (Limkar et al. 2019). The typical “one factor at a time” bioprocess design ignores variable interactions and fails to calculate the cumulative influence of factors on enzyme production. Response surface methodology (RSM) is a statistical approach for optimizing processes when numerous variables influence the response of interest (Baş and Boyacı 2007). However, low reusability, poor thermal/long-term stability, and high cost hinder industrial enzyme applications (Karami et al. 2022; Badoei-Dalfard et al. 2023). To improve these problems, the immobilization technique can be a cost-effective and environmentally friendly method and improves enzyme performance under severe conditions (Aggarwal et al. 2021; Zhang et al. 2021). Enzyme immobilization enhances enzyme efficiency in many biotechnological applications (Mylkie et al. 2021). Also, immobilization enables biocatalyst recovery, salability, process development, and cost reduction through diverse reactor designs (Sadaqat et al. 2022). However, enzyme-carrier interaction is required for immobilization efficiency. The carrier type significantly impacts the characteristics of the immobilized enzyme (Nunes et al. 2021). Generally, immobilization carriers should be biocompatible, stable, non-toxic, and eco-friendly. Carriers could be inorganic, hybrid, polymer, or metal–organic (Suo et al. 2020). Biomaterials, including κ-carrageenan, alginate, chitosan, chitin, and their derivatives, are widely investigated due to their abundance, biocompatibility, non-toxicity, and versatility in surface functional groups (Alnoch et al. 2020). κ-carrageenan is extensively utilized for enzyme immobilization and has numerous applications in biocatalysis, biosensor synthesis, decontamination, and energy storage (Awad et al. 2020).
The current study aims to screen different commercial substrates to produce alkaline protease and then optimize the production of the enzyme simultaneously in a single step by statistically optimizing key parameters such as substrate concentrations and inoculum size using central composite design (CCD). Subsequently, immobilizing the partially purified enzyme on a biopolymer carrier, called κ-Carrageenan. Both the free and immobilized alkaline proteases were analyzed for their kinetic characteristics as well as pH and thermal stability profiles. We assessed the reusability and shelf-stability of immobilized protease through consecutive experiments.
Materials and methods
Materials
Soluble casein, azocasein, gelatin, and folin reagent were purchased from Fluka Chemical Co. κ-Carrageenan (Gelcarin GP 812®) was obtained from Phyto Technology Laboratories®, USA. Polyethylenen-imine was acquired from Sigma, Germany. The other chemicals were of analytical grade.
Microorganisms and culture media
Streptomyces rochei strain NAM-19 was previously isolated from a soil sample collected from El-Giza Governorate, Giza, Egypt (Elsayed and Ahmed Abdelwahed 2020). The strain was maintained in (5% (v/v)) glycerol at −80 °C. The strain was regularly cultivated on 1% (w/v) casein-agar plates, incubated at 32 °C before use as an inoculum for protease production.
Solid state fermentation (SSF)
The one-variable at-a-time method was used to conduct an initial screening of the most essential carbon and nitrogen sources that would result in the highest protease production. A variety of substrates, including rice straw, rice bran, corn starch, oat flakes, cheese whey, and flaxseeds, were bought from a local market. We washed these substrates, except for cheese whey, with tap water and then distilled water to remove any surface dust particles. The blanching process involved submerging these substrates in hot water (75–80 °C) for 20 min, followed by drying them in an oven at 45 °C. The desiccated substance was pulverized using a mixer grinder, followed by sterilization at a temperature of 121 °C and a pressure of 15 lbs for 15 min. It was subsequently stored at a temperature of 4 °C until it was ready for further utilization. To evaluate the different substrates for Strep. AP production in SSF conditions, we started by taking 5.0 g of each substrate in a 250-mL Erlenmeyer flask. Then, we added a predetermined volume of 1 mL of 50 mM potassium phosphate buffer with a pH of 8.5. The mixture was thoroughly mixed and sterilized by autoclaving at 121 °C and 15 lbs of pressure for 15 min. The flasks were cooled to ambient temperature and thereafter inoculated with 2.0 mL of a 48-h-old Streptomyces culture (OD600 = 0.49–0.51) under aseptic conditions. The flasks were then incubated at a temperature of 32 °C. Flasks that were not inoculated were used as controls (Liu et al. 2023). Additional media screening (Table 1) was conducted for varied durations (24, 36, 48, 72, and 96 h) (Lin et al. 2024).
Recovery of the enzymatic extract
To isolate the protease enzyme produced under SSF, a specific amount of the fermented matter was combined with distilled water in a ratio of 1:5 (w/v). The mixture was stirred using a magnetic stirrer for 30 min at room temperature, approximately 25 °C. The slurry was thereafter passed through cheesecloth and then subjected to centrifugation at a force of 10,000 × g due to gravity for 10 min at a temperature of 4 °C to eliminate the substances that are not soluble (Mendoza-Cal et al. 2010).
Determination of Strep. AP activity
The activity of alkaline protease produced by S. rochei strain NAM-19 (Strep. AP) was assayed based on the method (Tsuchida et al. 1986). The substrate casein, with a concentration of 1% (w/v), is dissolved in a Tris–glycine buffer with a pH of 10 and a concentration of 0.05 M. A 0.5-mL solution of casein was mixed with an equal volume of enzyme solution that had been appropriately diluted. The mixture was then placed in an incubator at a temperature of 40 °C. The process was stopped after 10 min by adding 1 mL of 10% trichloroacetic acid. The reaction mixture was subjected to centrifugation, and then 5 mL of a 0.5 M Na2CO3 solution and 1 mL of a Folin Ciocalteau reagent that had been diluted by a factor of two were added to the supernatant. The color that formed after 30 min was measured at a wavelength of 660 nm using a UV–visible spectrophotometer (UV–vis Shimadzu). One unit of enzyme activity is defined as the amount of the enzyme that releases 1 μg of tyrosine/mL/min under the over mentioned assay conditions.
Optimization of Strep. AP using CCD statistical design
Variables selected through one-factor-at-a-time (ofat) were subjected to CCD at its five coded levels (− 2, − 1, 0, + 1, and + 2) using the statistical software package MINITAB (Release 17, PA, USA) and the average Strep. AP enzyme activity was used to calculate the response (Table 2). The impact of the parameters and their interaction terms on the response variable has been examined by conducting significance tests. Additionally, an analysis of variance (ANOVA) has been performed on the response variable to assess the adequacy of the model. A non-linear regression analysis was conducted using the data obtained according to CCD planning for response. This analysis yielded a second-order polynomial equation that incorporates the effects of linear, square, and interaction variables, which are process factors, on the response. The functional relationship between the response variable, Y, and the input variables is described by a polynomial quadratic equation (Eq. 1) to account for the non-linear calculation:
where Y is the predicted value of Strep. AP activity, β0, βi, βii, and βij represent the constant process effect in total, the linear, quadratic effect of Xi, and the interaction effect between Xi and Xj which are the coded independent variables, respectively, to produce Strep. AP enzyme. The detailed analysis of the effect of parameters and their interactions on the response was also done through surface plots using STATISTICA software version 8 through drawing 3D surface plots. A probability level of P < 0.05 was considered statistically significant. Later, an experiment was run using the optimum values for variables given by response optimization to confirm the predicted value of Strep. AP enzyme concentration. The main effect was calculated as the difference between the average of measurements made at the high setting and the average of measurements observed at the low setting for each variable (Morilla et al. 2023).
Protein determination
Protein content was determined according to the method (Lowry 1951) using crystalline bovine serum albumin as a standard.
Ammonium sulfate precipitation
The culture filtrate solution was partially purified by ammonium sulfate, which was added at different saturation percentages (0–20%, 20–70%, and 70–100%) then kept at 4 °C overnight. The precipitates were then collected by centrifugation (Lakshmi et al. 2018). This partially purified enzyme was used for the preparation of the immobilized enzyme.
Immobilization of Strep. AP
κ-Carrageenan (Carr) was obtained from Phyto Technology Laboratories®, USA. An 2% aqueous Carr solution was prepared via stirring in a 70 °C water bath. This solution was then dripped via a thin needle syringe onto a 3% KCl solution to obtain the Carr beads. The beads were kept overnight in the KCl solution (Wahba 2023b); afterwards, they were washed and immersed in a 3% (w/w) polyethylene-imine (PEI) solution at pH 8.65 for 2 h (Wahba 2020a). The beads were then meticulously washed and immersed in 3% glutaraldehyde (GA) for 1 h. Finally, the glutaraldehyde-polyethylene-imine- κ-carrageenan (GA-PEI-Carr) beads were washed and kept in distilled water.
Efficiency of immobilization
The efficiency of immobilization (EF) was calculated using the following equation:
where \(Ai\) is the specific activity of the immobilized enzyme = specific activity of the free enzyme (\(Af\)) − specific activity of the unbound enzyme.
Immobilization yield was calculated as reported by Ahmed et al. (2018) using the following equation:
where E0 = enzyme units taken for immobilization and Es = enzyme units lost in the supernatant.
Characterization of immobilized and free Strep. AP
Influence of immobilization on the physicochemical properties of Strep. AP was evaluated as follows:
Effect of temperature
The optimal temperature for evaluating the free and immobilized Strep. AP activity was determined by subjecting the reaction mixture of both enzymes to various temperatures, ranging from 30 to 90 °C, for 10 min (Qamar et al. 2020). The optimum temperature has been taken as 100% activity, and the relative activity at each temperature is expressed as a percentage % activity.
Effect of pH
The optimal pH for both the free and immobilized Strep. AP was determined by incubating the enzyme under optimal conditions for 1 h in 0.5 mL of casein solution with a pH range of 3–12 using different buffers (Qamar et al. 2020). The maximum enzyme activity is shown as 100%, and each pH is expressed relatively as a percentage of the activity.
Thermal stability
To demonstrate the stability of the immobilized enzyme under elevated temperatures, the enzymes were subjected to incubation in the enzyme’s buffer solution for durations of 30, 45, and 60 min at temperatures of 50, 60, and 70 °C, respectively. Subsequently, the enzymes were assessed for their enzymatic activity. The data were standardized to 100% activity. The enzyme activity reaches its maximum level when represented as 100%, and each temperature is expressed relatively as a percentage of the activity (Awad et al. 2020).
Effects of some metals, detergents, and enzyme inhibitors
The free and immobilized Strep. AP enzymes were pre-incubated with one of the following metal ions (1mM): CaCl2, MgCl2, CoCl2, CuSO4, MnCl2, ZnSO4, HgCl2, and KCl for 30 min at room temperature. The residual activity was assayed at optimal conditions (pH 10 and 40 °C) and compared to control (Abdella et al. 2023).
Substrate specificity
Substrate specificity of each free and immobilized Strep. AP was determined using soluble casein, azo-casein, and gelatin dissolved in Tris–glycine buffer pH 10 and using tyrosine as a control (Bakhtiar et al. 2005).
K m and V max
The Lineweaver–Burk plot, also known as the double reciprocal plot, was employed to derive the Michaelis–Menten kinetic models that accurately describe the hydrolysis of soluble casein by both the free and immobilized enzymes (Lineweaver and Burk 1934). The apparent Km and Vmax values of both free and immobilized Strep. AP were calculated by drawing a graph where the reciprocal of the substrate concentration (1/[S]) was plotted against the reciprocal of the reaction velocity (1/[V]).
Reaction time
Both free and immobilized enzymes, in equal quantities, were incubated in the assay mixture at a temperature of 40 °C for 10 min with a pH level of 10. Aliquots have been collected at regular intervals ranging from 5 to 45 min and subjected to analysis to determine the extent of casein hydrolysis (Qamar et al. 2020).
Reusability
To assess the storage durability of the immobilized enzyme, both the free and immobilized enzymes were stored at room temperature (about 25 °C) in a Tris–glycine buffer with a pH of 10. Subsequently, at consistent intervals, samples were analyzed to quantify the enzyme activity using the previously outlined procedure. The procedure was performed 20 times, and the original activity was 100%. The relative activity was expressed as a percentage of the starting operational activity (Abdella et al. 2023).
Shelf stability
The stability of the enzyme, both in its free form and when immobilized, was investigated for a duration of 6 weeks at a temperature of 4 °C. Every week, a sample of either the free or immobilized enzyme has been collected and tested to measure its enzymatic activity. The initial operational activity was considered to be 100% relative (Awad et al. 2020).
Statistical analysis
Each experiment was run three times, and the mean ± standard deviations produced by MS Excel are used to show the results. The optimization of the fermentation experiments were designed and analyzed using MINITAB (Release 17, PA, USA) and STATISTICA software version 8.
Results and discussion
Optimization of Strep. AP production
S. rochei strain NAM-19 was previously isolated from a soil sample collected from El-Giza Governorate, Giza, Egypt (Elsayed and Ahmed Abdelwahed 2020). It was additionally determined at a molecular level, exhibiting significant branching in both the substrate mycelia and aerial hyphae. These hyphae then developed into long, straight chains of spores with smooth surfaces, known as recti-flexibles. On starch nitrate agar media, the color of the colony ranges from white to gray. The partial nucleotide sequence of the strain’s 16S rRNA gene was matched to NCBI nucleotide databases using BLAST software. The results showed 99.31% to 99.41% similarity with numerous Streptomyces strains. The strain was deposited in the GenBank database with the accession number MN630193.
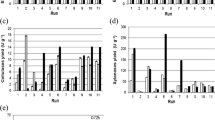
An initial investigation was conducted to screen a range of agro-based (wheat bran, rice bran, flaxseed grains, rice straw, corn starch, oat flakes) and industrial waste (cheese whey) residues. The residues can be used in SSF to support the growth of microbial cells and provide nutrients (Souza et al. 2015). Fig. 1 displays various protease activities depending on the substrate type. Media containing rice bran, cheese whey, and flaxseed exhibited higher protease activity at 78, 23.4, and 62.4 U/mL, respectively, compared to other substrates (Fig. 1). This modification highlights the significance of media composition in attaining high enzyme production. Rice bran, a by-product of the rice milling industry, comprises 15–20% extremely nutritious proteins and is a significant byproduct of rice processing, constituting 10% of the whole rice grain. Lin et al. (2024) also utilized black rice bran as an economical substrate for lactic acid bacteria fermentation (Tan et al. 2020; Lin et al. 2024). The researchers fermented rice bran to produce alkaline protease from Bacillus licheniformis NRRL 14209 (Limkar et al. 2019). Furthermore, the utilization of cheese whey as a cost-effective substrate was reported (Roncal et al. 2023). The production of cheese results in significant quantities of whey, a portion of which is discarded as waste, leading to significant environmental challenges. Whey is the aqueous component of milk that is extracted from cheese curds following the enzymatic (or acidic) coagulation of casein proteins. The composition of whey varies depending on the origin of the milk and the manufacturing methodology used. The primary constituent is water, comprising around 93–95% (w/w). The dry matter component consists of approximately 66–77% (w/w) lactose, 8–15% proteins, and 7–15% minerals (De Wit 2001; Panesar et al. 2007; Tsermoula et al. 2021). Additionally, it includes small amounts of non-protein nitrogen, such as amino acids, as well as vitamins and trace minerals. The concentrations of the two primary constituents of whey, lactose, and proteins are 46–52 g/L and 6–10 g/L, respectively (Buchanan et al. 2023). Whey is a significant resource that can be effectively and sustainably utilized, with fermentation methods being a viable choice (Roncal et al. 2023). The use of whey as a substrate for fermentation presents a possibility for producing beneficial products. Fresh whey was used as a substrate to produce alkaline proteases. Dias et al. produced alkaline protease from B. subtilis ATCC 6633, and Bacillus sp. UFLA 817CF by fermenting nutrient broth with cheese whey powder (Dias et al. 2008). Additionally, the medium supplemented with 50% whey, 1% skim milk, 10% NaCl, and 1% CaCl2 achieved the highest level of protease production. The fermentation was carried out at a pH of 7.0 and a temperature of 37 °C, employing B. thuringiensis (El-Gayar et al. 2020).
Effect of incubation time on screening of substrates
Most enzymes are synthesized during the logarithmic phase of cell proliferation, serving as primary metabolites. On the other hand, the stationary phase of cell growth leads to the production of secondary metabolites (Hesketh et al. 2002). To maximize the production of Strep. AP enzyme, the growth phase was optimized by harvesting the four-fermentation media at regular 24-h intervals and assessing them for enzyme production. Figure 2 shows that the production of Strep. AP reached its highest level after 48 h of incubation for media No. 2 and 4, with values of 147 and 139 U/mL, respectively. Other studies have investigated the impact of the fermentation process on the functional properties of flaxseeds, and its application in food products (Lorenc et al. 2022; Ye et al. 2022). Marambe and Wanasundara found that flaxseed contains approximately 22% protein which consists of nutritionally balanced levels of amino acids, indicating its potential as a protein source. Flaxseed protein contains high levels of branched-chain amino acids such as valine and leucine, as well as aromatic amino acids like tyrosine and phenylalanine (Marambe and Wanasundara 2024). Moreover, it has been shown that flaxseed protein and its hydrolysates offer numerous advantages for health (Ye et al. 2022). Hence, the incubation period of the fermentation process is critical for product accumulation. We observed a gradual decrease in protease production up to 96 h of incubation. Novelli et al. (2016) proposed that protease production correlates with varying fermentation periods among distinct strains. Other studies reported that the optimized incubation time for protease production was 3 days for B. subtilis (Yang et al. 2000) and 4 days for Streptomyces sp. in submerged fermentation (De Azeredo et al. 2004). Alkaline protease by Pseudomonas aeruginosa in 24 h (Meena et al. 2013).
Based on our evaluation of several waste substrates and incubation duration study, we determined that rice bran, cheese whey, and flaxseed significantly impacted the production of Strep AP. To further enhance this production, we employed CCD optimization.
Central composite design for optimization Strep. AP production
Strep. AP production in CCD varied from 38.766 U/mL to 176.630 U/mL in the thirty-one experiments, with a set incubation duration of 48 h (Table 3). The size of the introduced inoculum into the medium determines the growth of a microbial producer during fermentation. Research demonstrated a decrease in alkaline serine protease production at low inoculum levels but an increase in enzyme yield at higher inoculum levels (Niyonzima and More 2015). Large inoculum sizes cause rapid nutritional depletion, leading to decreased production of alkaline serine protease (Niyonzima and More 2015).
Table 3 provides the coefficients, t-values, and p-values for linear, quadratic, and mixed effects at a 95% significant level. The p-values were utilized to verify the significance of each coefficient, potentially revealing the pattern of interactions between variables. A smaller p-value signifies greater significance in the associated coefficient. The coefficient for the overall effect of the factors showed great significance (p = 0.000) on Strep. AP production. The impact of flaxseed (p = 0.000), rice bran (p = 0.001), cheese whey (p = 0.006), inoculum size (p = 0.009), and the squared interaction effect of flaxseed versus flaxseed (p = 0.000), rice bran versus rice bran (p = 0.001), cheese whey versus cheese whey (p = 0.001), and inoculum size versus inoculum size (p = 0.003) are identified as the most significant factors affecting Strep. AP production. There was no significant impact on Strep. AP production comes from the interactions between flaxseed and rice bran, flaxseed and cheese whey, flaxseed and inoculum size, rice bran and cheese whey, rice bran and inoculum size, and cheese whey and inoculum size. The regression equation reveals an R-squared value of 88.82%, indicating that the model can account for 88.82% of the data. The corrected coefficient, with an R-squared value of 79.03%, similarly demonstrates a high level of relevance in the experiments. The equation resulted in an empirical model that relates the measured response to the independent variables of the experiment:
ANOVA analysis of the regression model indicates that the model is highly significant, as shown by the estimated F-value (F model = 9.08) and probability value (p = 0.000). The linear and quadratic effects of the factors significantly influenced extracellular Strep. AP production. The high F-value of 9.08 suggests that the second-order polynomial model accurately represents the relationship between enzyme activity and the process variables: flaxseeds, rice bran, cheese whey, and inoculum size.
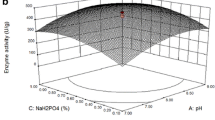
The 3D response surface plot visually represents the regression equation and illustrates the interaction between variables. Each figure illustrates the impact of two parameters on Strep. AP production, with the other two factors being maintained at the intermediate level. The data showed that Strep. AP production would rise with higher concentrations of flaxseed and rice bran, but going beyond the optimum concentration would cause a reversal in this trend (Fig. 3a). Combinations of flaxseed, cheese whey, rice bran, and inoculum size showed similar production effects (Fig. 3b). An increase in substrate concentration and inoculum size was observed to enhance Strep. AP activity, which then decreased after reaching a peak until the optimal point.
Response surface plot of alkaline protease production by Streptomyces rochei strain NAM-19 showing the interactive effects, A flaxseed concentration and whey concentration, B flaxseed concentration and rice bran concentration, C rice bran concentration and inoculum size, D flaxseed concentration and inoculum size, E whey concentration and rice bran concentration, and F whey concentration and inoculum size
An optimization plot was created to determine the optimum variable concentrations that would produce the highest response (enzyme production). The study aimed to determine the maximum Strep. AP production is at the desired value. The optimal condition, determined as the most effective combination of factor settings for obtaining the best response, was identified as 4.59 g of flaxseed, 4.31 g of rice bran, 4.17 mL of cheese whey, and 3.54 mL of inoculum size, resulting in a predicted response of 187.8 U/mL. A confirmatory experiment was conducted (at the optimum conditions), and the outcome of 186 U/mL indicated a significant closeness between the experimental and predicted results, which verified the accuracy and applicability of the developed optimum conditions. Results also revealed that Strep. AP activity demonstrated its authenticity with a desirability score of 1 (Fig. 4).
The optimization plot has many uses, such as getting the predicted response with a higher desirability score, adjusting the cost factor settings to almost optimal properties, studying how response variables change when factor settings are changed, and getting the needed response for a certain factor setting. Morilla et al. (2023) employed the statistical design CCD to produce protease using wheat bran agro-industrial waste in a SSF process. El Salamony et al. (2024) also used Plackett–Burman Design and CCD in a statistical–mathematical model to optimize the production of alkaline protease through the SSF of agriculture waste.
Partial purification of Strep. AP by ammonium sulfate
Optimized Strep. AP was partially purified from the culture filtrate by ammonium sulfate precipitation (20–70%). Data (Table 4) showed an efficient recovery (86.67%) with a high purification fold (5). Pant et al. (2015) precipitated alkaline protease from B. subtilis with ammonium sulphate at 75% (w/v) saturation, and the yield of protein was 2.31 mg/mL, with a total activity of 475.56 U/mL. While semi-purified alkaline protease from B. cereus strain S8 using 50% ammonium sulphate saturation gave 53.4% a recovery yield with a 1.67-purification fold (Lakshmi et al. 2018).
Immobilization efficiency
Strep. AP was immobilized via the GA-PEI-Carr beads with 90 ± 1.2% immobilization efficiency and 82 ± 1.5% immobilization yield. Such increased immobilization yield reflected the abundance of the covalently interactive residues on the GA-PEI-Carr beads (Wahba 2023a). The yield from the GA-PEI-Carr beads was higher than the > 70% immobilization yield that was reached by encapsulated B. brevis alkaline protease in alginate (Qamar et al. 2020). It was also a lot higher than the 35.51% immobilization yield that was reached when B. licheniformis alkaline protease was covalently bound to GA-PEI-(agar-gum tragacanth) disks (Wahba 2022).
Characterization of free and immobilized Strep. AP
Effect of temperature
The free Strep. AP optimal temperature was raised from 40 to 50 °C following its immobilization (Figs. 5 and 6). Furthermore, the immobilized Strep. AP shows 80, 65, and 50% activities at 60, 70, and 80 °C, respectively, whereas only 40, 20, and 5% activities were shown by the free Strep. AP, respectively. Such improved thermal tolerance could reflect the enhanced thermal stability of the immobilized Strep. AP (Wahba 2022). Similarly, Qamar et al. (2020) reported that the alginate-entrapped B. brevis alkaline protease recorded an optimal temperature higher than its free one, and the immobilized protease also demonstrated higher activities at elevated temperatures. Moreover, immobilizing B. licheniformis alkaline protease onto the GA-PEI-(agar-gum tragacanth) discs raised and widened its optimal temperature and improved its thermal tolerance (Wahba 2022).
Effect of pH
Figure 7 reveals that pH value of 10 was the optimal for both the free and immobilized Strep. AP. Nonetheless, the immobilized Strep. AP demonstrated higher activities at both low and high pH values. For instance, it demonstrated 70, 80%, 95%, and 75% activities at pH values of 7, 8, 11, and 12, respectively. However, the free Strep. AP demonstrated 40%, 50%, 85%, and 55% activities, respectively, at the same pH values. It has been seen that immobilizing β-galactosidase (Wahba and Soliman 2018) and alkaline protease (Hu et al. 2015) does not change the optimal pH value and increases the pH tolerance at both low and high pH values. Some studies discussed that immobilization induced only minor alterations in the electronic valence of the enzyme active site, thereby not altering the optimal pH value of the enzyme. However, the bonds created between the enzyme and the immobilizer stabilized its construction and raised its pH tolerance (Hu et al. 2015).
Thermal stability
Figure 8 demonstrates the enhanced thermal stability of the immobilized Strep. AP. After being maintained at 50 °C, 60 °C, and 70 °C, respectively, for 1 h, the immobilized Strep. AP showed 50, 40, and 35% activity, respectively. For thermal stability evaluation of the free Strep. AP (Fig. 7), the free enzyme demonstrated only 33%, 25%, and 5% activities, respectively. Similarly, the thermal stability of alkaline proteases from B. aryabhattai and B. licheniformis was improved when they were trapped in alginate beads and then bonded to GA-PEI-(agar-gum tragacanth) discs (Adetunji and Olaniran 2023; Wahba 2020b). Furthermore, the covalent binding of B. licheniformis alkaline protease to porous chitosan beads also enhanced its thermal stability (Tang et al. 2023). The enhancement in the thermal stability of covalently bound enzymes could be ascribed to their construction rigidification, which was a consequence of their multi-point covalent cross-links with the immobilizer (Rodrigues et al. 2021).
Effect of some metal ions
All the investigated metal ions lowered the free Strep. AP activity, however, Ca2+ slightly raised its activity (Table 5). Metal ions could bind to the proteinaceous enzymes or to other moieties, which are associated with the enzymes, and this might inactivate or activate the enzymes. As an example, the activation of Aspergillus niger endo-glucnase occurred in the presence of Ca2+ ions, while it was deactivated by Fe2+, Cu2+, and Co2+ (de Cassia Pereira et al. 2017). A similar pattern was observed in our studied Strep. AP (Table 5).
Immobilizing Strep. AP via the GA-PEI-Carr beads reduced its sensitivity to inhibition via K+, Mn2+, Zn2+, Co2+, Hg2+, and Fe2+ (Table 5). Such reduced sensitivity could be ascribed to the PEI layer in the GA-PEI-Carr beads (Wahba 2022). PEI would constitute a flexible polymeric bed between the immobilizer, and the enzyme might penetrate this PEI bed (Virgen-Ortiz et al. 2017). PEI presents cationic charges even at an elevated pH of 10 (Borkovec and Koper 1997). These cationic charges might repel the cationic metal ions away from the immobilized Strep. AP. Thus, the concentration of the metal ions between the immobilized Strep. AP would be reduced, and their inhibitory action would also be reduced.
Table 5 also revealed the activity of the immobilized Strep. AP was raised in the presence of Mg2+ and Cu2+, which were inhibitory to its free one. Notably, the influence exerted by certain moieties on the enzyme’s activity could be concentration-dependent. For example, methanol and ethanol were found to activate the β-galactosidase in Aspergillus oryzae, but only up to a certain concentration. Above that concentration, the methanol and ethanol inhibited the activity of the β-galactosidase (Wahba 2023b). This may also be the case with the immobilized Strep GA-PEI-Carr. AP. As argued earlier, the cationic PEI in the GA-PEI-Carr beads would repel and reduce the concentration of the metal ions amidst the immobilized Strep. AP. Reducing the Mg2+ and Cu2+concentrations would convert their influence from inhibitory to stimulatory if such influence was concentration dependent.
Substrate specificity
Various proteinaceous substrates were used to assess the substrate specificity of both free and immobilized proteases. Both enzymes effectively hydrolyzed all the substrates, as shown in Table 6. The free and immobilized enzymes had relative activities of 85 and 90%, respectively, in hydrolyzing soluble azo-casein under similar reaction conditions. This was compared to a control sample of soluble casein, which had an activity of 100%. Similarly, both the free and immobilized Strep. AP were able to hydrolyze gelatin. The free Strep. AP had a relative activity of 80%, while the immobilized Strep. AP had a relative activity of 95% (Table 6). The enzymes, whether free or immobilized, could hydrolyze natural substrates such as casein and gelatin, as well as synthetic substrates like azo-casein. Bakhtiar et al. (2005) found the alkaline protease produced by Nesterenkonia sp. AL20 exhibited high efficacy in hydrolyzing casein and hemoglobin while displaying limited effectiveness with other protein-based substrates. B. brevis SSA1 produces an alkaline protease that exhibits activity over a wide spectrum of substrates, including casein, BSA, gelatin, and hemoglobin (Aftab et al. 2006).
K m and V max
Immobilizing Strep. AP via the GA-PEI-Carr beads raised its Km, from 19.73 to 26.52 mM and its Vmax from 56.7 to 62.5 mmol min−1L−1 (Fig. 9). Similarly, covalently binding laccase and B. safensis protease to the grafted Nylon-6 films and the GA-(PEI-sericin)-agar disks, respectively, raised their Km and Vmax (Fatarella et al. 2014; Gomaa et al. 2022). Moreover, the Km of B. licheniformis protease was raised following its immobilization via the GA-PEI-(agar-gum tragacanth) disks (Wahba 2022). Such a raised Km value was ascribed to the lowered protease-substrate affinity, as immobilization would lay restrictions on the substrate’s diffusion. Furthermore, the modification and rigidification of protease construction, following its covalent binding, could have reduced its proficiency in forming the enzyme–substrate complex, and this would lower its substrate affinity (Wahba 2022).
Reaction time
Figure 10 shows that the initial casein hydrolysis rate was higher in the case of the immobilized Strep. AP. After 10 and 20 min, the immobilized Strep. AP offered 65 and 80% of the activities, whereas the free Strep. AP presented only 10 and 50% of the activities, respectively. These findings are in accordance with the larger Vmax presented by the immobilized Strep. AP. Notably, the Vmax values recorded for the alginate-entrapped B. brevis and B. aryabhattai alkaline proteases were higher than their free one (Adetunji and Olaniran 2023; Qamar et al. 2020).
Reusability
The immobilized Strep. AP retained 80% of its initial activity during the 20th cycle (Fig. 11). Such operational stability was higher than that presented by the B. brevis alkaline protease, which was entrapped in alginate, and the B. licheniformis alkaline proteases, which were covalently bound to porous chitosan beads and to GA-PEI-(agar-gum tragacanth) discs. These proteases presented only 68, 70.3, and 52.85% activities during their 10th, 7th, and 10th catalytic cycles, respectively (Qamar et al. 2020; Wahba 2020b; Tang et al. 2023). Previously, scientists attributed the reduced activity during the recycling of covalently bound enzymes to the accumulation of enzymatic products at the active sites, which impeded substrate diffusion. Moreover, the interaction with the substrates could have distorted the 3-D construction of the enzymes active sites, which would also reduce their activity (Wahba 2023b).
Storage stability
Upon storing the free and the immobilized Strep. AP, the free Strep. AP lost all its activity after 7 weeks of storage, whereas the immobilized Strep. AP maintained 50% of its initial activity (Fig. 12). The immobilized Strep. AP enhanced stability could be ascribed to its ionic binding within the PEI polymeric bed of the GA-PEI-Carr beads. Such a PEI polymeric bed could form a favorable stabilizing nano-environment between the immobilized Strep. AP as it could, for instance, partition the immobilized enzyme away from destabilizing agents, such as cationic metal ions (Virgen-Ortiz et al. 2017). Furthermore, the multi-point covalent cross-links with GA would rigidify the immobilized Strep. AP and this would increase its resistance to destabilizing factors. Similarly illustrated by Rodrigues et al. (2021). Comparing the storage stability of the immobilized Strep. AP to that of B. subtilis alkaline protease, which was adsorbed via silica nanoparticles (El Salamony et al. 2023), both immobilized samples retained 65% activity after 6 weeks of storage, as shown in Fig. 12.
Conclusion
The study investigated the production of alkaline protease via waste-solid-state fermentation by S. rochei strain NAM-19. The study found that flaxseed, rice bran, cheese whey, and inoculum size were the most significant factors in the production process. Using CCD design for production optimization has been shown to be an effective and dependable way of enhancing enzymatic production by optimizing bioprocess variables. This design aided in identifying statistically significant factors and establishing the appropriate concentration of these components in the production medium and increased the enzyme production by 27.2%. The immobilization of optimized alkaline protease was successfully achieved using GA-PEI-Carr beads. The results indicated that the covalent immobilization efficiency was 90 ± 1.2%, while the immobilization yield was 82 ± 1.5%. Alkaline proteases exhibited their highest activity at a pH of 10.0 and a temperature of 50 °C. This resulted in a significant enhancement in the thermal stability and storage stability of the immobilized enzyme compared to the free one. In addition, the immobilized enzyme showed better substrate affinity and catalytic efficiency. It also showed almost the same initial activity during up to 20 reaction cycles, which suggests its potential suitability for industrial use. This study offers numerous benefits, as it successfully addresses waste management and meets the demand of the global industrial enzyme market by developing a cost-effective potential industrial enzyme. Organizations consider waste management to be a delicate subject, and sustainable development is crucial for the preservation of the environment. The increasing demand for industrial enzymes in various applications, such as food processing and detergent production, propels the market’s expansion.
Data availability
Data will be made available on request.
References
Abdella MA, Ahmed SA, Hassan ME (2023) Protease immobilization on a novel activated carrier alginate/dextrose beads: improved stability and catalytic activity via covalent binding. Int J Biol Macromol 230:123–139. https://doi.org/10.1016/j.ijbiomac.2023.123139
Adeoye A, Adegbola G, Lateef A (2022) New insights into valorization of agro-industrial wastes for production of citric acid: effects of mutation and optimization–a review. EJOSAT 2(5):102–137
Adetunji AI, Olaniran AO (2023) Biocatalytic profiling of free and immobilized partially purified alkaline protease from an autochthonous Bacillus aryabhattai Ab15-ES. Reactions 4(2):231–245. https://doi.org/10.3390/reactions4020013
Aftab S, Ahmed S, Saeed S, Rasool SA (2006) Screening, isolation and characterization of alkaline protease producing bacteria from soil. Pak J Biol Sci 9(11):2122–2126. https://doi.org/10.3923/pjbs.2006.2122.2126
Aggarwal S, Chakravarty A, Ikram S (2021) A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int J Biol Macromol 167:962–986. https://doi.org/10.1016/j.ijbiomac.2020.11.052
Ahmed SA, Mostafa FA, Ouis MA (2018) Enhancement stability and catalytic activity of immobilized α-amylase using bioactive phospho-silicate glass as a novel inorganic support. Int J Biol Macromol 112:371–382. https://doi.org/10.1016/j.ijbiomac.2018.01.162
Alnoch RC, Alves dos Santos L, Marques de Almeida J, Krieger N, Mateo C (2020) Recent trends in biomaterials for immobilization of lipases for application in non-conventional media. Catalysts 10(6):697. https://doi.org/10.3390/catal10060697
Awad GEA, Ghanem AF, Abdel Wahab WA, Wahba MI (2020) Functionalized κ-carrageenan/hyperbranched poly(amidoamine)for protease immobilization: thermodynamics and stability studies. Int J Biol Macromol 148:1140–1155. https://doi.org/10.1016/j.ijbiomac.2020.01.122
Badoei-Dalfard A, Saeed M, Karami Z (2023) Protease immobilization on activated chitosan/cellulose acetate electrospun nanofibrous polymers: biochemical characterization and efficient protein waste digestion. Biocatal Biotransfor 41(4):279–298. https://doi.org/10.1080/10242422.2022.2056450
Bakhtiar S, Estiveira RJ, Hatti-Kaul R (2005) Substrate specificity of alkaline protease from alkaliphilic feather-degrading Nesterenkonia sp. AL20. Enzyme Microb Technol 37(5):534–540. https://doi.org/10.1016/j.enzmictec.2005.04.003
Bálint B, Bagi Z, Tóth A, Rákhely G, Perei K, Kovács KL (2005) Utilization of keratin-containing biowaste to produce biohydrogen. Appl Microbiol Biotechnol 69:404–410. https://doi.org/10.1007/s00253-005-1993-3
Baş D, Boyacı İH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78(3):836–845. https://doi.org/10.1016/j.jfoodeng.2005.11.024
Borkovec M, Koper GJ (1997) Proton binding characteristics of branched polyelectrolytes. Macromolecules 30(7):2151–2158. https://doi.org/10.1021/ma961312i
Buchanan D, Martindale W, Romeih E, Hebishy E (2023) Recent advances in whey processing and valorisation: technological and environmental perspectives. Int J Dairy Technol 76(2):291–312
De Azeredo L, Freire D, Soares R, Leite S, Coelho R (2004) Production and partial characterization of thermophilic proteases from Streptomyces sp. isolated from Brazilian cerrado soil. Enzyme Microb Technol 34(3–4):354–358. https://doi.org/10.1016/j.enzmictec.2003.11.015
de Cassia Pereira J, Giese EC, de Souza Moretti MM, dos Santos Gomes AC, Perrone OM, Boscolo M, da Silva R, Gomes E, Martins DAB (2017) Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In: Senturk M (ed) Enzyme inhibitors and activators. In Tech, Croatia, pp 139–164
De Wit J (2001) Lecturer’s handbook on whey and whey products. European Whey Products Association, Brussels
Dias DR, Vilela DM, Silvestre MPC, Schwan RF (2008) Alkaline protease from Bacillus sp. isolated from coffee bean grown on cheese whey. World J Microbiol Biotechnol 24:2027–2034. https://doi.org/10.1007/s11274-008-9706-6
El-Gayar KE, Essa AM, Abada EA (2020) Whey fermentation for protease production using Bacillus thuringiensis Isolated from mangrove rhizosphere soil in Jazan, Saudi Arabia. Pol J Environ Stud 29(3):1–10. https://doi.org/10.15244/pjoes/110583
El Salamony DH, El Gayar DA, El Mahdy AR, Zaghloul TI (2023) Preparation and characterization of silica nanoparticles as an efficient carrier for two bio-detergents based enzymes. J Surfact Deterg 26:577–592. https://doi.org/10.1016/j.biomaterials.2014.12.034
El Salamony DH, Salah Eldin Hassouna M, Zaghloul TI, Moustafa Abdallah H (2024) Valorization of chicken feather waste using recombinant Bacillus subtilis cells by solid-state fermentation for soluble proteins and serine alkaline protease production. Bioresour Technol 393:130110. https://doi.org/10.1016/j.biortech.2023.130110
Elsayed EA, Ahmed Abdelwahed N (2020) Medium optimization by response surface methodology for improved cholesterol oxidase production by a newly isolated streptomyces rochei NAM-19 strain. BioMed Res Int 2020:1870807. https://doi.org/10.1155/2020/1870807
Espoui AH, Larimi SG, Darzi GN (2022) Optimization of protease production process using bran waste using Bacillus licheniformis. Korean J Chem Eng 39(3):674–683
Fatarella E, Spinelli D, Ruzzante M, Pogni R (2014) Nylon 6 film and nanofiber carriers: preparation and laccase immobilization performance. J Mol Catal B Enzym 102:41–47. https://doi.org/10.1016/j.molcatb.2014.01.012
Gervais P, Molin P (2003) The role of water in solid-state fermentation. Biochem Eng J 13(2–3):85–101
Gomaa SK, Zaki RA, Wahba MI, Taleb MA, El-Refai HA, El-Fiky AF, El-Sayed H (2022) Green method for improving performance attributes of wool fibres using immobilized proteolytic thermozyme. 3 Biotech 12(10):254. https://doi.org/10.1007/s13205-022-03323-y
Gupta R, Beg Q, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32. https://doi.org/10.1007/s00253-002-0975-y
Hesketh A, Fink D, Gust B, Rexer HU, Scheel B, Chater K, Wohlleben W, Engels A (2002) The GlnD and GlnK homologues of Streptomyces coelicolor A3 are functionally dissimilar to their nitrogen regulatory system counterparts from enteric bacteria. Mol Microbiol 46:319–330
Hu T-G, Cheng J-H, Zhang B-B, Lou W-Y, Zong M-H (2015) Immobilization of alkaline protease on amino-functionalized magnetic nanoparticles and its efficient use for preparation of oat polypeptides. Ind Eng Chem Res 54(17):4689–4698. https://doi.org/10.1021/ie504691j
Karami Z, Tamri H, Badoei-dalfard A (2022) Immobilization of protease KHB3 onto magnetic metal–organic frameworks and investigation of its biotechnological applications. Catal Lett 152:2256–2269. https://doi.org/10.1007/s10562-021-03808-0
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 17(7):561–594. https://doi.org/10.1016/S0734-9750(99)00027-0
Kumari Chitturi CM, Lakshmi V (2016) Development of semi-solid state fermentation of Keratinase and optimization of process by cheaper and alternative agricultural wastes. Development 4(2):1–4
Lakshmi B, Kumar DM, Hemalatha K (2018) Purification and characterization of alkaline protease with novel properties from Bacillus cereus strain S8. J Genet Eng Biotechnol 16(2):295–304. https://doi.org/10.1016/j.jgeb.2018.05.009
Limkar MB, Pawar SV, Rathod VK (2019) Statistical optimization of xylanase and alkaline protease co-production by Bacillus spp using Box-Behnken design under submerged fermentation using wheat bran as a substrate. Biocatal Agric Biotechnol 17:455–464. https://doi.org/10.1016/j.bcab.2018.12.008
Lin S, Zhang X, Wang J, Li T, Wang L (2024) Effect of lactic acid bacteria fermentation on bioactive components of black rice bran (Oryza sativa L.) with different milling fractions. Food Biosci 58:103684. https://doi.org/10.1016/j.fbio.2024.103684
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666
Liu D, Guo Y, Yolandani MH (2023) Production of value-added peptides from agro-industrial residues by solid-state fermentation with a new thermophilic protease-producing strain. Food Biosci 53:102534. https://doi.org/10.1016/j.fbio.2023.102534
Lorenc F, Jarošová M, Bedrníček J, Smetana P, Bárta J (2022) Structural characterization and functional properties of flaxseed hydrocolloids and their application. Foods 11(15):2304
Lowry OH (1951) Measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marambe HK, Wanasundara JP (2024) Flaxseed (Linum usitatissimum L.) for protein based products. In: Marambe HK, Wanasundara JP (eds) Sustainable protein sources, 2nd edn. Elsevier, pp 339–356
Matrawy AA, Marey HS, Embaby AM (2024) The agro-industrial byproduct wheat bran as an inducer for alkaline protease (ALK-PR23) production by pschyrotolerant Lysinibacillus sphaericus strain AA6 EMCCN3080. Waste Biomass Valori 15:1943–1958
Meena P, Tripathi AD, Srivastava S, Jha A (2013) Utilization of agro-industrial waste (wheat bran) for alkaline protease production by Pseudomonas aeruginosa in SSF using Taguchi (DOE) methodology. Biocatal Agric Biotechnol 2(3):210–216
Mendoza-Cal A, Cuevas-Glory L, Lizama-Uc G, Ortiz-Vázquez E (2010) Naringinase production from filamentous fungi using grapefruit rind in solid state fermentation. Afr J Microbiol Res 4(19):1964–1969
Morilla EA, Stegmann PM, Tubio G (2023) Enzymatic cocktail production by a co-cultivation solid-state fermentation for detergent formulation. Food Bioprod Process 140:110–121. https://doi.org/10.1016/j.fbp.2023.05.001
Mylkie K, Nowak P, Rybczynski P, Ziegler-Borowska M (2021) Polymer-coated magnetite nanoparticles for protein immobilization. Materials 14(2):248
Niyonzima FN, More SS (2015) Purification and characterization of detergent-compatible protease from Aspergillus terreus gr. 3 Biotech 5:61–70
Nouri N, Sadeghi L, Marefat A (2024) Production of alkaline protease by Aspergillus niger in a new combinational paper waste culture medium. J Biosci Bioeng 137(3):173–178. https://doi.org/10.1016/j.jbiosc.2023.12.010
Novelli PK, Barros MM, Fleuri LF (2016) Novel inexpensive fungi proteases: production by solid state fermentation and characterization. Food Chem 198:119–124
Nunes YL, de Menezes FL, de Sousa IG, Cavalcante ALG, Cavalcante FTT, da Silva MK, de Oliveira ALB, Mota GF, da Silva Souza JE, de Aguiar Falcao IR (2021) Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int J Biol Macromol 181:1124–1170
Panesar PS, Kennedy JF, Gandhi DN, Bunko K (2007) Bioutilisation of whey for lactic acid production. Food Chem 105(1):1–14
Pant G, Prakash A, Pavani J, Bera S, Deviram G, Kumar A, Panchpuri M, Prasuna RG (2015) Production, optimization and partial purification of protease from Bacillus subtilis. J Taibah Univ Sci 9(1):50–55
Qamar SA, Asgher M, Bilal M (2020) Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal Lett 150:3572–3583
Rizzello CG, De Angelis M, Di Cagno R, Camarca A, Silano M, Losito I, De Vincenzi M, De Bari MD, Palmisano F, Maurano F (2007) Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: new perspectives for celiac disease. Appl Environ Microbiol 73(14):4499–4507
Rodrigues RC, Berenguer-Murcia Á, Carballares D, Morellon-Sterling R, Fernandez-Lafuente R (2021) Stabilization of enzymes via immobilization: multipoint covalent attachment and other stabilization strategies. Biotechnol Adv 52:107821
Roncal T, Maestro B, Prieto-Fernández S (2023) Fermentative production of 2,3-butanediol from cheese whey by a non-engineered mutant strain of Lactococcus lactis. Bioresour Technol Rep 24:101637. https://doi.org/10.1016/j.biteb.2023.101637
Sadaqat B, Sha C, Dar MA, Dhanavade MJ, Sonawane KD, Mohamed H, Shao W, Song Y (2022) Modifying thermostability and reusability of hyperthermophilic mannanase by immobilization on glutaraldehyde cross-linked chitosan beads. Biomolecules 12(7):999
Souza PMd, Bittencourt MLdA, Caprara CC, Freitas Md, Almeida RPCd, Silveira D, Fonseca YM, Ferreira Filho EX, Pessoa Junior A, Magalhães PO (2015) A biotechnology perspective of fungal proteases. Braz J Microbiol 46:337–346
Suo H, Xu L, Xue Y, Qiu X, Huang H, Hu Y (2020) Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: improvement of catalytic performance. Carbohydr Polym 234:115914. https://doi.org/10.1016/j.carbpol.2020.115914
Tan BL, Norhaizan ME, Tan BL, Norhaizan ME (2020) Production of rice by-products. In: Tan BL, Norhaizan ME, Tan BL, Norhaizan ME (eds) Rice by-products: phytochemicals and food products application, 1st edn. Springer, Cham, pp 13–39. https://doi.org/10.1007/978-3-030-46153-9
Tang Y, Wang P, Zeng H, Rui Z (2023) Construction of porous chitosan macrospheres via dual pore-forming strategy as host for alkaline protease immobilization with high activity and stability. Carbohydr Polym 305:120476. https://doi.org/10.1016/j.carbpol.2022.120476
Tsermoula P, Khakimov B, Nielsen JH, Engelsen SB (2021) WHEY-the waste-stream that became more valuable than the food product. Trends Food Sci Technol 118:230–241. https://doi.org/10.1016/j.tifs.2021.08.025
Tsuchida O, Yamagata Y, Ishizuka T, Arai T, Yamada J-I, Takeuchi M, Ichishima E (1986) An alkaline proteinase of an alkalophilic Bacillus sp. Curr Microbiol 14:7–12. https://doi.org/10.1007/BF01568094
Tunga R, Shrivastava B, Banerjee R (2003) Purification and characterization of a protease from solid state cultures of Aspergillus parasiticus. Process Biochem 38(11):1553–1558. https://doi.org/10.1016/S0032-9592(03)00048-7
Virgen-Ortiz JJ, Dos Santos JC, Berenguer-Murcia Á, Barbosa O, Rodrigues RC, Fernandez-Lafuente R (2017) Polyethylenimine: a very useful ionic polymer in the design of immobilized enzyme biocatalysts. J Mater Chem B 5(36):7461–7490. https://doi.org/10.1039/C7TB01639E
VMR (2019) Global Rebaudioside A (Reb A) market market size, status and forecast to 2028. Verified Market Research, New York, NY, USA
Wahba MI (2020a) Calcium pectinate-agar beads as improved carriers for β-d-galactosidase and their thermodynamics investigation. 3 Biotech 10(8):356. https://doi.org/10.1007/s13205-020-02341-y
Wahba MI (2020b) Mechanically stable egg white protein based immobilization carrier for β-D-galactosidase: thermodynamics and application in whey lactose hydrolysis. React Funct Polym 155:104696. https://doi.org/10.1016/j.reactfunctpolym.2020.104696
Wahba MI (2022) Gum tragacanth for immobilization of Bacillus licheniformis protease: optimization, thermodynamics and application. React Funct Polym 179:105366. https://doi.org/10.1016/j.reactfunctpolym.2022.105366
Wahba MI (2023a) Glutaraldehyde-copper gelled chitosan beads: characterization and utilization as covalent immobilizers. Biocatal Agric Biotechnol 50:102668. https://doi.org/10.1016/j.bcab.2023.102668
Wahba MI (2023b) Glutaraldehyde-pea protein grafted polysaccharide matrices for functioning as covalent immobilizers. Sci Rep 13(1):9105. https://doi.org/10.1038/s41598-023-36045-z
Wahba MI, Soliman TN (2018) Whey protein isolate for the preparation of covalent immobilization beads. Biocatal Agric Biotechnol 14:328–337. https://doi.org/10.1016/j.bcab.2018.04.003
Yafetto L (2022) Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: a review and bibliometric analysis. Heliyon 8:e09173. https://doi.org/10.1016/j.heliyon.2022.e09173
Yang J-K, Shih L, Tzeng Y-M, Wang S-L (2000) Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzy Microb Technol 26(5–6):406–413. https://doi.org/10.1016/S0141-0229(99)00164-7
Ye X-P, Xu M-F, Tang Z-X, Chen H-J, Wu D-T, Wang Z-Y, Songzhen Y-X, Hao J, Wu L-M, Shi L-E (2022) Flaxseed protein: extraction, functionalities and applications. Food Sci Technol 42:1–13. https://doi.org/10.1590/fst.22021
Zhang H, Bai Y, Zhu N, Xu J (2021) Microfluidic reactor with immobilized enzyme-from construction to applications: a review. Chin J Chem Eng 30:136–145. https://doi.org/10.1016/j.cjche.2020.12.011
Zhou M-Y, Chen X-L, Zhao H-L, Dang H-Y, Luan X-W, Zhang X-Y, He H-L, Zhou B-C, Zhang Y-Z (2009) Diversity of both the cultivable protease-producing bacteria and their extracellular proteases in the sediments of the South China Sea. Microb Ecol 58:582–590. https://doi.org/10.1007/s00248-009-9506-z
Acknowledgements
The authors would like to thank Chemistry of Natural and Microbial Products Department, Biochemistry Department and Centre of Scientific Excellence-Group of Advanced Materials and Nanotechnology, National Research Center, Dokki, Cairo, Egypt for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest regarding the publication of this paper.
Ethics approval
Ethical approval was not required for this research.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Shazly, A.I., Wahba, M.I., Abdelwahed, N.A.M. et al. Immobilization of alkaline protease produced by Streptomyces rochei strain NAM-19 in solid state fermentation based on medium optimization using central composite design. 3 Biotech 14, 161 (2024). https://doi.org/10.1007/s13205-024-04003-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-024-04003-9