Abstract
Highly sophisticated and synchronized interactions of various cells and hormonal signals are required to make organisms competent for reproduction. GnRH neurons act as a common pathway for multiple cues for the onset of puberty and attaining reproductive function. GnRH is not directly receptive to most of the signals required for the GnRH secretion during the various phases of the ovarian cycle. Kisspeptin neurons of the hypothalamus convey these signals required for the synchronized release of the GnRH. The steroid-sensitive anteroventral periventricular nucleus (AVPV) kisspeptin and arcuate nucleus (ARC) KNDy neurons convey steroid feedback during the reproductive cycle necessary for GnRH surge and pulse, respectively. AVPV region kisspeptin neurons also communicate with nNOS synthesizing neurons and suprachiasmatic nucleus (SCN) neurons to coordinate the process of the ovarian cycle. Neurokinin B (NKB) and dynorphin play roles in the GnRH pulse stimulation and inhibition, respectively. The loss of NKB and kisspeptin function results in the development of neuroendocrine disorders such as hypogonadotropic hypogonadism (HH) and infertility. Ca2+ signaling is essential for GnRH pulse generation, which is propagated through gap junctions between astrocytes-KNDy and KNDy-KNDy neurons. Impaired functioning of KNDy neurons could develop the characteristics associated with polycystic ovarian syndrome (PCOS) in rodents. Kisspeptin-increased synthesis led to excessive secretion of the LH associated with PCOS. This review provides the latest insights and understanding into the role of the KNDy and AVPV/POA kisspeptin neurons in GnRH secretion and PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive function requires multiple central and peripheral cues that ensure the perpetuation of the species. GnRH conveys all these regulatory cues to the hypothalamic-pituitary–gonadal axis (HPG axis) shown in Fig. 1. Episodically release of the GnRH regulates the LH secretion essential for normal reproductive function [1]. The activity of the GnRH system is regulated by various factors that include neuronal interactions (kisspeptin, KNDy, nNOS, gonadotropin-inhibitory hormone (GnIH), vasoactive intestinal peptide (VIP), arginine vasopressin (AVP), cellular interactions (astrocytes, tanycytes, and vascular endothelial cells), protein–ligand interactions (leptin, ghrelin, adiponectin), neurotransmitters (nitric oxide, γ-aminobutyric acid (GABA), excitatory amino acid), gonadal steroids, and non-peptides (dopamine, serotonin, epinephrine, norepinephrine, histamine) [2]. Two populations of kisspeptin synthesizing neurons are present in the rodent’s hypothalamus, in the ARC and AVPV region [3, 4] that mainly project to GnRH neurons. The ARC, kisspeptin neurons co-express NKB, and dynorphin, therefore also named KNDy neurons [5, 6]. The ARC (KNDy neurons) and AVPV region kisspeptin neurons act as a “GnRH pulse generator” and “GnRH surge generator,” respectively [7,8,9,10,11]. Bimodal release of GnRH, i.e., tonic as well surge, is under the control of gonadal steroids through positive and negative feedback mechanisms in both sexes [12, 13]. Inactivation of the kisspeptin and NKB gene causes infertility and sub-fertile phenotype in rodents, respectively [14,15,16]. Kisspeptin is a ligand of G-protein-coupled-receptor Kiss1R (formerly known as GPR54); mutations in KISS1 or KISS1R (encodes kisspeptin and KISS1 receptor) lead to HH in humans as well as mice [15, 17, 18]. Moreover, kisspeptin–GPR54 signaling is also crucial in controlling NO synthesizing neurons in the POA that play a role in releasing GnRH [19, 20]. Kisspeptin and NOS-containing neurons morphologically interact in the preoptic area [21] and are essential for GnRH secretion [15, 22, 23]. During proestrus, activation of nNOS (phosphorylation of the serine-1417) by activation of the PI3/AKT pathway is dependent on kisspeptin, which is essential for GnRH surge generation. Topalogalu et al. demonstrated that the inactivation of gene TAC3 (encodes NKB) or TAC3R (encodes NK3R receptor) results in HH and infertility in humans [24]. Inactivation of kisspeptin and NKB gene causes infertility and subfertile phenotype in rodents, respectively [14,15,16]. Mechanisms underlying the role of dynorphin in GnRH release are not clearly understood. KOR antagonist norbinalt-orphimine (nor-BNI) results in an increase in MUA volleys and the increase in LH pulse [25]. Cell–cell interactions are also involved in the structural plasticity of the GnRH neurons. A recently putative cellular mechanism is also proposed for the pulse generation by KNDy neurons involving astrocyte interplay and Ca2+ signal propagation through gap junction [26, 27] discussed later. KNDy neurons impaired functioning is associated with the development of PCOS-like characteristics in rodents. In human studies, increased secretion of kisspeptin was found to be associated with PCOS.
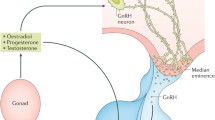
Schematic diagram for the regulation of hypothalamus-pituitary–gonadal axis (HPG) in females: GnRH neurons are present in the brain’s preoptic and ventral areas. These neuron processes extend to the median eminence (ME), where the GnRH is released into a hypophyseal portal blood vessel. In the hypothalamus, multiple neuronal populations affect the release of the GnRH. Kisspeptin neurons interact with the GnRH neurons in the POA. KNDy neurons interact with the GnRH neurons at the terminal level in the ME and play an essential role in GnRH pulse generation. In females, the direct action of kisspeptin on the GnRH plays a vital role in the surge and pulsatile release of the GnRH/LH. In females, the surge generation is regulated by the SCN neuronal populations. The SCN neurons (VIP and AVP) interact with the GnRH and AVPV kisspeptin neurons in the hypothalamic regions and play an essential role in the GnRH/LH surge necessary for ovulation. AVP neurons, via activation of AVPV kisspeptin neurons, during the LH surge, coordinate in the positive estradiol feedback and remove the estradiol negative feedback by suppressing the RFRP-3. VIP neurons can directly activate the GnRH neurons by interacting with their cell bodies and suppressing RFRP-3 neurons’ activity during LH surge. The nNOS neurons convey hormonal and neuronal cues to the GnRH neurons necessary for the GnRH/LH surge generation. Sex steroid hormone provides positive (solid line) and negative feedback (dashed line) to these neuronal populations within the brain during the various phases of the reproductive cycle. Preoptic area (POA); arcuate nucleus of the hypothalamus (ARC); anteroventral periventricular nucleus (AVPV); organum vasculosum of the lamina terminalis (OVLT); dorsomedial hypothalamus (DMH) vasoactive intestinal peptide (VIP); arginine vasopressin (AVP): RFamide-related peptides (RFRP-3)
GnRH Neurons
GnRH neurons were discovered in the 1970s. These individual neurons are the final frequent target for various neuropeptides and hormonal signals in the control of reproductive function and puberty onset. GnRH neurons originate from the neural crest in the olfactory placode and migrate to the basal forebrain before birth [28]. These neurons migrate during embryonic development (in rodents, 12.5 embryonic days) to distinct areas in different species [29, 30]. Early, GnRH neurons migrate with the mixed populations of the olfactory axons, neural crest, and placode-derived migratory cells, collectively called “migratory mass” (MM) in rodents [31]. Migration of the GnRH neurons is influenced by lots of factors that include anosmin1, neuropilins, leukemia inhibitory factor, fibroblast growth factor (FGFs), cell adhesions molecules, and interaction of GnRH neurons with astrocytes, olfactory ensheathing cells (OECs) [32,33,34]. Flaws in migration of the GnRH neurons result in Kallmann syndrome, characterized by hypogonadotropic hypogonadism and anosmia [35]. Morphologically GnRH cell bodies are present in the pre-optic area (POA), anterior hypothalamic area, and medial septum. The timing of the differentiation influences the ability of the post-mitotic GnRH neurons to navigate to the location, which is vital in wiring the adult network [36]. GnRH neuron somas are present in the POA and are extended to the median eminence (ME) region of the hypothalamus [37], where GnRH is released into the hypophyseal portal system to release LH/FSH from the anterior lobe of the pituitary. The projections of the GnRH neurons in ME have properties of axons and dendrites called dendrons [38]. These dendrons multiply in the ME into many short axons, which project onto portal blood vessels where GnRH is released. A recent study found that dendrons are the attribute of GnRH neurons [39]. These neurons are not directly receptive to all external and internal guidance cues that lead to the onset of puberty. In controlling sexual maturation and reproduction, the Kiss1 neurons act as gatekeepers in transmitting signals to GnRH neurons [40]. There is an increase in the percentage of GnRH depolarization in response to kisspeptin through various stages from juvenile to adult, suggesting that GnRH neurons acquire kisspeptin sensitivity across puberty [41]. Most GnRH neurons in the POA and mediobasal hypothalamus (MBH) express the GPR54 (also known as Kiss1 receptors) across various species [41, 42]. GnRH neurons in the POA and MBH receive synaptic inputs from KNDy neurons in sheep and ewe [43, 44], essential for GnRH pulse generation. Tract tracing studies in mice [45, 46] and rats [47] show minimal contact of KNDy neurons with GnRH neuron’s cell bodies. Matsuyama et al.’s study on castrated male goats found that immunoreactive (IR) fibers containing kisspeptin made direct contact with GnRH-IR fibers [48]. The study has shown no synaptic structure between kisspeptin and GnRH immunoreactive fibers suggesting that the ARC kisspeptin neurons in male goats regulate the GnRH secretion in the ME region of the brain by acting on GnRH neurons [48]. Similarly, studies in the rat showed that the ARC kisspeptin neurons and the GnRH neurons in the ME communicate through the axo-axonal non-synaptic mode [49].
Role of Estrogen Feedback in GnRH Surge and Pulse Generation
GnRH neurons are the final common target of all the internal and external cues that control the GnRH/LH release. GnRH neurons tune with the gonadal status by the gonadal steroid feedback mechanism (negative and positive) during the ovarian cycle. GnRH neurons do not express ERα receptors [50]; ERα expressing afferents transmit estradiol feedback to GnRH neurons. The biphasic effect of estradiol (estrogen) in the gonadotropin release is essential for a regular reproductive cycle. Steroid-specific input to the GnRH neurons is provided by the ERα expressing kisspeptin and KNDy neurons [6, 51]. Initially, the negative feedback causes the suppression of the FSH and the follicle’s selection, followed by positive feedback necessary for the ovulation and LH release [52]. The sex steroid hormone feedback is provided to the GnRH neurons by AVPV and ARC kisspeptin neurons [6, 41, 53, 54]. AVPV region Kiss1 neurons act as positive feedback mediators [55]. Estrogen positive feedback is transmitted through the kisspeptin neurons expressing ERα. Dubois et al. and Greenwald et al. [56, 57] found that kisspeptin-specific ERα knockout (KERKO) does not exhibit estradiol-induced LH surge. During the estrogen positive feedback mechanism, estrogen increases histone acetylation of Kiss1 promoter and results in enhanced expression of kisspeptin in AVPV region Kiss1 neurons [58]. Estrogen-ERα binds to the estrogen response element (ERE) in the hypermethylated region of the Kiss1 promoter resulting in the recruitment of the histone acetyltransferases that cause acetylation of the H3K9/14 [58, 59]. This histone modification results in the chromatin loop formation between the 3′ downstream region and promoter region of the Kiss1 locus. The loop formation results in the expression of the Kiss1 and induction of the GnRH/LH surge necessary for ovulation [58, 59]. During negative feedback, estrogen induces deacetylation of H3 in the Kiss1 promoter region of the ARC neurons and results in the decreased expression of the Kiss1 [27, 58, 59]. KNDy neurons [6] mediate negative steroid feedback to the GnRH neurons and generate GnRH episodic release, as shown in Fig. 2. The expression of the KNDy peptides depends on the status and circulating level of the gonadal steroids. There is much compelling evidence supporting steroids’ negative feedback mechanisms by KNDy neurons. The ablation of ERα in mice, especially in the ARC region, results in impaired negative feedback mechanisms and fertility [60]. In OVX mice [55] and ewe [61], nadir steroid hormone level leads to increased expression of the NKB and kisspeptin. At the same time, the treatment of these OVX animals with estrogen shows a reverse decrease in the expression of both neuropeptides, which supports the estradiol negative feedback mechanism. Estradiol (E2) has divergent effects on regulating the Kiss1 neurons of the ARC and AVPV region. High levels of E2 lead to change in the transition of the neurotransmission in ARC kisspeptin neurons from the peptidergic to glutamatergic transmission [62, 63]. Activation of the ARC kisspeptin neurons was sufficient to increase LH-like surge in intact mice and ARC stimulation terminated in the absence of the E2 and OVX mice [64]. The treatment of the OVX female mice with E2 has shown increased mRNA expression of Slc17a6 (encodes vGluT2) in ARC kisspeptin neurons [62]. During the proestrus (high level of E2) in ARC kisspeptin neurons, the expression of Kiss1 is attenuated, and the vesicular glutamate transporter 2 (vGluT2) expression increases required for the release of the glutamate. Glutamate release from ARC neurons provides excitatory signals to AVPV Kiss1 neurons and GnRH neurons’ distal processes [65] that help in initiating GnRH/LH surge as shown in Fig. 2 [63]. Deleting the ERα from proopiomelanocortin (POMC) neurons impairs estradiol negative feedback, and fertility suggests that these non-KNDy neurons also participated in estradiol negative feedback [66].
Role of AVPV/PeN kisspeptin and KNDy neurons in GnRH surge and pulse generation: AVPV Kiss1 neurons during the proestrus drive the LH surge by directly activating GnRH neurons via Kiss1-GPR54 signaling. a Prior to ovulation, rising estrogen levels cause the release of the glutamate from KNDy neurons that stimulates the firing of the AVPV/PeN Kiss1 neurons and contributes to kisspeptin-mediated activation of the GnRH neurons. During the proestrus, the high estrogen level gives positive feedback to the AVPV/PeN kiss1 neurons, leading to the synthesis of kisspeptin that acts on the GnRH neurons and results in GnRH/LH surge essential for ovulation. b Low estrogen levels provide negative feedback to the KNDy neurons that regulate the GnRH/LH pulse generation at the terminals region of GnRH neurons in the ME region. GnRH/LH pulses are essential for folliculogenesis and spermatogenesis. The elements used in the figure are just for illustration purposes
AVPV/POA Kisspeptin Neurons: GnRH Surge Generator
Anterior hypothalamic kisspeptin neurons cell bodies in females are localized mainly in the AVPV region (rodents), periventricular nucleus region (PeN)(pigs) POA region (ruminants, primates) [67,68,69,70,71,72]. The fibers of the kisspeptin neurons of the AVPV region are found in close appositions with the GnRH neurons cell body in mice [73]. The role of these kisspeptin neurons was earlier described by microinjecting kisspeptin antibody in the POA that abolishes the LH surge induced by the estradiol [3]. Similarly, in sheep, the central injection of kisspeptin antagonist (p-271) attenuated the LH surge [42]. After the estradiol treatment, LH surges, and c-Fos expression increases in the AVPV/POA region kisspeptin neurons in castrated male and OVX female goats [68]. The selective optogenetic activation of the rostral periventricular area of the third ventricle (RP3V) kisspeptin neurons resulted in the increased secretion of the LH, similar to endogenous LH surge [74]. The kisspeptin neurons of the hypothalamus also co-express other co-transmitter (GABA, tyrosine hydroxylase (TH), dopamine), which along with kisspeptin, play an essential role in the excitation of the GnRH neurons [75,76,77,78]. Mice lacking TH in kisspeptin cells (THKOs) exhibit normal reproduction, suggesting that GABA and kisspeptin are essential for the excitation of the GnRH neurons [78]. RP3V kisspeptin neurons provide kisspeptin-GABA co-transmission as an excitatory input to the GnRH neurons essential for ovulation [74]. In musk shrew, increased c-Fos expression was found in the POA kisspeptin neurons, and these neurons are essential for mating-induced ovulation [70]. The estrogen treatment leads to increased expression of Kiss1 in the AVPV region kisspeptin neurons [68, 69]. Estradiol implantation around the POA results in GnRH surge, whereas the lesion of the AVPV area results in the loss of the preovulatory surge [79] [12]. The ovariectomized (OVX) rats treated with estradiol showed an increased percentage of Kiss1 cells in the AVPV region before the LH surge [69, 80]. The immunohistochemical study found higher c-Fos expression and Kiss1-ISH in the medial preoptic area (mPOA) region after the high dosage of E2 in castrated males and OVX female goats [68].
Similarly, immunohistochemical studies found kisspeptin expressing cells in the POA and ARC regions of the brain in heifers [81]. The GnRH surge system is required for ovulation, so it is reasonable to believe that this system may be absent in males. Numerous studies demonstrated that the GnRH generating surge system is sexually dimorphic. The treatment of the high dosage of estrogen induces LH surge in the female sheep but not in the male [82]. In gonadectomized male rats, the high dose of estrogen does not induce LH surges [83, 84]. Castrated male goats were able to generate LH surge after the high dosage injection of the estradiol and found that the GnRH/LH surge generation system was retained in male goats [68]. In primates and human, the GnRH system is not fully sexually differentiated; in castrated male monkey, administration of the estradiol was able to produce the LH surge [72, 85]. Overall, these studies suggest that in some species (sheep, rat), the GnRH/LH system is absent in male, but in other species (primates, goat), the GnRH/LH system retain its function. In female rats, the GnRH/LH surge generation during the proestrus is under the circadian clock’s control. Two neuronal populations from the SCN, where the master clock is present, are believed to play an essential role in the GnRH/LH surge and ovulation, as shown in Fig. 1. The SCN neurons synthesizing the vasoactive intestinal peptides (VIP) are projected mono-synaptically to the GnRH neurons present in the POA [86]. These SCN VIP neurons activated and communicated with GnRH neurons to successfully generate the LH surge and ovulation [86, 87]. The treatment of GT1-7 cells with VIP found increased secretion of the GnRH peptides [88]. Application of VIP to the brain slices of the female mice results in the firing of the GnRH neurons independent of the estrous cycle [89]. Male mice brain sections treated with VIP also showed spontaneous activation of GnRH neurons and increased Ca2+ concentration in these neurons [89]. During the proestrus, the GnRH receiving the VIPergic afferents has shown the expression of c-Fos. Similarly, the arginine vasopressin (AVP) synthesizing neurons of the SCN is also projected towards the AVPV region kisspeptin neurons and plays an important role in the GnRH/LH surge generation [90] [87]. Intracerebroventricular (ICV) injection of the AVP to the Clock/Clock mutant mice demonstrated a surge-like increase in the LH compared to the control group [91]. In rodents, exogenous AVP administration in the afternoon can increase the release of the LH and suggest time-dependent sensitivity of the kisspeptin cells to the AVP. The time-dependent stimulation of the kisspeptin cell to AVP was not found in Syrian Hamsters, GnRH neurons were found responsive to daily changing kisspeptin and suggest another autonomous circadian control downstream of SCN [87, 90]. In vitro study is done to find the intrinsic daily rhythm of the Clock genes at the cellular level using GT1-7 cell lines [88]. The study found that the time of stimulation affected the differential GnRH release. Increased GnRH release was observed at 28 and 48 h after kisspeptin treatment and 4, 22, and 46 h after VIP treatment [88]. AVPV-area kisspeptin neurons are activated by AVPergic neurons, dependent on estrogen levels. [92]. The circadian pattern of kiss1 activation in the AVPV region was missing in the OVX mice. Spontaneous firing of the RP3V kisspeptin neurons in the brain slices of the mice was observed after the 1-min bath application of the AVP [93]. Central administration of the AVP and VIP receptor antagonist results in the attenuation of LH surge amplitude. RFamide-related peptide (RFRP-3) neurons of the dorsomedial hypothalamus (DMH), along with the SCN neurons, play an important role in integrating circadian and [94] estrogenic signaling by interacting directly with the GnRH neurons and neuronal population upstream to GnRH in various mammalian species [95]. Mainly, these neurons suppress the gonadotropic secretion and help in mediating the estrogen negative feedback [95, 96] but in seasonal breeder (male Siberian hamster) stimulates the LH concentrations [97, 98]. RFRP-3 neurons receive projection from both AVP and VIP neurons and send their projection to GnRH neurons [99, 100]. These neuronal population is sensitive to the milieu of the steroid and has shown increased activity during diestrus and reduced activity during proestrus [101]. During a high estrogen level, VIP neurons suppress the activity of these neurons and remove that estrogen negative feedback and stimulate LH surge [102]. A recent study in male mice also showed that the central administration of the RFRP-3 neurons results in the dose-dependent increase of the LH levels [94]. In castrated male mice, the administration of RFRP-3 found sex steroid independent increase in the LH level also highlights the sex and species difference in the working of these neurons [94].
Role of Kisspeptin in GnRH Surge Generation
Kisspeptin neurons of the hypothalamus (AVPV and ARC regions) are responsible for inducing GnRH surge and pulse, respectively, which are under the influence of estradiol, as shown in Fig. 2 [4, 23, 69, 103]. In ewes, estradiol-mediated positive feedback after the onset of the preovulatory surge occurs in both ARC (KNDy) and POA region (Kiss1) neurons [104]. Kisspeptin causes the activation of GnRH neurons by transsynaptic and direct mechanisms, and indirect activation reduces in the absence of estradiol. Seminara et al. and de Roux et al. [22, 105] documented that deletion and inactivating mutations in the GPR54 gene result in defective gonadotropin secretion and infertility. Kisspeptin causes the activation of c-Fos in GnRH neurons and depolarizes these neurons to the various responses [106]. There is much compelling evidence that kisspeptin directly activates the GnRH neurons and mediates the activation of non-GnRH neurons that play an essential role in GnRH/LH release through ionotropic GABA and glutamate receptors [107].
Kisspeptin released from the AVPV kisspeptin neurons modulates the catalytic activity of NOS during the ovarian cycle by a posttranscriptional modification that includes phosphorylation [20, 108, 109] and regulates the release of the GnRH. All preoptic nNOS neurons express ERα [110, 111] and NMDA receptors [112]. During the proestrus stage, when the circulating estrogen is at the highest level, there is an increase in NO production and the phosphorylation-based activation of nNOS [108]. Physical association of the P-nNOS with the NR2B/PSD-95 (NR2B subunit of NMDA receptor) is highest during the proestrus [108]. Kisspeptin promotes the phosphorylation of the nNOS (serine1412) by activating PI3/AKT pathway in the preoptic nNOS neurons [113] [109] but not in the tuberal region of the hypothalamus [108]. The treatment of GPR54/nNOS null mice with kisspeptin-10 results in LH release similar to proestrus [108]. Hence, NO acts as a tonic brake in LH release during estradiol-mediated negative feedback [108]. Estradiol induces the formation of the NMDAR-nNOS complex to enhance NO production [112, 114]. Administration of estradiol in GPR54 null mice does not induce nNOS activation by phosphorylation [108]. Kisspeptin/GPR54 signaling promotes the estradiol-dependent preovulatory activation of nNOS neurons. Estradiol-mediated activation of nNOS through kisspeptin-GPR54 is a switch that is required for the peak and pulsatile release of GnRH [115]. In GnRH-GFP mice, the effect of kisspeptin analysis using patch-clamp studies of acute brain slices revealed that little exposure to kisspeptin has a long-lasting effect on GnRH neurons [41, 107, 116].
KNDy Neurons: a Pulse Generator
The population of KNDy neurons resides in the ARC region of the hypothalamus across many mammalian species [5, 7, 25, 47, 81, 117] [118]. KNDy neurons are an integral part of the GnRH system that regulates the pulsatile release of GnRH/LH shown in Fig. 3. Moreover, KNDy neuronal fibers are apposed with the axonal terminals of GnRH neurons in the ME [48, 49, 119]. Goodman et al. also demonstrated that GnRH receives projections from these ARC neurons in sheep using light and electron microscopic studies [120]. The immunohistochemical studies were performed to localize KNDy neurons’ populations using immune reactivity against different markers present on these neurons (NKB, Dynorphin) [5, 121, 122]. Earlier mapping studies found that kisspeptin fibers and cell bodies are found throughout the ARC of the hypothalamus in rats and mice [67, 119, 123]. The ME and ARC are regions with a dense population of these neurons, and in the ARC region, kisspeptin-IR fibers surround themselves. In sheep, triple immunohistochemistry for GnRH and two KNDy neuropeptides found close juxtapositions of GnRH cells with KNDy neurons [43]. One distinguishable attribute of these neurons is that KNDy-KNDy cells form a reciprocal interconnected network in the sheep and rat ARC region [124, 125] [126]. Dual-label studies showed that KNDy neurons co-localize gonadal steroid receptors (estrogen, progesterone, androgen receptors) [124, 125, 127, 128]. Neuroanatomical evidence and the essential role of KNDy neuropeptides in GnRH release lead to the “KNDy hypothesis” [6, 7, 129, 130]. KNDy hypothesis states that (1) kisspeptin from KNDy neurons acts as an output signal. (2) NKB and dynorphin, respectively, function as stimulatory and inhibitory signals for the GnRH pulse. (3) KNDy forms a reciprocal circuit and causes a synchronized GnRH pulse. KNDy hypothesis is supported by many neuroanatomical, immunohistochemical, pharmacological, and microscopic studies. Double immunohistochemical studies in ovine demonstrated that 88% of dynorphin cells are positive for NKB, and 84% of NKB neurons are positive for dynorphin in the ARC region of the hypothalamus [122]. The idea of reciprocal connection is supported by evidence from the rodent study, which demonstrates that NKB/Dynorphin-IR fibers are found closely appose with NKB/dynorphin-IR cell bodies [121]. In sheep [42] and rats [131], the absence of the Kiss1r mRNA in KNDy neurons strengthens the idea of kisspeptin as an output signal from KNDy neurons. Pharmacological studies using NK3R antagonists and agonists [132] and KOR [25] also supported the stimulatory and inhibitory effect of NKB and dynorphin on the GnRH pulses generation in the ARC region of sheep, respectively [44]. The role of the Kiss1 in ARC region was evaluated by an optogenetics study and demonstrated that the selective optogenetic activation of the kisspeptin neurons ARC region was correlated with the LH pulse, and these neurons are the long-elusive pulse generator [133]. Oral or intravenous administration of the NK3R agonist (SB223412) in the E2-treated OVX goats showed a suppressive effect on the GnRH pulses generation [134]. Volitios et al. carried in vivo optogenetic study to find out the origin of the GnRH pulse generator and found that KNDy produces a sustain dynamics GnRH pulse [135]. NKB causes the auto stimulation of KNDy and acts as a bistable switch that allows neurons to fire at a high or low rate; negative feedback of dynorphin plays an essential role in pulse inhibition [135]. A recent study by Nagae et al. provides the first direct evidence that KNDy neurons maintain folliculogenesis and gonadotropin pulses [10]. Transfection of the Kiss1 gene intoTac3 neurons of the ARC in Kiss1 knock-out female rats restores the normal gonadotropin release and folliculogenesis [10].
Schematic representation of GnRH pulse generation in mammals: During the pulse onset, NKB (blue) release acts reciprocally within the KNDy-KNDy circuit and causes the neurons’ synchronized firing. a. Firing of the KNDy neurons involves Ca.2+ signaling, which propagates between astrocyte-KNDy neurons through the gap junction. Stimulation of KNDy neurons results in the release of kisspeptin (orange) from these neurons that can directly act on the GnRH neurons, resulting in pulse generation. On the other, during the pulse inhibition, there is the release of dynorphin (black) from the KNDy neurons, which acts through reciprocal connections and causes the inhibition of the release of NKB, and dynorphin (?) may also directly act on GnRH neurons and cause GnRH pulse inhibition. KNDy neurons also interact with many other upstream non-KNDy neurons (pink) that may play a role in stimulating and inhibiting (?) GnRH pulse. The color and shape given to each component are just for ease of understanding
Narayanaswamy et al. carried out a human study to investigate whether the KNDy hypothesis applies to humans or not. The participants were administered kisspeptin, NKB, and naltrexone hydrochloride (opioids receptor antagonist) to see their effect on the LH pulse. LH pulse number is significantly increased in most groups compared to the vehicle except for kisspeptin and NKB groups. Similarly, kisspeptin-administered group showed a significant rise in the FSH, naltrexone-NKB-administered group also showed a significant increase in the FSH compared to the vehicle. The co-administration of NKB and kisspeptin, which was never done before this study in humans, found a new interaction between these two peptides. The study found that NKB limits the ability of the kisspeptin to stimulate the increase in the LH when co-administrated with kisspeptin in humans [136]. The study cannot conclude or provide robust evidence on whether KNDy applies to humans or not. The KNDy neurons alone can generate GnRH pulse generator which is also challengeable as studies in primates provide evidence that neuropeptide Y(NPY) pulses occur synchronously with GnRH pulses [137]. Immunohistochemical studies in humans found that most NKB-IR in the infundibular nucleus (Inf) and infundibular stalk (InfS) were devoid of kisspeptin labeling [138]. This study questions the validity of the concept of the KNDy neuron in humans and suggests that the abundance of these peptides depends upon age, sex, and species differences [138]. Later immunohistochemical studies for the tachykinin peptide substance P (SP) in the post-mortem hypothalamic tissue found significant SP neurons in postmenopausal women’s compared to aged men’s Inf [139]. The study provides evidence of the colocalization of SP within kisspeptin and NKB fibers and the close apposition of SP IR fibers with terminals of the GnRH neurons in the infundibular stalk (Ifs) [140]. Co-localization of SP, kisspeptin, and NKB in humans highlights the pulse generation’s complexity and suggests that the SP can modulate the effects of NKB and kisspeptin on GnRH neurons [139]. Human studies showed that the patients with the TAC3 mutation on the infusion of the opioids antagonist and kisspeptin exhibit LH pulse and suggest multiple layers in controlling the GnRH pulse generation [141]. Due to the limited number of studies, evidence, and knowledge gaps, it is still unclear that the “KNDy Hypothesis” applies to human and nonhuman primates [142]. Viral-tract tracing studies in mice demonstrated that the proper functioning of KNDy neurons also requires input from the upstream populations of non-KNDy neurons in the ARC region of the hypothalamus [143]. KNDy neurons also participate in other physiological processes like thermoregulation, homeostasis, and weight control [144, 145].
Role of Kisspeptin in GnRH Pulse Generation
The ARC region KNDy neurons are involved in estradiol-mediated negative feedback control of GnRH secretion [55]. The study also found that testosterone (T) inhibits the expression of the Kiss1 mRNA in the ARC region and stimulates the expression of the Kiss1 mRNA in AVPV /PeN neurons. These results suggest that both the androgen and estrogen receptors are required to regulate the Kiss1 mRNA in the AVPV/PeN and ARC region of the brain. Later studies also provide evidence that KNDy neurons act as a mediator of ovarian steroid feedback. In low E2 levels (OVX rats), administration of senktide (NK3R agonist) suppresses LH pulse via Dyn/KOR signaling [146, 147], and NK3R activation evokes kisspeptin-induced LH pulse in the prepubertal female rats [148]. Kiss1 expression increased upon administering senktide in the hypothalamic neurons, and the stimulatory effect diminished in GPR54 knockout males [149, 150]. On the other hand, the administration of GnRH antagonists eliminates kisspeptin’s stimulatory effects on LH secretion and confirms GnRH-mediated kisspeptin action [54, 151,152,153]. Kisspeptin from ARC KNDy neurons acts as an output signal that directly can act on the GnRH neurons at the terminal region and lead to GnRH pulse. The in situ hybridization and immunohistochemical studies provide evidence for the lack of expression of the GPR54 receptors by the ARC region kisspeptin neurons in sheep [42] and rats [131], suggesting that kisspeptin does not act through reciprocal connections between the KNDy-KNDy circuits. There is much compelling evidence that ablation of ARC region kisspeptin neurons is associated with a disruption in the estrus cyclicity and alteration of the LH frequency. Knock-out studies using virally delivered kisspeptin antisense in female rats result in low LH frequency and a disrupted estrus cycle [154, 155]. Applications of kisspeptin on the brain slices of rats do not induce any electrical activity in ARC kisspeptin neurons [156], further supporting that kisspeptin does not act through reciprocal connections. During the pulse onset, the release of NKB from the KNDy neurons acts on reciprocally interconnected neurons and further causes the release of kisspeptin that can directly modulate the activity of GnRH neurons. Due to the limited number of in vivo model, it is challenging to dissect the independent role of kisspeptin synthesized from the AVPV kisspeptin and KNDy neurons. Most in vivo models available target the ARC region Kiss1 either via toxin-based or viral-based ablation/silencing of Kiss1 postnatally.
Recently, embryonic target conditional Kiss1 KO was generated with prodynorphin IRES-Cre (Pdyn-Cre/Kiss1fl/fl KO) mouse line that showed hypogonadism [157]. The adult Pdyn-Cre/Kiss1fl/fl KO showed larger antral follicles, majorly early-stage follicles, and an irregular estrous cycle. The LH pulse frequency also decreases in both male and female conditional ARC region-specific Kiss1 knock-out mice. The female (Pdyn-Cre/Kiss1fl/fl KO) mice are infertile, and the male (Pdyn-Cre/Kiss1fl/fl KO) were found sub-fertile, having less reproductive success rate [157]. This study highlights the ARC region kisspeptin’s importance and provides evidence of the KNDy neurons as the pulse generator. Interestingly, the study also found that the ARC region kisspeptin is not required for puberty in mice. Although much research had done on kisspeptin, more research is required to understand the exact mechanism of the ARC region kisspeptin and other targets of kisspeptin and neuronal interplay in ARC and adjacent areas of the brain in the regulation of the GnRH neurons.
Role of KNDy Peptides in GnRH Secretion
Neurokinin B (NKB)
Tachykinin (NKB) comprises 10–11 amino acids in length [158]. TAC3 and Tac2 genes encode NKB in primates and rodents, respectively [158, 159]. TAC3 positive neurons are predominately identified in the Inf, anterior hypothalamic area, septal region, diagonal band of Broca, bed nucleus of the stria terminalis, amygdala, and neocortex in humans [160, 161]. Tac3 mRNA expression was found predominately cerebral cortex, hippocampus, amygdaloid complexes, a bed of stria terminalis, ventral, pallidum, habenula, olfactory bulb, dorsomedial nucleus, ventromedial nucleus, lateral hypothalamic area, caudate-putamen, medial preoptic area, ARC, and lateral mammillary bodies in rat brain [149, 162]. In the ME region of the hypothalamus, there were differences in the abundance and percentage of kisspeptin and NKB fiber localization in various species (humans, monkeys, and rodents) [51, 67, 118, 163, 164]. Dynorphin, NKB, and their receptors (KOR and NK3R) are co-synthesized by ARC Kiss1 neurons of the hypothalamus but not in the kisspeptin neurons of the AVPV region of the hypothalamus [5]. A previous study by Burke. et al. demonstrated that NKB/Dyn fibers are found in the ME and mPOA regions [121]. NKB act specifically through its specific receptors (NK3R) [124]. High-affinity receptors for NKB (NK3R) are present on the KNDy neurons in several species (rats, mice, sheep) [7, 165]. Immunohistochemical studies found that GnRH neurons do not express NK3R in rat [166, 167] and sheep [168] brains. While the axonal terminal region of the GnRH expressed NK3R in the rats [167], the activation of GnRH neurons at the terminal region by NKB is independent of kisspeptin [169]. An initial study in the human ARC region found an mRNA increase for NKB in postmenopausal women [170]. This study leads the researcher to find out the role of the NKB in GnRH/LH release and localization of NKB in various regions of the brain to get detailed insight into NKB action. While unraveling the role of NKB initial study found that the central injection of senktide in OVX rats suppresses LH release [171]. Later studies in humans found that mutations in TAC3 and TACR3 genes lead to defects in the release of GnRH and HH [24, 172] and provide evidence for the stimulatory effect of NKB in GnRH pulse generation. Further stimulation of LH secretion after the administration of senktide was also reported in various species, including goat [25], rat [149], mouse [7], sheep [44, 173], and monkey [118, 174], supporting the stimulatory effects of NKB in the GnRH pulse. In ewe, senktide stimulates GnRH neuron activity and LH secretion [163, 173]. The oral administration of SB223412 (selective NK3R antagonist) in estradiol-treated OVX goats suppresses the GnRH pulses [134]. Intravenous administration of the SB223412 leads to an increase in the interval of MUA volleys [134]. The inter-pulse interval of the LH pulse is significantly increased compared to the vehicle, suggesting that NKB is directly acting on the KNDy neurons for the regulation of the GnRH pulses [134]. Immunohistochemical studies in sheep [122] and rats [121] later found specific cells co-localized NKB and dynorphin in the ARC region of the hypothalamus. The absence of NK3R expression by GnRH neurons suggests that NKB does not directly modulate these neurons’ activity [166,167,168]. However, studies in various species found redundancy in the tachykinin signaling [175]. In control of the regulation of GnRH neurons in rodents, the redundancy occurs between various tachykinins, which include (i) NKB-NK3R, (ii) SP-NK1R, and (iii) neurokinin A (NKA–NK2R). The earlier idea of redundancy in tachykinin signaling is provided using the Tacr3 knockout mice that show mild reproductive defects [16]. Later studies found that the antagonist of all three receptors is required to suppress GnRH pulse [176]. In the rats, the antagonist of the specific NK3R results in delayed puberty, suggesting that the redundancy may vary with age and hormonal status [177]. On the other hand, the species redundancy is also demonstrated using agonist for NK1R and NK2R in mice results in the surge increase of LH [178], while in rats, only the agonist NK2R seems to be stimulatory [179]. In goat [180] and sheep [181], most studies suggest that all three tachykinins act through NK3R. Further research is required to understand the redundancy in tachykinins signaling in different species precisely. Biallelic study in humans [141] also provides evidence of NKB independent generation of the GnRH-induced LH pulse and opens a new dimension to studying other new players and mechanisms in controlling these important physiological processes.
Dynorphin
Dynorphin endogenous opioid act by KOR inhibits the LH secretion [182]. Among the three KNDy neuropeptides, dynorphin is the longest subject of neuroendocrine research. The KOR distribution is first reported by Weems et al. [183] in sheep. This study analyzes the distributed KOR in the POA, ARC, anterior hypothalamic area, supraoptic (SON), paraventricular nuclei (PVN), dorsomedial, ventromedial, and lateral hypothalamus using in situ hybridization and immunohistochemical analysis. Approximately 41% and 90% of KNDy express KOR in male mice [163] and sheep [183]. Previous studies are also unable to provide adequate evidence for the expression of the KOR in GnRH neurons in the mPOA. It is hypothesized that dynorphin released from the KNDy acts in an autocrine and paracrine way to inhibit the activity of KNDy neurons.
There is evidence supporting the hypothesis that the KNDy neurons cause the suppression of the GnRH pulse generation by inhibiting kisspeptin stimulation that is dependent on Dyn/KOR signaling. Evidence that sustains this hypothesis is that (i) morphology of KNDy neurons allows the negative autoregulation by dynorphin [7, 124] and (ii) administration of U50488 (Dyn analog) reduces the firing of KNDy neurons in murine [156, 163]. An electrophysiological study found that central administration of the dynorphin has an inhibitory action on LH pulse and is associated with multiple unit activity (MUA) volleys in goats [25] which also support the inhibitory action of dynorphin. MUA is the average neuronal population spiking adjacent to the placed microelectrode. Dynorphin also inhibits the transmission of a glutamatergic signal to KNDy neurons and inhibits pre-synaptic activity [184]. Dynorphin acts reciprocally only within the KNDy circuit, but a recent study using multiple labels IHC found that approximately 94% (KNDy) and 75% (GnRH) neurons in the rat and sheep co-localized KOR [16]. The synapse of dynorphin-IR fibers between GnRH neurons, cell bodies, and dendrites is found in the POA and MBH regions [120]. A total of 94.7% of the GnRH cell shows KOR colocalization in POA [185]. Dynorphin causes the GnRH pulse termination through the KOR within the KNDy circuit and inhibits kisspeptin release, leading to pulse termination [44]. The binding of dynorphin to KOR causes desensitization of receptors, resulting in internalization of the KOR after repeated exposure to the receptor agonist [186]. Initial studies found that the opioid receptor antagonist WIN 44,441–3 activates the MBH GnRH neurons, not POA GnRH neurons [187]. Endocytosis of the KOR after binding to the agonist recently unraveled as an essential process that causes a delay in pulse onset and pulse termination in various species.
Non-KNDy neurons also interact with the KNDy neurons in the ARC region [188, 189]. In sheep, the study found that the end of the pulse may also involve non-KNDy neurons, which leads to the endocytosis of KOR [44, 190]. In ewes, administration of NKB increases the internalization of KOR receptors in the ARC region [185]. There is no effect of the episodic LH release after administration of the nor-BNI in OVX rat which suggests additional neurotransmitter besides dynorphin in GnRH pulse termination [147]. The GnRH neurons in the MBH region are in proximity with KNDy neurons and play an essential role in the pulsatile secretion of GnRH [185], while POA GnRH neurons are required for the LH surge. Some studies also highlight the role of the hypothalamic paraventricular nucleus (PVN) dynorphin neurons in negative feedback via suppressing kisspeptin release. The OVX rat treated with the negative feedback level of the estrogen increased the expression of the Pdyn gene expression in the PVN region but not in the ARC region of the rats [191]. Dynorphin may act on different targets during the GnRH pulse termination and pulse onset within the different regions of the brain.
Cellular Mechanism for GnRH Pulse Generation in Mammals
The cellular and molecular mechanism by which KNDy neurons play an essential role in the GnRH pulse generation is not well explored. Voltage clamp recording of brain slices of the male mouse demonstrated that senktide results in a robust inward current that causes the increased firing of the ARC Kiss1-GFP neurons [192]. However, the application of senktide does not affect the firing of the GnRH-GFP neurons [192]. Blocking the glutamate and ionotropic GABA receptors does not affect senktide-induced firing activity of the KNDy neurons. This study proves that fast synaptic transmission does not require NK3R-mediated excitation of KNDy neurons [163]. Recent studies also shed light on the cellular and molecular mechanisms involved in GnRH pulse generation. Ikegami et al. highlight the role of the gap junction and glial-neuronal communication in the GnRH pulse generation, as shown in Fig. 3. The intracellular level of Ca2+ was examined as an indicator of the activity of KNDy neurons and their synchronized activity. In vitro study using dye coupling analysis showed that the KNDy and glial cells communicate through the gap junction. Ca2+ oscillation frequency increased in the Kiss1-GFP cells after senktide treatment. Inhibiting the gap junctions leads to the attenuation of the Ca2+ oscillations induced by senktide in the Kiss1-GFP cells [26]. The NKB-NK3R signaling facilitates the activation of the KNDy and synchronizes their activity by neuro-glia and neuron-neuron communications through gap junction [26]. Recently, based on the evidence and studies, Ikegamai et al. proposed a putative cellular mechanism for the working of the KNDy neurons in GnRH pulse generation. According to the proposed model, the activation of NKB-NK3R signaling results in high-frequency firing and increased membrane potential via altering the intrinsic cationic channels. The high-frequency firing of the KNDy neurons may lead to the activation of the voltage-gated Ca2+ channels, leading to the Ca2+ influx that results in increased intracellular Ca2+ concentration, which is propagated within the KNDy-KNDy and KNDy-astrocyte networks through gap junction and play a role in GnRH pulse onset as shown in Fig. 3a. The increase in the intracellular Ca2+ level may be due to typical Gq-GPCR signaling, resulting in increased inositol 1,4,5-trisphosphate (IP3) via phospholipase C (PLC). Increased level of the IP3 causes the release of the Ca2+ from the endoplasmic reticulum (ER) and causes the activation of the KNDy neurons and plays role in the pulse generation. The Dyn-KOR signaling terminates the pulse by decreasing the intracellular Ca2+ and firing of the KNDy neurons [26, 27].
PCOS and KNDy Association in Rodents
Polycystic ovarian syndrome (PCOS) is a hormonal disorder in females characterized by the abnormal production of the male hormone androgen by ovaries, ovulatory dysfunction, and increased level of serum LH. Ovarian steroid hormone plays a critical role in providing feedback to the hypothalamus to drive normal secretion of the GnRH and LH/FSH. Impaired steroid hormone feedback can result in abnormal activation of GnRH neurons, leading to the altered GnRH/LH pulse frequency and contributing to PCOS progression. In PCOS patients, high LH pulse frequency causes increased synthesis of the sex steroids (estrogen and androgen) by the ovaries and leads to hyperandrogenemia. KNDy neurons have been shown to play an essential role in generating the GnRH pulse frequency via steroid negative feedback mechanism. Studies using prenatal androgenized (PNA) PCOS animal models provide evidence that the KNDy neuron’s impaired functioning contributes to the PCOS. The abnormal level of the sex steroid changes the gene expression and morphology of the KNDy neurons, leading to changes in the pulse frequency of GnRH and LH, which is common in PCOS. A recent study by Moore et al. demonstrated that the PNA-treated mice show increased gene expression of the androgen receptors, decreased progesterone receptors, and dynorphin gene expression in the KNDy neurons. Overall, this study shows that in PCOS, the androgen directly acts on the KNDy neurons and impairs that negative progesterone feedback [193]. Changes in the gene expression within the KNDy cells result in an impaired negative steroid feedback mechanism and an increase in the serum LH levels associated with PCOS [193]. Prenatally, testosterone (T)-treated ewe showed an increase in the somal size of the ARC region kisspeptin neurons and had no effect on the POA kisspeptin neurons. Reduced KNDy-KNDy synaptic inputs and decreased KNDy output to the GnRH neurons are also observed in the prenatal T ewes that may contribute to the defects in the steroidal control of GnRH and contributes to the progression of the PCOS. The increase in kisspeptin immunoreactive cell bodies was observed in the prenatal dihydrotestosterone (DHT) treated rat’s ARC region [194]. There was also an increase in the number of NKB-positive cell bodies in the prenatally DHT-treated rats compared to the control. The PNA rats also show increased NKB and LepR mRNA expression before puberty and during the pubertal stages.
After the onset of puberty, increased kisspeptin mRNA expression was also observed in PNA female rats [195]. In the ARC region of PNA rats, kisspeptin and NKB-positive cells were also found to co-express LepR [195]. The treatment with a kisspeptin antagonist cannot suppress the elevated LH serum level stimulated by the leptin and speculate that NKB and leptin play an essential role in increasing the LH pulse and GnRH activation at puberty in PNA female rats [195]. The letrozole-induced PCOS rat model showed an increased expression of the Kiss1 mRNA in the ARC than the control group but comparable levels in the AVPV among the groups [196]. The study provides evidence that the increased LH levels in PCOS patients may be due to increased activity of the KNDy neurons [196]. The PCOS women treated with NK3R antagonist AZD4901 showed decreases in the LH pulse frequency, highlighting the importance and association of NKB signaling with PCOS [197].
Correlation Between Kisspeptin and PCOS in Humans
Recent studies highlight that dysregulation of the HPG can also be a factor in PCOS development and progression. The overexpression of kisspeptin increases the activity of the HPG axis, which is further associated with PCOS development and progression. Kisspeptin is one of the critical players in regulating the HPG axis, and it plays an essential role in ovulation and folliculogenesis. The hypothesis is that the increased secretion of kisspeptin leads to increased secretion of the GnRH, coupled with the increased LH secretion associated with PCOS. Many clinical studies were carried out to understand the correlation between kisspeptin and PCOS. These studies found a higher kisspeptin level positively correlated with LH levels, as expected in women with PCOS. In contrast, others found no positive correlation between kisspeptin and LH levels in PCOS women and normal women. Pandis et al. first performed a clinical study to measure kisspeptin levels in non-PCOS and PCOS patients. The study found a higher LH level in PCOS women than in control, and no significant differences in kisspeptin levels were found in the PCOS and control women [198]. Although this study provides evidence that insulin resistance increases the free androgen level and is associated with a decrease in the kisspeptin level [198]. Chen et al. did a human study to find the correlation between kisspeptin and PCOS. A total of sixty-two women were recruited to conduct the study, out of which twenty adolescent women as a control group, 19 adolescent women with PCOS, and 23 adult PCOS women. Blood samples from all three groups were collected in the morning, and LH and kisspeptin concentration measurements were done using chemiluminescence and enzyme-linked immunosorbent assay (ELISA). Slightly increased kisspeptin levels were found in the adolescent PCOS compared to adult PCOS women. In adult and adolescent women with PCOS, kisspeptin levels were more than in the adolescent control group, and LH and kisspeptin levels were positively correlated [199]. The study suggests that the increase in the kisspeptin in adolescent women may be used as a marker to recognize PCOS. RBP4 is associated with developing insulin resistance in type II diabetes and obese patients [200]. Retinol binding protein 4 (RBP4) belongs to the lipocalin family and mainly plays a role in transporting vitamin A in circulation [201]. RBP4 is also associated with insulin resistance in obese and type 2 diabetes mellitus patients [200]. A study was conducted to find a correlation between kisspeptin, RBP4, and leptin in PCOS. Ninety women, including 56 PCOS women and 36 non-PCOS women, were recruited for the study. The blood sample from non-PCOS is collected during 3–8 days of normal menstrual cycle and from PCOS during the spontaneous bleeding period. Hormonal, kisspeptin, leptin, and RBP4 concentration measurements were done using immune-radio assay, ELISA, and Bradford assay. A positive correlation between the serum RPB4 and plasma kisspeptin and leptin levels was found in PCOS patients. The study found a higher level of LH in PCOS patients with no correlation between LH and kisspeptin levels in PCOS patients [202]. This study suggested that kisspeptin is positively correlated with the FAI (free androgen index) in obese PCOS patients and is not directly affected by obesity and insulin resistance in PCOS. A total no. of 447 women were recruited by Ozay et al., out of which 285 were with PCOS and 162 as control non-PCOS women, to find out the correlation between kisspeptin and PCOS. The blood samples were taken between 3 and 5 days of the menstrual cycle in the morning. There is no significant difference between kisspeptin and leptin found in PCOS and the control group. However, LH and leptin levels were positively correlated with kisspeptin in all PCOS patients and suggested further evaluation of kisspeptin and associated biochemical pathways [140]. The immunohistochemical studies showed increased expression of the KISS1 and KISS1R expression in the endometrium and ovarian biopsies of the PCOS patients compared to the control group [203]. A human study also reported that single nucleotide polymorphism (SNP) rs4889 C/G in kisspeptin gene is predominant in PCOS women compared to non-PCOS women [204], due to the polymorphism of the rs4889 arginine being substituted by proline at position 81. The blood samples were collected in the morning between 3 and 6 days of the menstrual cycle, and the kisspeptin and hormonal measurements were done using an ELISA. A positive correlation between plasma LH and kisspeptin levels was found in PCOS patients. This genetic variation is considered one of the factors contributing to PCOS development and suggested a large-scale study to validate the exact role of this genetic variation in PCOS [204]. A human study was conducted to find the early marker of PCOS, which includes finding kisspeptin’s correlation with various parameters (triglycerides, HDL). Blood samples were collected during the 2–3 days early follicular phase, and kisspeptin and hormonal measurements were done using ELISA. The study found kisspeptin negatively correlated with triglycerides and a positively correlated with HDL. An increase in LH levels was found with the absence of an increase in kisspeptin in PCOS women. The study suggests various early markers (LH/FSH, testosterone, progesterone, waist-hip ratio (WHR), sex hormone binding globulin (SHGB), and vascular endothelial growth factor (VEGF) lipid profile that could be used for the detection of PCOS [205]. Similarly, Gorkem et al. found no correlation between kisspeptin serum levels and LH levels in PCOS patients. A total of 154 participants were recruited and divided into three groups: (i) 60 women with high ovarian reserve pattern (PCOS), (ii) 57 women with adequate ovarian reserve pattern (AOR), and (iii) women with diminished ovarian reserve pattern (DOR). Blood samples were collected on the 2–5 days of the menstrual cycle in the morning, and hormonal and kisspeptin measurements were done electrochemiluminescence immunoassay (ECLIA) and ELISA. Higher level of kisspeptin in PCOS patients was found positively correlated with total testosterone (TT) and dehydroepiandrosterone sulfate (DHEAS) and negatively correlated with serum FSH levels. The study suggests that the positive correlation of kisspeptin with androgen might be related to hyperandrogenism associated with PCOS [206]. Later, the study by Kaya et al. found conflicting results with the Gorkem et al.’s study and found that the increased expression of kisspeptin elicits secretion of the LH in PCOS women [207]. A total of ninety women were included in the study; blood sample were collected on day 2–5 of menstrual cycle in morning. Hormonal and kisspeptin levels were measured using ECLIA and ELISA. PCOS patients without oligomenorrhoea showed coupling of the LH pulse with kisspeptin secretion. The study suggests that the increased level of kisspeptin is associated with higher antral follicle count (AFC), higher body mass index (BMI), and PCOS [207]. Human studies showed that kisspeptin level is more in PCOS than normal, but most studies regarding the correlation between kisspeptin and other metabolic factors in PCOS are inconclusive. The difference in the results found in studies can be due to various reason that may include the variation in diagnosis criteria to identify the PCOS patients, and there are multiple phenotypes of the PCOS that has multiple metabolic characteristics that could lead to different results in different studies, a varying number of the sample size may lead to sample error that is needed to be addressed in future studies to find the exact correlation between kisspeptin and metabolic factors in PCOS patients. Recently, using the bioinformatics tool study has been done to better understand the pathophysiology of the PCOS and found that insulin (INS) as the main hub. Most of the pathophysiological defects of PCOS act downstream to the INS and only kisspeptin and glucagon act upstream to the INS [208]. This study also highlights the importance of the insulin in the pathophysiology of PCOS and that it will be helpful in developing therapeutics for PCOS.
Conclusion and Future Perspective
GnRH is receptive to a limited number of cues, and kisspeptin neurons act as a gatekeeper in transmitting the signal to the GnRH [40]. AVPV/POA kisspeptin neurons play a key role in the preovulatory GnRH/LH surge and ovulation. KNDy neurons are essential for the GnRH pulse generation necessary for folliculogenesis and steroidogenesis. Ablations of the KNDy peptides from the ARC region demonstrated deficits in the normal estrous cycle, hypogonadism, and infertility. More insight into the cellular mechanism responsible for pulse generation can further be utilized to increase the breeding efficacy and treat the reproductive disorder of domestic animals. Weems et al. [185] demonstrated the pattern of internalization of the KOR receptor during pulse onset and pulse termination in the GnRH neurons at POA, MBH, as well as in KNDy neurons in the ARC region [183]. This study showed that the ICV injection of NKB during the pulse onset does not cause the KOR internalization in the MBH GnRH neurons. At the same time, there is a significant increase in KOR endocytosis in these neurons at the time of pulse termination [185]. The ICV injection of NKB does not affect the internalization of the KOR in GnRH neurons of POA [185]. Does the internalization of the KOR associated with the GnRH secretion is yet to explore. The dynorphin receptors on GnRH neurons in some species also highlight the direct action of dynorphin on the GnRH, suggesting more research is needed to validate dynorphin’s direct action on GnRH neurons. KNDy neurons also interact with the soma of the GnRH neurons in the POA, where nNOS immunoreactive fibers are present. So, it could be postulated that the KNDy neurons interact directly or indirectly with nNOS neurons in the brain’s POA and MBH. Impaired negative feedback results in the abnormal activation of the GnRH neurons, resulting in altered LH frequency and increased serum LH levels associated with PCOS. Understanding the correlation of kisspeptin with the other metabolic factors will help develop medical therapy for PCOS. With the limitation of the current technology, the precise role of these KNDy neurons is still unclear. Recent advancements in immunohistochemical, electrophysiological, and optical tissue imaging will help in elucidating these neurons’ purpose in the reproductive axis. Exploring the role of non-KNDy and KNDy neuron interactions also helps to find new pathways or mechanisms regulating the GnRH secretion and allow to develop medical therapies for neuroendocrine pathologies.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol. 1987;49(1):365–82. https://doi.org/10.1146/annurev.ph.49.030187.002053.
Spergel DJ. Modulation of gonadotropin-releasing hormone neuron activity and secretion in mice by non-peptide neurotransmitters, gasotransmitters, and gliotransmitters. Front Endocrinol (Lausanne). 2019;10:329. https://doi.org/10.3389/fendo.2019.00329.
Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–6. https://doi.org/10.1210/en.2005-0195.
Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687. https://doi.org/10.1523/JNEUROSCI.1618-06.2006.
Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–60. https://doi.org/10.1210/en.2007-0961.
Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–89. https://doi.org/10.1210/en.2010-0022.
Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859. https://doi.org/10.1523/JNEUROSCI.1569-09.2009.
Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–36. https://doi.org/10.1210/en.2018-00653.
Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452–66. https://doi.org/10.1038/nrendo.2016.70.
Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci U S A. 2021;118(5):e2009156118. https://doi.org/10.1073/pnas.2009156118.
Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol (Lausanne). 2021;12:724632. https://doi.org/10.3389/fendo.2021.724632.
Wang L, Moenter SM. Differential roles of hypothalamic AVPV and arcuate kisspeptin neurons in estradiol feedback regulation of female reproduction. Neuroendocrinology. 2020;110(3–4):172–84. https://doi.org/10.1159/000503006.
Herbison AE. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front Neuroendocrinol. 2020;57:100837. https://doi.org/10.1016/j.yfrne.2020.100837.
Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104(25):10714. https://doi.org/10.1073/pnas.0704114104.
Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–63. https://doi.org/10.1016/j.bbrc.2003.11.066.
Yang JJ, Caligioni CS, Chan Y-M, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–508. https://doi.org/10.1210/en.2011-1949.
de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972. https://doi.org/10.1073/pnas.1834399100.
George JT, Seminara SB. Kisspeptin and the Hypothalamic control of reproduction: lessons from the human. Endocrinology. 2012;153(11):5130–6. https://doi.org/10.1210/en.2012-1429.
Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, Anglemont de Tassigny X, et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci. 2012;32(3):932. https://doi.org/10.1523/JNEUROSCI.4765-11.2012.
Prashar V, Arora T, Singh R, Sharma A, Parkash J. Interplay of KNDy and nNOS neurons: a new possible mechanism of GnRH secretion in the adult brain. Reprod Biol. 2021;21(4):100558. https://doi.org/10.1016/j.repbio.2021.100558.
Bellefontaine N, Hanchate NK, Parkash J, Campagne C, de Seranno S, Clasadonte J, et al. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology. 2011;93(2):74–89. https://doi.org/10.1159/000324147.
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. https://doi.org/10.1056/NEJMoa035322.
Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147(3):1154–8. https://doi.org/10.1210/en.2005-1282.
Topaloglu A, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–8. https://doi.org/10.1038/ng.306.
Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124. https://doi.org/10.1523/JNEUROSCI.5848-09.2010.
Ikegami K, Minabe S, Ieda N, Goto T, Sugimoto A, Nakamura S, et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol. 2017;29:6. https://doi.org/10.1111/jne.12480.
Ikegami K, Watanabe Y, Nakamura S, Goto T, Inoue N, Uenoyama Y, et al. Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals. Front Neuroendocrinol. 2021;64:100968. https://doi.org/10.1016/j.yfrne.2021.100968.
Casoni F, Malone SA, Belle M, Luzzati F, Collier F, Allet C, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969. https://doi.org/10.1242/dev.139444.
Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161. https://doi.org/10.1038/338161a0.
Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132–6. https://doi.org/10.1073/pnas.86.20.8132.
Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31(18):6915–27. https://doi.org/10.1523/JNEUROSCI.6087-10.2011.
Cho H-J, Shan Y, Whittington NC, Wray S. Nasal placode development, GnRH neuronal migration and Kallmann syndrome. Front Cell Dev Biol. 2019;7:121. https://doi.org/10.3389/fcell.2019.00121.
Cariboni A, Pimpinelli F, Colamarino S, Zaninetti R, Piccolella M, Rumio C, et al. The product of X-linked Kallmann’s syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum Mol Genet. 2004;13(22):2781–91. https://doi.org/10.1093/hmg/ddh309.
Pellegrino G, Martin M, Allet C, Lhomme T, Geller S, Franssen D, et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat Neurosci. 2021;24(12):1660–72. https://doi.org/10.1038/s41593-021-00960-z.
Mitchell AL, Dwyer A, Pitteloud N, Quinton R. Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory. Trends Endocrinol Metab. 2011;22(7):249–58. https://doi.org/10.1016/j.tem.2011.03.002.
Jasoni Christine L, Porteous Robert W, Herbison Allan E. Anatomical location of mature GnRH neurons corresponds with their birthdate in the developing mouse. Dev Dyn. 2009;238(3):524–31. https://doi.org/10.1002/dvdy.21869.
Gibson MJ, Ingraham L, Dobrjansky A. Soluble Factors guide gonadotropin-releasing hormone axonal targeting to the median eminence*. Endocrinology. 2000;141(9):3065–71. https://doi.org/10.1210/endo.141.9.7656.
Herde M, Iremonger K, Constantin S, Herbison A. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J Neurosci. 2013;33:12689–97. https://doi.org/10.1523/JNEUROSCI.0579-13.2013.
Moore AM, Prescott M, Czieselsky K, Desroziers E, Yip SH, Campbell RE, et al. Synaptic innervation of the GnRH neuron distal dendron in female mice. Endocrinology. 2018;159(9):3200–8. https://doi.org/10.1210/en.2018-00505.
Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, et al. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides. 2009;30(1):49–56. https://doi.org/10.1016/j.peptides.2008.08.004.
Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349. https://doi.org/10.1523/JNEUROSCI.3328-05.2005.
Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–12. https://doi.org/10.1210/en.2010-1225.
Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–35. https://doi.org/10.1111/jne.12293.
Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–69. https://doi.org/10.1210/en.2013-1331.
Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–94. https://doi.org/10.1210/en.2015-1131.
Yeo S-H, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–99. https://doi.org/10.1210/en.2011-0164.
True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. https://doi.org/10.1111/j.1365-2826.2010.02076.x.
Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, et al. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinol. 2011;94(4):323–32.
Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011;23(10):863–70. https://doi.org/10.1111/j.1365-2826.2011.02199.x.
Hrabovszky E, Steinhauser Ar, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler In, et al. Estrogen receptor -β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142(7):3261–4. https://doi.org/10.1210/endo.142.7.8176.
Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the brain. Endocr Rev. 2009;30(6):713–43. https://doi.org/10.1210/er.2009-0005.
Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207(4437):1371.
Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C, Boehm U. Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology. 2015;156(1):32–8. https://doi.org/10.1210/en.2014-1671.
Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761. https://doi.org/10.1073/pnas.0409330102.
Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of kiss1 gene expression in the brain of the female mouse. Endocrinol. 2005;146(9):3686–92. https://doi.org/10.1210/en.2005-0488.
Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinol. 2015;156(3):1111–20. https://doi.org/10.1210/en.2014-1851.
Greenwald-Yarnell ML, Marsh C, Allison MB, Patterson CM, Kasper C, MacKenzie A, et al. ERα in Tac2 neurons regulates puberty onset in female mice. Endocrinol. 2016;157(4):1555–65. https://doi.org/10.1210/en.2015-1928.
Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci U S A. 2012;109(20):E1294. https://doi.org/10.1073/pnas.1114245109.
Uenoyama Y, Tomikawa J, Inoue N, Goto T, Minabe S, Ieda N, et al. Molecular and epigenetic mechanism regulating hypothalamic kiss1 gene expression in mammals. Neuroendocrinol. 2016;103(6):640–9. https://doi.org/10.1159/000445207.
Yeo S-H, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinol. 2014;155(8):2986–95. https://doi.org/10.1210/en.2014-1128.
Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 Messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinol. 2007;148(3):1150–7. https://doi.org/10.1210/en.2006-1435.
Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, Palmiter RD, et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. Elife. 2018;7:e35656. https://doi.org/10.7554/eLife.35656.
Stincic TL, Kelly MJ. Estrogenic regulation of reproduction and energy homeostasis by a triumvirate of hypothalamic arcuate neurons. J Neuroendocrinol. 2022;23:e13145. https://doi.org/10.1111/jne.13145.
Lin X-H, Lass G, Kong L-S, Wang H, Li X-F, Huang H-F, et al. Optogenetic activation of arcuate kisspeptin neurons generates a luteinizing hormone surge-like secretion in an estradiol-dependent manner. Front Endocrinol (Lausanne). 2021. https://doi.org/10.3389/fendo.2021.775233.
Stincic TL, Qiu J, Connors AM, Kelly MJ, Rønnekleiv OK. Arcuate and preoptic kisspeptin neurons exhibit differential projections to hypothalamic nuclei and exert opposite postsynaptic effects on hypothalamic paraventricular and dorsomedial nuclei in the female mouse. eNeuro. 2021;8:4. https://doi.org/10.1523/eneuro.0093-21.2021.
Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2019;29(5):1232. https://doi.org/10.1016/j.cmet.2019.04.006.
Clarkson J, D’Anglemont De Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673–82. https://doi.org/10.1111/j.1365-2826.2009.01892.x.
Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, et al. The luteinising hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol. 2015;27(1):57–65. https://doi.org/10.1111/jne.12235.
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–78. https://doi.org/10.1262/jrd.18146.
Inoue N, Sasagawa K, Ikai K, Sasaki Y, Tomikawa J, Oishi S, et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci U S A. 2011;108(42):17527. https://doi.org/10.1073/pnas.1113035108.
Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinol. 2007;148(4):1774–83. https://doi.org/10.1210/en.2006-1540.
Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, et al. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26(12):909–17. https://doi.org/10.1111/jne.12227.
Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinol. 2006;147(12):5817–25. https://doi.org/10.1210/en.2006-0787.
Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant neuropeptide cotransmission in kisspeptin-GABA regulation of GnRH neuron firing driving ovulation. J Neurosci. 2018;38(28):6310. https://doi.org/10.1523/JNEUROSCI.0658-18.2018.
Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Atkin S, Bookout AL, et al. Characterization of kiss1 neurons using transgenic mouse models. Neurosci. 2011;173:37–56. https://doi.org/10.1016/j.neuroscience.2010.11.022.
Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22(6):2313. https://doi.org/10.1523/JNEUROSCI.22-06-02313.2002.
Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23(4):293–301. https://doi.org/10.1111/j.1365-2826.2011.02107.x.
Stephens SBZ, Rouse ML, Tolson KP, Liaw RB, Parra RA, Chahal N, et al. Effects of selective deletion of tyrosine hydroxylase from kisspeptin cells on puberty and reproduction in male and female mice. eNeuro. 2017;4(3):0150–17. https://doi.org/10.1523/ENEURO.0150-17.2017.
Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Neuroendocrinol. 1980;31(2):147–57. https://doi.org/10.1159/000123066.
Matsuda F, Ohkura S, Magata F, Munetomo A, Chen J, Sato M, et al. Role of kisspeptin neurons as a GnRH surge generator: Comparative aspects in rodents and non-rodent mammals. J Obstet Gynaecol Res. 2019;45(12):2318–29. https://doi.org/10.1111/jog.14124.
Hassaneen A, Naniwa Y, Suetomi Y, Matsuyama S, Kimura K, Ieda N, et al. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev. 2016;62(5):471–7. https://doi.org/10.1262/jrd.2016-075.
Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the reovulatory discharge of luteinizing hormone in sheep1,2. Endocrinol. 1975;97(2):373–9. https://doi.org/10.1210/endo-97-2-373.
Crowley WR. Sex differences in the responses of hypothalamic luteinizing hormone-releasing hormone and catecholamine systems to ovarian hormones and naloxone: implications for sexual differentiation of luteinizing hormone secretion in rats. Brain Res. 1988;461(2):314–21. https://doi.org/10.1016/0006-8993(88)90261-2.
Henderson SR, Baker C, Fink G. Effect of oestradiol-17β exposure on the spontaneoUS secretion of gonadotrophins in chronically gonadectomized rats. J Endocrinol. 1977;73(3):455–62. https://doi.org/10.1677/joe.0.0730455.
Stearns EL, Winter JSD, Faiman C. Positive feedback effect of progestin upon serum gonadotropins in estrogen-primed castrate men1. J Clin Endocrinol Metab. 1973;37(4):635–8. https://doi.org/10.1210/jcem-37-4-635.
Kriegsfeld LJ, Silver R, Gore AC, Crews D. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J Neuroendocrinol. 2002;14(9):685–90. https://doi.org/10.1046/j.1365-2826.2002.00818.x.
Gotlieb N, Moeller J, Kriegsfeld L. Development and modulation of female reproductive function by circadian signals. 2020. https://doi.org/10.1007/978-3-030-40002-6_16.
Zhao S, Kriegsfeld LJ. Daily Changes in GT1–7 Cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinol. 2009;89(4):448–57. https://doi.org/10.1159/000192370.
Piet R, Dunckley H, Lee K, Herbison AE. Vasoactive intestinal peptide excites GnRH neurons in male and female mice. Endocrinol. 2016;157(9):3621–30. https://doi.org/10.1210/en.2016-1399.
Williams WP III, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinol. 2011;152(2):595–606. https://doi.org/10.1210/en.2010-0943.
Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and clock mutant mice1. Bio Reprod. 2006;75(5):778–84. https://doi.org/10.1095/biolreprod.106.052845.
Kriegsfeld L, Williams IIIW. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne). 2012. https://doi.org/10.3389/fendo.2012.00060.
Piet R, Fraissenon A, Boehm U, Herbison AE. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J Neurosci. 2015;35(17):6881. https://doi.org/10.1523/JNEUROSCI.4587-14.2015.
Ancel C, Inglis MA, Anderson GM. Central RFRP-3 Stimulates LH Secretion in male mice and has cycle stage–dependent inhibitory effects in females. Endocrinol. 2017;158(9):2873–83. https://doi.org/10.1210/en.2016-1902.
Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinol. 2008;149(11):5811–21. https://doi.org/10.1210/en.2008-0575.
Kriegsfeld LJ, Jennings KJ, Bentley GE, Tsutsui K. Gonadotrophin-inhibitory hormone and its mammalian orthologue RFamide-related peptide-3: discovery and functional implications for reproduction and stress. J Neuroendocrinol. 2018;30(7):e12597-e. https://doi.org/10.1111/jne.12597.
Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinol. 2012;153(1):373–85. https://doi.org/10.1210/en.2011-1110.
Ancel C, Bentsen AH, Sébert M-E, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male syrian hamster: the exception proves the rule. Endocrinol. 2012;153(3):1352–63. https://doi.org/10.1210/en.2011-1622.
Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916(1):172–91. https://doi.org/10.1016/S0006-8993(01)02890-6.
Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–43. https://doi.org/10.1111/j.1365-2826.2011.02162.x.
Gibson EM, Humber SA, Jain S, Williams WP III, Zhao S, Bentley GE, et al. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinol. 2008;149(10):4958–69. https://doi.org/10.1210/en.2008-0316.
Russo KA, La JL, Stephens SBZ, Poling MC, Padgaonkar NA, Jennings KJ, et al. Circadian control of the female reproductive axis through gated responsiveness of the RFRP-3 system to VIP signaling. Endocrinol. 2015;156(7):2608–18. https://doi.org/10.1210/en.2014-1762.
Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, et al. Characterization of the Potent Luteinizing Hormone-Releasing Activity of KiSS-1 Peptide, the Natural Ligand of GPR54. Endocrinol. 2005;146(1):156–63. https://doi.org/10.1210/en.2004-0836.
Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinol. 2012;153(11):5406–14. https://doi.org/10.1210/en.2012-1357.
de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Nat Acad Sci U S A. 2003;100(19):10972–6. https://doi.org/10.1073/pnas.1834399100.
Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2011;8:40. https://doi.org/10.1038/nrendo.2011.147.
Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinol. 2008;149(4):1979–86. https://doi.org/10.1210/en.2007-1365.
Parkash J, D’Anglemont De Tassigny X, Bellefontaine N, Campagne C, Mazure D, Buée-Scherrer V, et al. Phosphorylation of N-methyl-D-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle. Endocrinol. 2010;151(6):2723–35. https://doi.org/10.1210/en.2010-0007.
Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, et al. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27(13):3445. https://doi.org/10.1523/JNEUROSCI.4799-06.2007.
Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043(1):205–13. https://doi.org/10.1016/j.brainres.2005.02.074.
Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor α and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453(4):336–44. https://doi.org/10.1002/cne.10413.
de Anglemont Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain J-C, et al. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci. 2007;27(23):6103. https://doi.org/10.1523/JNEUROSCI.5595-06.2007.
de d’Anglemont Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiol. 2010;25(4):207–17. https://doi.org/10.1152/physiol.00009.2010.
De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain J-C, et al. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci. 2004;24(46):10353. https://doi.org/10.1523/JNEUROSCI.3228-04.2004.
Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544–77. https://doi.org/10.1210/er.2009-0023.
Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinol. 2008;149(9):4605–14. https://doi.org/10.1210/en.2008-0321.
Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause1. J Clin Endocrinol Metab. 1999;84(6):2111–8. https://doi.org/10.1210/jcem.84.6.5689.
Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinol. 2010;151(9):4494–503. https://doi.org/10.1210/en.2010-0223.
Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinol. 2008;149(9):4387–95. https://doi.org/10.1210/en.2008-0438.
Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinol. 2004;145(6):2959–67. https://doi.org/10.1210/en.2003-1305.
Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–26. https://doi.org/10.1002/cne.21086.
Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–41. https://doi.org/10.1111/j.1365-2826.2006.01445.x.
Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010;22(10):1101–12. https://doi.org/10.1111/j.1365-2826.2010.02053.x.
Burke Michelle C, Letts Penny A, Krajewski Sally J, Rance Naomi E. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–26. https://doi.org/10.1002/cne.21086.
Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinol. 2002;143(11):4366–74. https://doi.org/10.1210/en.2002-220586.
Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neurosci. 2010;166(2):680–97. https://doi.org/10.1016/j.neuroscience.2009.12.053.
Goubillon M-L, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus**This work was supported by the United Kingdom Biotechnology and Biological Sciences Research Council (to J.E.R. and A.E.H.) and a European Community Marie Curie Research Training Grant (to M.L.G.). Endocrinol. 2000;141(11):4218–25. https://doi.org/10.1210/endo.141.11.7743
Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–30. https://doi.org/10.1016/j.neulet.2006.03.039.
Maeda K-I, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, et al. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010;1364:103–15. https://doi.org/10.1016/j.brainres.2010.10.026.
Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–28. https://doi.org/10.1016/j.brainres.2010.08.059.
Higo S, Iijima N, Ozawa H. Characterisation of kiss1r (Gpr54)-expressing neurones in the arcuate nucleus of the female rat hypothalamus. J Neuroendocrinol. 2017;29:2. https://doi.org/10.1111/jne.12452.
Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinol. 2015;101(2):161–74. https://doi.org/10.1159/000377702.
Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216. https://doi.org/10.1073/pnas.1713897114.
Sasaki T, Sonoda T, Tatebayashi R, Kitagawa Y, Oishi S, Yamamoto K, et al. Peripheral administration of SB223412, a selective neurokinin-3 receptor antagonist, suppresses pulsatile luteinizing hormone secretion by acting on the gonadotropin-releasing hormone pulse generator in estrogen-treated ovariectomized female goats. J Reprod Dev. 2020;66(4):351–7. https://doi.org/10.1262/jrd.2019-145.
Voliotis M, Li XF, De Burgh R, Lass G, Lightman SL, Byrne KT, et al. The origin of GnRH pulse generation: an integrative mathematical-experimental approach. J Neurosci. 2019;39(49):9738. https://doi.org/10.1523/JNEUROSCI.0828-19.2019.
Narayanaswamy S, Prague JK, Jayasena CN, Papadopoulou DA, Mizamtsidi M, Shah AJ, et al. Investigating the KNDy hypothesis in humans by coadministration of kisspeptin, neurokinin B, and naltrexone in men. J Clin Endocrinol Metab. 2016;101(9):3429–36. https://doi.org/10.1210/jc.2016-1911.
Terasawa E. Mechanism of pulsatile GnRH release in primates: unresolved questions. Mol Cell Endocrinol. 2019;498:110578. https://doi.org/10.1016/j.mce.2019.110578.
Hrabovszky E, Sipos MT, Molnár CS, Ciofi P, Borsay BÁ, Gergely P, et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinol. 2012;153(10):4978–89. https://doi.org/10.1210/en.2012-1545.
Hrabovszky E, Borsay BÁ, Rácz K, Herczeg L, Ciofi P, Bloom SR, et al. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PloS one. 2013;8(8):e72369-e. https://doi.org/10.1371/journal.pone.0072369.
Emekci Ozay O, Ozay AC, Acar B, Cagliyan E, Seçil M, Küme T. Role of kisspeptin in polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2016;32(9):718–22. https://doi.org/10.3109/09513590.2016.1161019.
Lippincott MF, León S, Chan Y-M, Fergani C, Talbi R, Farooqi IS, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin B and dynorphin signaling. J Clin Endocrinol Metab. 2019;104(10):4304–18. https://doi.org/10.1210/jc.2019-00146.
Lehman MN, He W, Coolen LM, Levine JE, Goodman RL. Does the KNDy model for the control of gonadotropin-releasing hormone pulses apply to monkeys and humans? Semin Reprod Med. 2019;37(02):071–83. https://doi.org/10.1055/s-0039-3400254.
Moore AM, Coolen LM, Lehman MN. Kisspeptin/neurokinin B/dynorphin (KNDy) cells as integrators of diverse internal and external cues: evidence from viral-based monosynaptic tract-tracing in mice. Sci Rep. 2019;9(1):14768. https://doi.org/10.1038/s41598-019-51201-0.
Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109(48):19846–51. https://doi.org/10.1073/pnas.1211517109.
Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinol. 2012;153(6):2800–12. https://doi.org/10.1210/en.2012-1045.
Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinol. 2012;153(1):307–15. https://doi.org/10.1210/en.2011-1641.
Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinol. 2012;153(10):4894–904. https://doi.org/10.1210/en.2012-1574.
Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PloS one. 2012;7(9):e44344. https://doi.org/10.1371/journal.pone.0044344.
Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2010;300(1):E202–10. https://doi.org/10.1152/ajpendo.00517.2010.
García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MAM, Manfredi-Lozano M, Romero-Ruiz A, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinol. 2012;153(1):316–28. https://doi.org/10.1210/en.2011-1260.
Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920. https://doi.org/10.1523/JNEUROSCI.5740-08.2009.
Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PloS one. 2009;4(12):e8334. https://doi.org/10.1371/journal.pone.0008334.
Millar RP, Roseweir AK, Tello JA, Anderson RA, George JT, Morgan K, et al. Kisspeptin antagonists: unraveling the role of kisspeptin in reproductive physiology. Brain Res. 2010;1364:81–9. https://doi.org/10.1016/j.brainres.2010.09.044.
Beale KE, Kinsey-Jones JS, Gardiner JV, Harrison EK, Thompson EL, Hu MH, et al. The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinol. 2014;155(3):1091–8. https://doi.org/10.1210/en.2013-1544.
Hu MH, Li XF, McCausland B, Li SY, Gresham R, Kinsey-Jones JS, et al. Relative importance of the arcuate and anteroventral periventricular kisspeptin neurons in control of puberty and reproductive function in female rats. Endocrinol. 2015;156(7):2619–31. https://doi.org/10.1210/en.2014-1655.
de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinol. 2013;154(8):2750–60. https://doi.org/10.1210/en.2013-1231.
Nandankar N, Negrón AL, Wolfe A, Levine JE, Radovick S. Deficiency of arcuate nucleus kisspeptin results in postpubertal central hypogonadism. Am J Physiol Endocrinol Metab. 2021;321(2):E264–80. https://doi.org/10.1152/ajpendo.00088.2021.
Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11(15):2045–81. https://doi.org/10.2174/0929867043364748.
Bonner TI, Affolter HU, Young AC, Young WS 3rd. A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Brain Res. 1987;388(3):243–9.
Chawla Monica K, Gutierrez Graciela M, Young WS, McMullen Nathaniel T, Rance Naomi E. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1998;384(3):429–42. https://doi.org/10.1002/(SICI)1096-9861(19970804)384:3.
Pinto FM, Almeida TA, Hernandez M, Devillier P, Advenier C, Candenas ML. mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur Jour Pharmacol. 2004;494(2–3):233–9. https://doi.org/10.1016/j.ejphar.2004.05.016.
Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. J Comp Neurol. 1992;317(4):341–56. https://doi.org/10.1002/cne.903170403.
Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinol. 2013;154(8):2761–71. https://doi.org/10.1210/en.2013-1268.
Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984–98. https://doi.org/10.1111/j.1460-9568.2010.07239.x.
Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–86. https://doi.org/10.1002/cne.20626.
Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neurosci. 1992;51(2):317–45. https://doi.org/10.1016/0306-4522(92)90318-V.
Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–86. https://doi.org/10.1002/cne.20626.
Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin b cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. https://doi.org/10.1111/j.1365-2826.2009.01930.x.
Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinol. 2013;154(11):3984–9. https://doi.org/10.1210/en.2013-1479.
Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women*. Endocrinol. 1991;128(5):2239–47. https://doi.org/10.1210/endo-128-5-2239.
Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026(2):307–12. https://doi.org/10.1016/j.brainres.2004.08.026.
Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94(10):3633–9. https://doi.org/10.1210/jc.2009-0551.
Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–46. https://doi.org/10.1210/en.2010-0174.
Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinol. 2011;94(3):237–45. https://doi.org/10.1159/000329045.
Chrysanthi F, Víctor MN. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reprod. 2017;153(1):R1–14. https://doi.org/10.1530/REP-16-0378.
Noritake K-I, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409–15. https://doi.org/10.1262/jrd.11-002S.
Li SY, Li XF, Hu MH, Shao B, Poston L, Lightman SL, et al. Neurokinin B receptor antagonism decreases luteinising hormone pulse frequency and amplitude and delays puberty onset in the female rat. J Neuroendocrinol. 2014;26(8):521–7. https://doi.org/10.1111/jne.12167.
Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinol. 2015;156(2):627–37. https://doi.org/10.1210/en.2014-1651.
Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, Sánchez-Garrido MA, Roa J, et al. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinol. 2015;156(2):576–88. https://doi.org/10.1210/en.2014-1026.
Okamura H, Yamamura T, Wakabayashi Y. Mapping of KNDy neurons and immunohistochemical analysis of the interaction between KNDy and substance P neural systems in goat. J Reprod Dev. 2017;63(6):571–80. https://doi.org/10.1262/jrd.2017-103.
Fergani C, Navarro VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reprod. 2016;153(1):R1-r14. https://doi.org/10.1530/rep-16-0378.
Chavkin C, James IF, Goldstein A, Chavkin C. Dynorphin is a specific endogenous ligand of the -opioid receptor. Science. 1982;215(4531):413–5. https://doi.org/10.1126/science.6120570
Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinol. 2016;157(6):2367–79. https://doi.org/10.1210/en.2015-1763.
Zhen S, Gallo RV. The effect of blockade of kappa-opioid receptors in the medial basal hypothalamus and medial preoptic area on luteinizing hormone release during midpregnancy in the rat. Endocrinol. 1992;131(4):1650–6. https://doi.org/10.1210/en.131.4.1650.
Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, et al. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinol. 2018;159(9):3187–99. https://doi.org/10.1210/en.2018-00435.
Li JG, Luo LY, Krupnick JG, Benovic JL, Liu-Chen LY. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin- and dynamin-dependent mechanism .Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem. 1999;274(17):12087–94. https://doi.org/10.1074/jbc.274.17.12087.
Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion1. Endocrinol. 1999;140(12):5929–36. https://doi.org/10.1210/endo.140.12.7216.
Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19(7):2658–64. https://doi.org/10.1523/jneurosci.19-07-02658.1999.
Brown CH, Leng G, Ludwig M, Bourque CW. Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells. J Neurophysiol. 2006;95(5):3235–44. https://doi.org/10.1152/jn.00062.2006.
Foradori CD, Goodman RL, Lehman MN. Distribution of preprodynorphin mRNA and dynorphin-a immunoreactivity in the sheep preoptic area and hypothalamus. Neurosci. 2005;130(2):409–18. https://doi.org/10.1016/j.neuroscience.2004.08.051.
Tsuchida H, Mostari P, Yamada K, Miyazaki S, Enomoto Y, Inoue N, et al. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinol. 2020;161(11):bqqa161. https://doi.org/10.1210/endocr/bqaa161.
Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, et al. Regulation of NKB pathways and their roles in the control of kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinol. 2011;152(11):4265–75. https://doi.org/10.1210/en.2011-1143.
Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinol. 2021;162:11. https://doi.org/10.1210/endocr/bqab158.
Osuka S, Iwase A, Nakahara T, Kondo M, Saito A, Bayasula, et al. Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinol. 2017;158(2):367–77. https://doi.org/10.1210/en.2016-1333.
Yan X, Yuan C, Zhao N, Cui Y, Liu J. Prenatal androgen excess enhances stimulation of the GNRH pulse in pubertal female rats. J Endocrinol. 2014;222(1):73–85. https://doi.org/10.1530/JOE-14-0021.
Matsuzaki T, Tungalagsuvd A, Iwasa T, Munkhzaya M, Yanagihara R, Tokui T, et al. Kisspeptin mRNA expression is increased in the posterior hypothalamus in the rat model of polycystic ovary syndrome. Endocr J. 2017;64(1):7–14. https://doi.org/10.1507/endocrj.EJ16-0282.
George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–21. https://doi.org/10.1210/jc.2016-1202.
Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, et al. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85(6):1778–83. https://doi.org/10.1016/j.fertnstert.2005.11.044.
Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):72–6. https://doi.org/10.1016/j.ejogrb.2009.11.018.
Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–62. https://doi.org/10.1038/nature03711.
Steinhoff JS, Lass A, Schupp M. Biological functions of RBP4 and Its relevance for human diseases. Front Physiol. 2021. https://doi.org/10.3389/fphys.2021.659977.
Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2013;75(4):268–74. https://doi.org/10.1159/000350217.
Yarmolinskaya MI, Ganbarli NF, Tkachenko NN, Nikolaeva VI, Tolibova GK, Tral TG, et al. Kisspeptin and polycystic ovary syndrome - is there any connection? JOWD. 2017;66(6):73–80. https://doi.org/10.17816/JOWD66673-80
Albalawi FS, Daghestani MH, Daghestani MH, Eldali A, Warsy AS. rs4889 polymorphism in KISS1 gene, its effect on polycystic ovary syndrome development and anthropometric and hormonal parameters in Saudi women. J Biomed Sci. 2018;25(1):50. https://doi.org/10.1186/s12929-018-0452-2.
Daghestani MH. Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int J Gynaecol Obstet. 2018;142(2):162–9. https://doi.org/10.1002/ijgo.12527.
Gorkem U, Togrul C, Arslan E, Sargin Oruc A, Buyukkayaci Duman N. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol Endocrinol. 2018;34(2):157–60. https://doi.org/10.1080/09513590.2017.1379499.
Kaya C, Alay İ, Babayeva G, Gedikbaşı A, Ertaş Kaya S, Ekin M, et al. Serum kisspeptin levels in unexplained infertility, polycystic ovary syndrome, and male factor infertility. Gynecol Endocrinol. 2019;35(3):228–32. https://doi.org/10.1080/09513590.2018.1519792.
Geronikolou SA, Pavlopoulou A, Cokkinos DV, Bacopoulou F, Chrousos GP. Polycystic οvary syndrome revisited: an interactions network approach. Eur J Clin Invest. 2021;51(9):e13578. https://doi.org/10.1111/eci.13578.
Acknowledgements
Dr. Jyoti Parkash is thankful to the University Grants Commission (UGC) for start-up grant F.30-318/2016 (GP-105) and DST-Science and Engineering Research Board (SERB) for Early Carrier Research Grant ECR/2015/000240 (GP-90), and Core Research Grant CRG/2020/003257 (GP-176). Mr. VP is thankful to SERB, DST, for the Junior and Senior Research Fellowship. TA and RS are also thankful to CSIR for the JRF and SRF in carrying out this research work. Dr. Jyoti Parkash is also thankful to the Central University of Punjab for RSM-CUPB/CC/16/00/13 for providing financial assistance, space, and infrastructure to carry out this research. The authors acknowledge the use of Inkscape 1.2 licensed by a GNU General Public License, version 3, and Servier Medical Art bank (https://smart.servier.com) licensed by a Creative Commons Attribution 3.0 Unported License to create schematic Figs. 1, 2, and 3.
Funding
This work is supported by the DST- Science and Engineering Research Board (SERB), Early Carrier Research grant ECR/2015/000240 (GP-90), Core Research Grant CRG/2020/003257 (GP-176), the University Grants Commission (UGC start-up grant F.30–318/2016 GP-105), and the Central University of Punjab RSM grant- CUPB/CC/16/00/13.
Author information
Authors and Affiliations
Contributions
VP drafted and wrote the article. TA and RS reviewed the article, and AS and JP reviewed and approved the article’s final version.
Corresponding author
Ethics declarations
Ethical Consents
Ethical consents do not apply to this study since the study did not involve an animal and human subjects.
Consent to participate
Not applicable.
Consent of Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Prashar, V., Arora, T., Singh, R. et al. Hypothalamic Kisspeptin Neurons: Integral Elements of the GnRH System. Reprod. Sci. 30, 802–822 (2023). https://doi.org/10.1007/s43032-022-01027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01027-5