Abstract
The reproductive neuropeptide gonadotropin-releasing hormone (GnRH) has two modes of secretion. Besides the surge mode, which induces ovulation in females, the pulse mode of GnRH release is essential to cause various reproductive events in both sexes, such as spermatogenesis, follicular development, and sex steroid synthesis. Some environmental cues control gonadal activities through modulating GnRH pulse frequency. Researchers have looked for the anatomical location of the mechanism generating GnRH pulses, the GnRH pulse generator, in the brain, because an artificial manipulation of GnRH pulse frequency is of therapeutic importance to stimulate or suppress gonadal activity. Discoveries of kisspeptin and, consequently, KNDy (kisspeptin/neurokinin B/dynorphin) neurons in the hypothalamus have provided a clue to the possible location of the GnRH pulse generator. Our analyses of hypothalamic multiple-unit activity revealed that KNDy neurons located in the hypothalamic arcuate nucleus might play a central role in the generation of GnRH pulses in goats, and perhaps other mammalian species. This chapter further discusses the possible mechanisms for GnRH pulse generation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
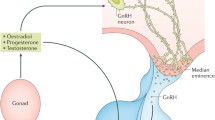
There are two modes of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion: one mode is the surge, necessary for ovulation in females, and the other is the pulse, required for the tonic support of reproductive function in both sexes. For example, GnRH pulses are needed to initiate the process of reproductive cycles, such as estrous cycles, in females. Follicular development is stimulated by the increase in frequency of GnRH/LH pulses, resulting in a surge-like secretion of estrogen from the mature follicles. The increased estrogen acts in the brain to cause the GnRH surge to induce ovulation in females. In contrast, males do not generate GnRH surges, and therefore only have the pulse mode of GnRH secretion, to maintain testicular activities such as spermatogenesis and steroidogenesis. Therefore, manipulation of the activity of the GnRH pulse generator is of therapeutic potential in both sexes, and the GnRH pulse generator is a good target for the development of drugs that might control fertility. This chapter focuses on the involvement of kisspeptin, and other related peptides, in the generation of GnRH pulses in mammals.
Discovery of Pulsatile LH Secretion
Pulsatile secretion of LH was first described, in monkeys, in 1970 by Knobil [1]. This was only a few years after the establishment of a radioimmunoassay for LH in the blood [2]. Knobil had noticed that the concentration of LH in the blood fluctuated significantly from assay to assay, or from time to time, in monkeys. He then utilized frequent blood collections in monkeys to determine the cause of these fluctuations. The resultant data exposed a beautiful series of plasma LH concentrations displaying repetitive abrupt increases in LH followed by an exponential decrease, the distinguishing feature of pulses [1].
The discovery of LH pulses changed the concept of hormone actions, because gonadal activity was subsequently shown in rhesus macaques to be controlled by the “frequency” of LH pulses [3–5]. The greater the LH pulse frequency, the greater the resultant gonadal activity. Knobil’s experiments elegantly proved that gonadal activity is completely dependent on the pulse frequency of LH release. After the discovery of pulsatile LH secretion in monkeys, reproductive endocrinologists began to reveal the pulsatile nature of LH secretion in various other mammalian species, including rats [6], sheep [7], cows [8], pigs [9], and horses [10], although frequent blood sampling was sometimes difficult in some species under no anesthesia and freely moving conditions. These data reiterated the importance of LH pulse frequency for the regulation of gonadal activities. Consistent across species, more frequent LH pulses are found during the follicular phase, whereas the pulse frequency is lower during luteal phase [8, 11, 12]. In seasonal animals, such as sheep, LH pulses are more frequent during the breeding season and less frequent in the nonbreeding season [13]. Interestingly, the frequency of the pulse is negatively correlated with the size of the body [14].
Discovery of Gonadotropin-Releasing Hormone Pulses and Surges
There is little doubt that the pulsatile nature of pituitary LH secretion is caused by the pulsatile release of GnRH from nerve terminals located in the median eminence, because GnRH is considered to be the single hypothalamic releasing factor stimulating pituitary LH secretion. Initially, however, this was only a belief and not based on solid evidence. The pulsatile nature of GnRH secretion was first seen in 1982 in a landmark study by Clarke and Cummins [15] and later examined in greater detail by Moenter et al. in the early 1990s. Both groups used a skillful technique of portal cannulation in sheep and very frequent portal blood collections (e.g., 30-s intervals!) to demonstrate beautiful GnRH pulses, each of which corresponded to simultaneous LH pulses [16]. The width of GnRH pulses was found to be narrower than LH pulses, suggesting that the half-life of GnRH in the portal blood is much shorter than the half-life of LH in the peripheral circulation [16]. This pioneering work demonstrated the clear relationship between GnRH and LH secretion, and supported the earlier studies by Knobil’s group demonstrating that when pulses of GnRH were infused to monkeys bearing hypothalamic lesions and abolished pulsatile LH secretion, LH secretion was restored in a pulsatile fashion, with each LH pulse corresponding beautifully to each experimental GnRH pulse [17]. Additionally, artificial pulsatile infusion of GnRH, with 1-h intervals, stimulated the ovary to produce complete menstrual cycles [3], whereas monkeys exposed to less frequent GnRH pulses showed no sign of ovarian activity [18].
In addition to identifying GnRH pulses in their sheep portal samples, Moenter et al. also observed robust periodic GnRH surges [19]. The discovery of GnRH surges leads to a dramatic turnaround in the theory of the LH surge formation, because researchers had previously believed that a high frequency of LH pulses during the preovulatory period caused a surge. This model held that when the frequency of LH pulses was too high to be effectively cleared from the circulation, the blood LH concentration would not decline and would keep increasing (i.e., a surge) until pulse frequency eventually drops. However, this idea was rejected after the discovery that a huge amount of GnRH is released just prior to LH surges, and the GnRH surge release continues even after the end of LH surges [19]. Currently, researchers believe that the GnRH and LH surges are generated by a mechanism different from that generating GnRH/LH pulses.
Anatomical Location of the GnRH Pulse Generator
The anatomical location of the GnRH pulse-generating mechanism has always been a big puzzle for reproductive endocrinologists. The first work describing the possible location of the center for pulsatile GnRH secretion was conducted by Halasz and Pupp [20], who utilized a micro “Halasz” knife in rats to isolate specific brain regions from the rest of the brain. They found that isolating the mediobasal hypothalamus (MBH), including the pituitary, abolishes ovulation but not follicular development [20]. This was confirmed later by Blake and Sawyer [21], who demonstrated that complete hypothalamic deafferentation spares LH pulses in ovariectomized (OVX) rats. These experiments clearly showed that the brain center generating GnRH/LH pulses was located within the hypothalamic area isolated by the Halasz’s knife, namely, the MBH. According to this data, the GnRH pulse generator may not involve GnRH neurons themselves, because very few GnRH cell bodies are located in the MBH of most animal species (with the exception of primates, in which most of GnRH neurons are located in the area [22]). The MBH location of the GnRH pulse generator was also confirmed by fetal MBH transplantation in rats that had brain lesions which abolished GnRH pulses [23]. A type of deafferentation called posterior-anterior deafferentation (PAD), which cuts the anterior part of the arcuate nucleus (ARC) off, abolished pulsatile LH secretion in rats, but the pulse was restored with transplantation of fetal MBH tissues (but not fetal cortical tissues). These findings indicate the presence of a GnRH pulse-generating mechanism in the MBH region.
On the other hand, evidence also suggests that GnRH neurons themselves are equipped with an intrinsic GnRH pulse-generating mechanism. This was first demonstrated in GT-1 cells, which are immortalized by introducing T antigen to the mouse genome to induce GnRH-producing tumor cells. GT-1 cells show periodic excitation, resulting in pulsatile GnRH release into the culture medium [24]. Further evidence came from primary cultures of rhesus monkey GnRH neurons taken from the fetal olfactory placode, the anatomical region where GnRH neurons originate and migrate from to the hypothalamus during development. The idea to obtain a pure population of GnRH neurons from the monkey fetus came from the laboratory of Terasawa and enabled the demonstration of pulsatile activation of GnRH neurons in vitro. These primary GnRH neurons displayed periodic increases in intracellular calcium concentrations [25]. Terasawa’s group also found that the periodic increases in intracellular calcium levels in cultured GnRH neurons are synchronized with each other [26]. The authors considered that these calcium increases cause GnRH pulses.
It is evident that GnRH is released in fixed intervals from GnRH neuronal terminals. The synchronized release of GnRH from each nerve terminal appears to require coordinated activation of GnRH neurons from neuronal afferents. There are three mechanistic possibilities for synchronizing GnRH neuronal output. First, GnRH cell bodies make contacts with each other, as evidenced by reports of morphological contacts between GnRH neuronal processes [27]. However, somatosomatic or dendrodendritic contacts between GnRH neurons are quite rare in the POA of rats [27]. Second, the synchronization of GnRH releases from each nerve terminal might be achieved by contact between multiple GnRH terminals in the median eminence, because the median eminence is one of the sites where there is a convergence of various bioactive substances acting to regulate the release GnRH [28]. There might be the third possibility that GnRH cells may all be synchronized by an upstream “clock” that affects all GnRH cells at the same time, resulting in simultaneous GnRH output from the various GnRH cells. However, there is no experimental evidence yet to support the last possibility.
The discovery of kisspeptin might help to settle the controversy over the location of the GnRH pulse generator and synchronization of GnRH release. However, there are still difficulties we must overcome in order to unravel the mechanism of GnRH pulse generation. In the rest of this chapter, we will discuss the possibility that kisspeptin neurons play a major role in generating GnRH pulses in multiple mammalian species.
MUA Recording of the GnRH Pulse Generator Activity at Close Vicinity of Kisspeptin Neurons in the Arcuate Nucleus
The Knobil laboratory was the first to identify changes in the multiple-unit activity (MUA) corresponding to changes in LH pulses [29]. By recording electrical activity in the MBH, the neural activity of the putative GnRH pulse generator was successfully represented as periodic bursts of MUA (termed MUA volleys) in monkeys [29–35], rats [36–40], and goats [41–46]. Those studies unambiguously demonstrated that the pulsatile discharge of GnRH into the portal vessels is governed by neural substrates in the MBH that fire a high-frequency volley of action potentials. However, none of the aforementioned studies successfully identified a specific neuronal population within the MBH that was responsible for the generation of the MUA volley.
The MUA volley was observed in the MBH in all animals, regardless of the difference in the distribution of GnRH neurons between species; GnRH cells are relatively abundant in the MBH of monkeys [47, 48], moderately so in goats [49], and few, if any, in rats [50, 51]. Moreover, during the LH surge, when the activity of GnRH neurons was extremely enhanced, the basal MUA activity did not change and the MUA volley frequency decreased rather than increased [30, 31, 42, 44]. These findings strongly suggest that the MUA volley originates outside of the GnRH neuronal network. It was proposed that the observed bursts of MUA in the MBH might reflect the pulsatile activation of GnRH fibers as they traverse en passant to the ME; in this case, the GnRH pulse would be triggered by another unidentified group of oscillators. Thus, the neural substrate of the GnRH pulse generator was still to be determined.
When MUA is measured in goats through an electrode targeted to the posterior ARC (which is part of the MBH), in which a number of kisspeptin neurons are concentrated (Fig. 14.1a), rhythmic MUA volleys are found at regular intervals and are temporally associated with LH pulses (Fig. 14.1b) in both gonadectomized males [52] and females [53]. Furthermore, treatment of OVX goats with estradiol (E2) increases the intervolley interval (i.e., decreases the MUA frequency), while the duration of the volley is decreased (Fig. 14.2a–c). The frequency of the MUA volley in goats is also profoundly decreased by progesterone (P) (Fig. 14.2d) [53]. These results are likely to reflect the negative feedback actions of gonadal sex steroids. Because these results are consistent with those previously demonstrated [30, 35, 42], it is reasonable to conclude that the MUA volley observed at close vicinity of ARC kisspeptin neurons represents the GnRH pulse generator activity. These results lead us to propose a compelling idea that the population of ARC kisspeptin neurons is the intrinsic source of the GnRH pulse generator [53–55]. However, the argument remains circumstantial at this moment. Because the MUA is the summation of the electrical activity of multiple neurons around the electrode, it is still possible that the MUA volley originates from a population of anonymous non-kisspeptin neurons residing in the same vicinity as kisspeptin neurons.
MUA recording at close vicinity of kisspeptin neurons in the ARC. (a) A photomicrograph showing the placement of MUA recording electrode in a section immunostained for kisspeptin. Arrowheads indicate the area where a trace of a bundle of electrodes is observed. ARC arcuate nucleus; 3V third ventricle. Scale bar: 1 mm. (b) Representative profiles of the MUA and plasma LH concentrations in an OVX goat. Panel (b) was modified from Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010 Feb 24;30(8):3124–32. With permission from Journal of Neuroscience
Effects of ovarian steroids on the MUA and LH secretion. (a) Representative profiles of the MUA and plasma LH concentrations in an OVX goat. (b) Representative profiles of the MUA and plasma LH concentrations in an E2-treated OVX goat. (c) Changes in the intervolley interval (blank circle) and volley duration (solid square) of the MUA volley after the E2 treatment. Data were collected for 6 h (12:00–18:00) in each day, and values are expressed as mean ± SEM in three goats. **p > 0.01 compared with those on Day 0. (d) Representative profiles of the MUA and plasma LH concentrations in an E2 plus P-treated OVX goat. Note that the MUA volley is invariably accompanied by an LH pulse, regardless of the steroidal milieu. Panel (d) was reproduced from Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010 Feb 24;30(8):3124–32. With permission from Journal of Neuroscience
Anatomical Aspects of ARC Kisspeptin Neurons in Relation to the GnRH Pulse Generator
In theory, the GnRH pulse generator should possess several neural characteristics to perform its tasks, including the generation of rhythmic oscillations, electrophysiological synchronization, transmission of the signal of rhythmic oscillation to GnRH neurons, elicitation of a pulsatile GnRH discharge, and processing of the negative feedback action of gonadal steroids. It appears that the functional and anatomical characteristics of ARC kisspeptin neurons meet these requirements.
Using the ovine model, Goodman et al. [56] were the first to document that kisspeptin neurons in the ARC co-express neurokinin B (NKB) and dynorphin A (Dyn). Since then, the colocalization of kisspeptin with either NKB or Dyn—or both—in ARC neurons was identified in a variety of mammals, including mice [57, 58], rats [59], goats [53], monkeys [60], and humans [61]. Therefore, concomitant expression of these three peptides in single ARC neurons appears to be a common feature across mammalian species. Those neurons, therefore, have been referred to as KNDy (kisspeptin/NKB/Dyn) neurons [62].
Anatomical evidence indicates that KNDy neurons comprise a neuronal network interconnected by axon (and/or dendritic) collaterals. For example, in the rodent [63] and ovine [63] ARC, NKB/Dyn neurons receive close appositions from fibers containing NKB/Dyn. Dyn neurons in the ARC form synaptic contacts with Dyn fibers [64]. It is therefore not surprising that kisspeptin/NKB and kisspeptin/Dyn neurons are surrounded by their own dense network of fibers [53]. Moreover, an anterograde tracer study in rats revealed that NKB neurons in the ARC are bilaterally interconnected by NKB axons [65]. Importantly, NKB neurons in the ARC contain NKB receptors (NK3R) [63, 66] and ARC Kiss1 neurons express both NK3R and KOR [57]. These reports suggest that NKB/NK3R and Dyn/KOR signaling pathways might play a role in an auto-feedback loop (or paracrine feedback loop) of KNDy neurons [55, 57, 62, 67, 68]. However, it should be noted that one study recently reported that KNDy neurons in the male mouse do not to express KOR [58], which is inconsistent with this group’s earlier report. Other KOR-expressing interneurons mediating Dyn’s action might be involved in the auto-feedback loop of KNDy neurons in the ARC.
NKB/NK3R signaling is thought to play a role in stimulating neuronal activity [69], whereas Dyn/KOR (Dyn receptor) signaling is considered to participate in suppressing neuronal activity [70, 71]. By possessing these two opposing signaling mechanisms and forming an anatomical network structure, the population of ARC KNDy neurons seems to possess the required framework for a role as a GnRH pulse generator. For example, reciprocal interactions between NKB/NK3R and Dyn/KOR (or other inhibitory signaling mediating the Dyn action) signaling would make it possible to generate pseudo-pacemaking activities, providing the oscillatory drive of the GnRH pulse generator. The neural network would be suitable for electrophysiological synchronization of individual neurons.
Kisspeptin fibers make extensive associations with GnRH axons in the ME [72–75], and kisspeptin could therefore act as the output of the pulse generator to influence GnRH neurons. Electron microscopy has revealed that kisspeptin axon terminals are in fact in close apposition to GnRH axon terminals [73, 74]. Considering the fact that NKB is contained in KNDy neurons, but not in POA kisspeptin neurons (Fig. 14.3a–c), and that a majority of those kisspeptin fibers in the ME also contain NKB (Fig. 14.3d–f) [73, 75, 76], it is likely that KNDy neurons send, although not exclusively, dense projections to the ME [62, 68] and interact with Kiss1r on GnRH axon terminals. However, the presence of Kiss1r protein on GnRH axon fibers has yet to be demonstrated since there is currently not a good Kiss1r antibody.
Dual labeling of kisspeptin and NKB in the E2-treated OVX goat. Photomicrographs of sections of the POA (a–c) or ME (d–f) immunostained for kisspeptin (a and d) and NKB (b and e). (c, f) are computer-aided merged images of (a) and (b), or (d) and (e), respectively. The arrows in (a) and (c) indicate cell bodies containing exclusively kisspeptin immunoreactivity. The green, red, and yellow arrowheads show kisspeptin, NKB, and kisspeptin/NKB positive fibers. Note that a majority of kisspeptin positive fibers contain NKB immunoreactivity (f) at the ME. MEe the external layer of the ME; pt pars tuberalis. Scale bar: 50 μm
It is thought that the GnRH pulse generator is responsive to the negative feedback actions of gonadal steroids [77]. Although there are several populations of neurons that contain sex steroid receptors in the hypothalamus, such as GABA [78], neuropeptide Y [79], substance P [80], somatostatin [81], beta-endorphin [82], or dopamine [82] neurons, KNDy neurons are conspicuous in that virtually all of them express both estrogen receptor alpha [63, 83–86] and progesterone receptor [64, 87] in the female or androgen receptors in the male [88]. This anatomical property further supports the possibility that the KNDy neurons may comprise for the GnRH pulse generator.
Roles of NKB/NK3R, Dyn/KOR, and Kisspeptin/Kiss1r Signaling Pathways in the GnRH Pulse Generation
NKB/NK3R Signaling
The involvement of NKB in the control of GnRH/LH secretion was initially proposed based on morphological changes in NKB neurons in the ARC (the infundibular nucleus in primates) of postmenopausal women and experimental animals [67, 83]. The proposition is strongly supported by the finding that mutations in either Tac3 or Tacr3 (which encode NKB and NK3R, respectively) cause severe gonadotropin deficiency in humans [89, 90] and that Tacr3 null mice show reduced gonadal activities, although they are not completely infertile [91]. Those studies predicted the stimulatory action of NKB on GnRH/LH secretion, but initial reports provided a controversial view indicating that senktide (a selective NK3R agonist) decreased LH secretion in rats [92] and mice [57].
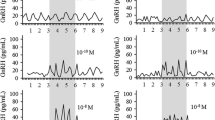
Our electrophysiological studies clarified the physiological role of NKB by examining effects of activation or blockade of the NKB/NK3R signaling pathway on the GnRH pulse generator activity in goats, using MUA recordings aimed at KNDy neurons. A bolus intracerebroventricular (icv) administration of NKB immediately induces multiple MUA volleys in the area where KNDy neurons reside, followed by a slight quiescent period before the resumption of spontaneous MUA volleys (Fig. 14.4a) [53]. When senktide was peripherally infused, the intervolley interval of the MUA volley was decreased and was maintained at a relatively constant level throughout the infusion period (Fig. 14.4b) [93]. On the other hand, the blockade of NKB/NK3R signaling by peripheral administration of an NK3R antagonist significantly decreased the occurrence of MUA volleys (Wakabayashi et al., unpublished data). These results suggest that the role of NKB/NK3R signaling is to stimulate the pulse generator activity in the ARC region, which may in fact be the GnRH pulse generator. Because KNDy neurons contain NKB receptors [57, 58, 63, 66], and icv administration of senktide induces cFos in KNDy neurons [59, 94], it is likely that a population of KNDy neurons in the ARC is at least one of the sites of NKB’s stimulatory action. Indeed, recent electrophysiological studies using Kiss1-CreGFP transgenic mice demonstrated that NKB elicits trains of action potentials in Kiss1 neurons in the ARC via NK3R [58].
Effects of NK3R agonists on the MUA and LH secretion. (a) Representative profiles of the MUA and plasma LH concentrations in an OVX goat that received a bolus icv injection of NKB at an indicated time point. (b) Representative profiles of the MUA and plasma LH concentrations in an E2-teated OVX goat received iv infusion of saline (upper) or senktide (lower) for 4 h. Note that the change in LH concentrations during the senktide infusion is an enhanced pulse frequency (although some pulses are ambiguous) but not increase or decrease in overall concentrations. Panel (a) was modified from Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010 Feb 24;30(8):3124–32. With permission from Journal of Neuroscience
MUA studies in goats also uncovered an important aspect in the pulse-generating mechanism. Since the activation of NK3R by either a bolus administration of NKB or continuous infusion of senktide resulted in intermittent MUA volleys, rather than a single sustained rise in the MUA, it is hypothesized that the stimulatory action of NKB/NK3R signaling on MUA firing is counteracted by some endogenous inhibitory drive, which operates immediately after the induction of the MUA volley and gradually reduces its inhibitory tone thereafter.
Dyn/KOR Signaling
It has been shown that administration of naloxone, a nonselective opioid receptor antagonist, increases the frequency of LH pulses [94] and bursts of the GnRH pulse generator [34, 43]. Moreover, a series of elegant studies in sheep indicated that the inhibitory effect of P on pulsatile GnRH/LH secretion is mediated by endogenous opioid peptides, namely, Dyn [95–97]. In support of this, icv administration of Dyn in goats suppresses the occurrence of the MUA volleys in the ARC region, resulting in a marked increase in the intervolley interval after the treatment (Fig. 14.5a). On the other hand, the blockade of Dyn/KOR signaling by icv administration of nor-binaltorphimine (nor-BNI, a selective KOR antagonist) reduced the intervolley interval and increased the volley duration (Fig. 14.5b, c) [53], indicating that the GnRH pulse generator activity is under a tonic suppression by endogenous Dyn. In vasopressin neurons of the supraoptic nucleus, Dyn/KOR signaling has been suggested to participate in termination of the phasic firing and the release of vasopressin by an autosynaptic loop [98, 99]. With an analogy to vasopressin neurons, it is proposed that Dyn/KOR signaling plays a role in extinguishing the bursts of KNDy neurons in the ARC and regulating the duration of nadir between each bout of bursts.
Effects of KOR agonist or antagonist on the MUA and LH secretion in an OVX goat. (a) Representative profiles of the MUA and plasma LH concentrations in an OVX goat that received a bolus icv injection of Dyn at the indicated time point. (b) Representative profiles of the MUA and plasma LH concentrations in the goat that received icv infusion of KOR antagonist (nor-BNI) for 2 h. (c) Changes in the intervolley interval and volley duration before (Pre, blank bar) and during (solid bar) the nor-BNI infusion periods. Values are expressed as mean ± SEM in four goats. **p > 0.01, *p > 0.05 compared with respective Pre values. Reproduced from Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010 Feb 24;30(8):3124–32. With permission from Journal of Neuroscience
Kisspeptin/Kiss1r Signaling
Peripheral injection of kisspeptin-10 [39, 54], or central administration of the full-length kisspeptin (Wakabayashi et al., unpublished data), which elicits a robust release of LH, has no effect on either amplitude or frequency of the MUA volley. In a preliminary experiment, we observed in goats that the blockade of kisspeptin/Kiss1r signaling by a continuous activation of Kiss1r resulted in a complete suppression of LH secretion and no detectable LH pulses in plasma, as demonstrated in other species [100–102], whereas the occurrence of MUA volleys was unchanged [103]. Furthermore, the expression of Kiss1r was not detected in KNDy neurons [75, 104]. These results suggest that kisspeptin/Kiss1r signaling is not involved in the GnRH pulse-generating mechanism per se. However, fibers surrounding KNDy neurons contain not only NKB and Dyn [53, 62, 63, 105] but also kisspeptin [54, 86, 106]. Moreover, treatment with a kisspeptin antagonist into the ARC suppresses pulsatile LH secretion [107]. Therefore, the possibility that kisspeptin may have some functions in the control of GnRH/LH secretion by acting on other cells than KNDy neurons still cannot be ruled out. However, it is also likely that the kisspeptin antagonist treatment did not affect the GnRH pulse generator, but rather diffused to the median eminence where it was able to block kisspeptin stimulation of GnRH fibers, resulting in suppressed LH secretion.
It is very likely that the primary role of kisspeptin/Kiss1r signaling in the GnRH pulse generation mechanism is to transmit volleys of action potentials from the pulse generator to GnRH neurons and regulate pulsatile GnRH secretion at the level of the ME. Several lines of evidence support this notion. First, in monkeys, kisspeptin is secreted into the ME episodically and is temporally associated with pulsatile GnRH secretion [108]. Second, kisspeptin stimulates GnRH release from the ME in vivo [108] and in vitro [74, 75, 109], potentially acting via Kiss1r [109]. Third, administration of a kisspeptin antagonist directly into the ME suppresses pulsatile GnRH release [110].
Interaction of NKB and Kisspeptin Signaling
Human genetic studies [89, 90, 111, 112] indicate that kisspeptin and NKB signaling play pivotal roles in the control of reproduction by facilitating GnRH secretion. In concert, it has been demonstrated in a variety of species that activation of Kiss1r [113] or NK3R [58–60, 114–117] increases LH secretion. Moreover, it has been shown that administration of antagonists for either Kiss1r [75, 110] or NKB receptor [60, 118] suppresses LH secretion. Their similar physiological characteristics and concomitant existence in KNDy neurons suggest an intimate association between kisspeptin and NKB signaling.
Recently it has been demonstrated that the blockade of kisspeptin/Kiss1r signaling by Kiss1r desensitization [115] or in Kiss1r KO mice [116] abrogates the stimulatory action of senktide on LH secretion, whereas the block of NKB/NK3R signaling by NK3R desensitization does not affect the ability of kisspeptin to stimulate LH secretion [115]. We have observed in goats that the blockade of kisspeptin/Kiss1r signaling completely eliminates LH pulses without affecting the MUA volley [103], whereas the occurrences of the MUA volley and LH pulses are concomitantly postponed after the injection of NK3R antagonist (Wakabayashi et al., unpublished data). Furthermore, GnRH neurons possess Kiss1r [75, 104, 119] but not NK3R [58, 66], but see Krajewski et al. [120], and NK3R agonists have no effect on electrophysiological activities of GnRH neurons in vitro [58]. Thus, it is plausible to conclude that NKB/NK3R signaling is upstream from kisspeptin/Kiss1r signaling, and that the activation of NK3R stimulates, via kisspeptin/Kiss1r signaling, a discharge of GnRH, and thus LH [59, 115–117].
We reported that icv administration of NKB induced a distinct MUA volley, with an accompanying LH pulse, in P-treated OVX goats, whereas the association of the MUA volley and LH pulse was ambiguous in some instances in OVX and E2-treated OVX goats, and overall LH secretion was reduced by a high dose (but not a low dose) of NKB [52]. However, with the latter, the initial event after NKB treatment was a discharge of LH, which was followed by a gradual decline of basal LH levels (Fig. 14.4a). In those animals with reduced LH secretion, several MUA volleys that had an extraordinarily shorter intervolley interval were induced, and there was a slight pause before the normal spontaneous MUA volley were reestablished. We assume that this pause resulted in an extended decline of basal LH levels, leading to an apparent reduction in LH secretion. Excessive activation of NK3R might therefore cause dysfunction among the NKB/Dyn-kisspeptin-GnRH-LH cascade, such as a hyperenhancement of the Dyn/KOR signaling tone, before the resumption of normal bursting activities of KNDy neurons. This may, at least in part, be responsible for the inconsistent results of LH responses to pharmacological NK3R agonist treatments [40, 53, 57, 92].
Electrophysiological Properties of the GnRH Pulse Generator
Knobil and colleagues uncovered the single unit components of the MUA underlying the operation of the GnRH pulse generator by cluster analysis in monkeys [33]. The results indicated that the MUA volley is the consequence of coincidental increases in the firing rate of individual cells that are active even during the intervals between volleys, rather than the activation of previously silent cells. Thus, neurons consisting of the GnRH pulse generator appear to have electrophysiological properties for both spontaneous and burst activities. In this context, it is of great interest that recent findings in Kiss1-CreGFP mice [121] and genetically intact guinea pigs [122] show that ARC kisspeptin neurons do possess such electrophysiological properties. Levine [123, 124] has proposed in his model of the GnRH pulse-generating mechanism that the random activity of any neurons within an interconnected network would initiate the process of the pulse-generating activity. It is conceivable that spontaneous activity in ARC kisspeptin neurons plays a role to generate such random activity, though this requires further investigation.
A Putative Mechanism of the GnRH Pulse Generation
Taken all together, we propose, although highly speculative, the following working hypothesis for the mechanism of GnRH pulse generation [55]:
-
1.
KNDy neurons in the ARC send projections to GnRH terminals in the ME, while their collaterals and/or dendrites form a bilateral neural network connecting each other (Fig. 14.6a).
Fig. 14.6 A speculative hypothesis for the role of KNDy neurons in the generation of pulsatile GnRH release. (a) A population of KNDy neurons forms a neural network connected by their axon collaterals (and/or dendrites). Through the reciprocal actions of NKB/NK3R and Dyn/KOR signaling in the KNDy neuron network, episodic bursts are periodically generated, each of which, in turn, induces pulsatile discharge of kisspeptin at the ME and hence, pulsatile GnRH release into Fig. 14.6 (continued)the portal circulation. (b) It is assumed that three components are involved in the generation of the burst: the random activity of any neuron within the network that initiates the burst, NKB/NK3r signaling that evokes synchronized bursting activities in the network, and Dyn/KOR signaling that inhibits the bursting activities. Progesterone enhances the inhibitory tone of Dyn/KOR signaling, which acts to reduce the frequency of the periodic burst. Estrogen attenuates the stimulatory tone of NKB/N3R signaling and the excitability of KNDy neurons, which act to shorten the duration of each burst and to reduce the frequency of the periodic burst, respectively. (c) A sustained activation of KNDy neurons by continuous administration of NK3R agonist results in an apparent rise in the random activity, leading to an increase in the frequency of the burst. (d) A sustained attenuation of KOR signaling by continuous administration of KOR antagonist also produces an increase in the frequency of the burst. See text for details
-
2.
The random activity of any neuron within the KNDy neuron network would propagate among other neurons in the network through NKB/NK3 signaling to evoke synchronized bursting activities (volleys of action potentials) among KNDy neurons, which may function as a kind of positive feedback mechanism.
-
3.
At the same time, Dyn would also be released by bursting activities in KNDy neurons, and Dyn/KOR signaling is considered to act, with a slight time lag (perhaps caused by differences of secretory mechanism or cellular signal transduction processes between NKB/NK3R and Dyn/KOR signaling), to extinguish these bursts, resulting in the net activity of the KNDy neuronal network to be an episodic oscillation (Fig. 14.6b).
-
4.
It is suggested that Dyn/KOR signaling then imposes a prolonged quiescence, or a refractory period, which lasts until the drive of Dyn/KOR signaling diminishes enough to allow the propagation of random activities again.
-
5.
The reciprocal interaction between the stimulatory tone of NKB/NK3R signaling and the inhibitory tone of Dyn/KOR signaling would generate intermittent oscillations, providing a pseudo-pacemaking activity in the KNDy neuron network (Fig. 14.6b).
-
6.
Each oscillation would induce a pulse of kisspeptin release at the ME, which in turn would trigger a discharge of GnRH through kisspeptin/Kiss1r signaling, producing a pulsatile mode of GnRH secretion into the portal circulation (Fig. 14.6a).
This hypothesis is in accord with that of other research laboratories who have established the KNDy cell model [57, 62, 68] as well as the model proposed by Levine [123, 124] before the discovery of kisspeptin.
Implications Based on the Hypothesis
The Source of the MUA Volley (GnRH Pulse Generator Activity)
The MUA volleys, which represent electrophysiological manifestations of the GnRH pulse generator, can be monitored at the posterior ARC (Fig. 14.1). Although there are several neuronal populations, such as NPY [46], dopamine [82], substance P [80], as well as other yet to be determined neurons in the ARC, the population of KNDy neurons might be the only one that is fully equipped with the prerequisite neural mechanisms to act as the GnRH pulse generator, i.e., generating rhythmic oscillation, synchronizing activities within the population, and transmitting the rhythmic activity to GnRH neurons. Moreover, the negative feedback action of E2 on LH secretion, which is mediated by the GnRH pulse generator [77], is completely diminished by a pharmacological ablation of KNDy neurons [125]. Thus, it is plausible that the population of KNDy neurons is the intrinsic source of the MUA volley observed at the posterior ARC in goats [53, 54] as well as in the MBH of monkeys [29–35], rats [36–40], and goats [41–46].
Putative Mechanisms Underlying the Negative Feedback Actions of Steroid Hormones
Mechanisms of the negative feedback action of gonadal steroids can be, at least in part, explained by the schema shown in Fig. 14.6b. Progesterone is a potent inhibitor of pulsatile GnRH secretion in many species. KNDy neurons contain Dyn and receptors for P [64, 87], and P increases the expression of Dyn [97]. Therefore, it is suggested that P enhances the inhibitory drive of Dyn/KOR signaling, leading to a reduction in the frequency of burst activities in KNDy neurons (Fig. 14.6b). This speculation is in concert with the previous finding that blockade of Dyn/KOR signaling reverses the inhibitory effect of P on pulsatile LH secretion in rats [126] and sheep [127]. It appears that Dyn/KOR signaling may play a critical role in determining the length of the refractory period after the burst in KNDy neurons.
One aspect of E2 negative feedback is a decrease in the amplitude (amount) of LH secretion. The expression of not only NKB [57, 128, 129] but also NK3R [57], in the ARC, is decreased by E2, suggesting that E2 acts to attenuate the stimulatory drive of NKB/NK3 signaling. Figure 14.6b indicates that such E2 action would lead to “thinning” of the burst of KNDy neurons, which might be reflected as a marked decrease in the duration of the MUA volley after E2 treatment (Fig. 14.2c). Given that the release of kisspeptin to GnRH neuronal projections in the ME is mainly under the control of the burst activity of KNDy neurons (Fig. 14.6a), the shortening of the burst of KNDy neurons by E2 would result in a decline in the amount of GnRH released during each pulse. This may represent one aspect of the negative feedback action of E2. Moreover, it has been indicated in many species that E2 also reduces the expression of kisspeptin in the ARC [85, 87, 106, 130], which may also contribute to the decreased amount of GnRH released per pulse.
The other aspect of the E2 action is its negative effect on the frequency of GnRH/LH pulses. It has been shown that E2 also reduces the frequency of the MUA volley and LH pulses in several species, including rats [38], monkeys [30], and goats (Fig. 14.2 [42, 44, 53]), although this action of E2 seems less conspicuous in sheep [131, 132]. Because the inhibitory effect of E2 is much smaller than P (Fig. 14.2), it seems unlikely that Dyn/KOR signaling mediates the E2 action. Instead, other mechanisms may also be involved in the negative feedback action of E2. One possible mechanism is the alteration of neuronal excitability. It is possible that E2 reduces the excitability of KNDy neurons through modifying electrophysiological properties of the cell membrane, as shown in mouse GnRH neurons [133], leading to the attenuation of spontaneous activity of individual neurons. This would decrease, in a stochastic manner, the occurrence of the random activity that initiates the bursting process in the KNDy neuron. Although highly speculative, it is suggested that neuronal mechanisms involving E2 actions in KNDy neurons, such as the excitability for example, are associated with the pathway of the control of GnRH secretion by nutrition, because the inhibitory influence of several nutritional stressors on the GnRH pulse generator is more conspicuous in the presence of E2 than its absence [32, 46, 134, 135].
Putative Mechanism for the Action of Pheromones on the GnRH Pulse Generator
In goats and sheep, exposure of seasonally anestrous females to the male pheromone results in an out-of-seasonal ovulation [136, 137]. Because the initial endocrine event following the reception of the pheromone is the stimulation of pulsatile GnRH/LH secretion, it is suggested that the central target of the pheromone signal is the GnRH pulse generator [45]. We examined whether the KNDy neuronal network was involved in the pheromone action in OVX goats using MUA recording with the electrode aimed at KNDy neurons. Exposure to the male pheromone, between two successive MUA volleys, immediately induced an MUA volley and an accompanying LH pulse [138]. This pheromone effect on the MUA volley and LH secretion was abrogated by the treatment with an NK3R antagonist (Sakamoto et al., unpublished data). Further, the pheromone evoked the MUA volley but not LH pulses when kisspeptin/KOR signaling was blocked (Sakamoto et al., unpublished data). Therefore, it seems conceivable that the action of the male pheromone is indeed mediated by the KNDy neuronal network [139]. Interestingly, the effect of the pheromone was time dependent, i.e., the pheromone was not able to induce the MUA volley immediately after the preceding MUA volley, and the ability of the pheromone in inducing the MUA volley increased towards the occurrence of the next MUA volley [138]. This suggests that pheromone action may be counteracted by the inhibitory tone of Dyn/KOR signaling, which we propose would gradually decrease from the maximum to the basal level during the refractory period (Fig. 14.6b). These pheromone studies also reveal a note of caution that should be taken into account when observing the GnRH/LH response to an experimental stimulation of KNDy neurons. If a stimulus acts at the level of Kiss1r (e.g., kisspeptin), one would be able to expect a consistent result. However, if the stimulus acts at the levels of NK3R (e.g., senktide), it is possible that the GnRH/LH response to the treatment is variable depending on the timing of the treatment between two spontaneously occurring bursts of KNDy neurons.
Perspective on the Application
GnRH neurons are charged with the role of maintaining the ever-present basal levels of circulating gonadotropins for the normal functioning of the gonads. Because continuous exposure of the gonadotrophs to GnRH results in the abolishment of gonadotropin secretion, a pulsatile mode of GnRH discharge is obligatory to produce sustained gonadotropin secretion [3]. In this context, it is of interest that continuous infusion of NKB (Fig. 14.4b) or nor-BNI (Fig. 14.5b) induced frequent MUA volleys rather than a sustained raise in the MUA. Our hypothesis envisages that the frequency of periodic bursts in KNDy neurons can be increased by continuously raising the stimulatory tone of NKB/NK3R signaling by NK3R agonists (Fig. 14.6c) or reducing the inhibitory tone of Dyn/KOR signaling by KOR antagonists (Fig. 14.6d). The preliminary result detailed in this chapter (Fig. 14.2b) partially supports this proposition. There are several occasions in which insufficient LH pulse frequency causes reproductive disorders, such as women with anorexia nervosa [138], exercise amenorrhea [140], or hyperprolactinemia [141]. Our proposed model implies that NKB agonists and KOR antagonists may hold promise as novel therapeutic drugs to accelerate or improve gonadal activities via their ability to enhance the GnRH pulse generator activity.
References
Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E (1970) Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology 87(5):850–853
Midgley AR Jr (1966) Radioimmunoassay: a method for human chorionic gonadotropin and human luteinizing hormone. Endocrinology 79(1):10–18
Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G (1980) Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 207(4437):1371–1373
Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E (1981) Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109(2):376–385
Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E (1983) Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology 112(6):2076–2080
Gay VL, Sheth NA (1972) Evidence for a periodic release of LH in castrated male and female rats. Endocrinology 90(1):158–162
Butler WR, Malven PV, Willett LB, Bolt DJ (1972) Patterns of pituitary release and cranial output of LH and prolactin in ovariectomized ewes. Endocrinology 91(3):793–801
Rahe CH, Owens RE, Fleeger JL, Newton HJ, Harms PG (1980) Pattern of plasma luteinizing hormone in the cyclic cow: dependence upon the period of the cycle. Endocrinology 107(2):498–503
Van de Wiel DF, Erkens J, Koops W, Vos E, Van Landeghem AA (1981) Periestrous and midluteal time courses of circulating LH, FSH, prolactin, estradiol-17 beta and progesterone in the domestic pig. Biol Reprod 24(2):223–233
Fitzgerald BP, I’Anson H, Loy RG, Legan SJ (1983) Evidence that changes in LH pulse frequency may regulate the seasonal modulation of LH secretion in ovariectomized mares. J Reprod Fertil 69(2):685–692
Foster DL, Lemons JA, Jaffe RB, Niswender GD (1975) Sequential patterns of circulating luteinizing hormone and follicle-stimulating hormone in female sheep from early postnatal life through the first estrous cycles. Endocrinology 97(4):985–994
Sollenberger MJ, Carlsen EC, Johnson ML, Veldhuis JD, Evans WS (1990) Specific physiological regulation of luteinizing hormone secretory events throughout the human menstrual cycle: new insights into the pulsatile mode of gonadotropin release. J Neuroendocrinol 2(6):845–852
Lincoln GA, Short RV (1980) Seasonal breeding: nature’s contraceptive. Recent Prog Horm Res 36:1–52
Minabe S, Uenoyama Y, Tsukamura H, Maeda K-I (2011) Analysis of pulsatile and surge-like luteinizing hormone secretion with frequent blood sampling in female mice. J Reprod Dev 57(5):660–664
Clarke IJ, Cummins JT (1982) The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111(5):1737–1739
Moenter SM, Brand RM, Midgley AR, Karsch FJ (1992) Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 130(1):503–510
Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E (1978) The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 102(1):52–62
Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E (1978) Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 202(4368):631–633
Moenter SM, Brand RC, Karsch FJ (1992) Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130(5):2978–2984
Halasz B, Pupp L (1965) Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotrophic area. Endocrinology 77(3):553–562
Blake CA, Sawyer CH (1974) Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology 94(3):730–736
Silverman AJ, Antunes JL, Ferin M, Zimmerman EA (1977) The distribution of luteinizing hormone-releasing hormone (LHRH) in the hypothalamus of the rhesus monkey. Light microscopic studies using immunoperoxidase technique. Endocrinology 101(1):134–142
Ohkura S, Tsukamura H, Maeda K-I (1992) Effects of transplants of fetal mediobasal hypothalamus on luteinizing hormone pulses impaired by hypothalamic deafferentation in adult ovariectomized rats. Neuroendocrinology 55(4):422–426
Martinez de la Escalera G, Choi AL, Weiner RI (1992) Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1-1 GnRH neuronal cell line. Proc Natl Acad Sci U S A 89(5):1852–1855
Terasawa E, Schanhofer WK, Keen KL, Luchansky L (1999) Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19(14):5898–5909
Richter TA, Keen KL, Terasawa E (2002) Synchronization of Ca2+ oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol 88(3):1559–1567
Witkin JW, Silverman AJ (1985) Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides 6(2):263–271
Halasz B, Kiss J, Molnar J (1989) Regulation of the gonadotropin-releasing hormone (GnRH) neuronal system: morphological aspects. J Steroid Biochem 33(4B):663–668
Knobil E (1981) Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol Reprod 24(1):44–49
Kesner JS, Wilson RC, Kaufman JM, Hotchkiss J, Chen Y, Yamamoto H, Pardo RR, Knobil E (1987) Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci U S A 84(23):8745–8749
O’Byrne KT, Thalabard JC, Grosser PM, Wilson RC, Williams CL, Chen MD, Ladendorf D, Hotchkiss J, Knobil E (1991) Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology 129(3):1207–1214
Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E (1992) Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology 56(5):666–673
Cardenas H, Ordog T, O’Byrne KT, Knobil E (1993) Single unit components of the hypothalamic multiunit electrical activity associated with the central signal generator that directs the pulsatile secretion of gonadotropic hormones. Proc Natl Acad Sci U S A 90(20):9630–9634
Grosser PM, O’Byrne KT, Williams CL, Thalabard JC, Hotchkiss J, Knobil E (1993) Effects of naloxone on estrogen-induced changes in hypothalamic gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Neuroendocrinology 57(1):115–119
O’Byrne KT, Knobil E (1993) Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod 8(suppl 2):37–40
Kawakami M, Uemura T, Hayashi R (1982) Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology 35(1):63–67
Nishihara M, Hiruma H, Kimura F (1991) Interactions between the noradrenergic and opioid peptidergic systems in controlling the electrical activity of luteinizing hormone-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology 54(4):321–326
Nishihara M, Sano A, Kimura F (1994) Cessation of the electrical activity of gonadotropin-releasing hormone pulse generator during the steroid-induced surge of luteinizing hormone in the rat. Neuroendocrinology 59(6):513–519
Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT (2008) Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology 149(3):1004–1008
Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O’Byrne KT (2012) The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153(1):307–315
Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, Hoshino K (1991) Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator activity in the goat. Neuroendocrinology 53(4):392–395
Tanaka T, Mori Y, Hoshino K (1992) Hypothalamic GnRH pulse generator activity during the estradiol-induced LH surge in ovariectomized goats. Neuroendocrinology 56(5):641–645
Ito K, Tanaka T, Mori Y (1993) Opioid peptidergic control of gonadotropin-releasing hormone pulse generator activity in the ovariectomized goat. Neuroendocrinology 57(4):634–639
Tanaka T, Mori Y, Hoshino K (1994) Long-term recording of hypothalamic GnRH pulse generation activity during programmed administration of progesterone and estradiol in the ovariectomized goat. J Reprod Dev 40(3):183–188
Hamada T, Nakajima M, Takeuchi Y, Mori Y (1996) Pheromone-induced stimulation of hypothalamic gonadotropin-releasing hormone pulse generator in ovariectomized, estrogen-primed goats. Neuroendocrinology 64(4):313–319
Ichimaru T, Mori Y, Okamura H (2001) A possible role of neuropeptide Y as a mediator of undernutrition to the hypothalamic gonadotropin-releasing hormone pulse generator in goats. Endocrinology 142(6):2489–2498
Silverman AJ, Antunes JL, Abrams GM, Nilaver G, Thau R, Robinson JA et al (1982) The luteinizing hormone-releasing hormone pathways in rhesus (Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: new observations on thick, unembedded sections. J Comp Neurol 211(3):309–317
Goldsmith PC, Thind KK, Song T, Kim EJ, Boggant JE (1990) Location of the neuroendocrine gonadotropin-releasing hormone neurons in the monkey hypothalamus by retrograde tracing and immunostaining. J Neuroendocrinol 2(2):157–168
Hamada T, Shimizu T, Ichikawa M, Mori Y (1992) Immunohistochemical study on gonadotropin-releasing hormone neurons in the Shiba goat brain. J Reprod Dev 38(2):133–142
Hoffman GE, Gibbs FP (1982) LHRH pathways in rat brain: ‘deafferentation’ spares a sub-chiasmatic LHRH projection to the median eminence. Neuroscience 7(8):1979–1993
Witkin JW, Paden CM, Silverman AJ (1982) The luteinizing hormone-releasing hormone (LHRH) systems in the rat brain. Neuroendocrinology 35(6):429–438
Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, Maeda K-I, Tsukamura H (2009) Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides 30(1):49–56
Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H (2010) Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30(8):3124–3132
Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda K-I, Okamura H (2009) Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21(10):813–821
Maeda K-I, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H (2010) Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res 1364:103–115
Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ (2007) Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148(12):5752–5760
Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA (2009) Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29(38):11859–11866
Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA (2011) Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152(11):4265–4275
Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA (2011) Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300(1):E202–E210
Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM (2010) Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151(9):4494–4503
Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I (2010) The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31(11):1984–1998
Lehman MN, Coolen LM, Goodman RL (2010) Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151(8):3479–3489
Burke MC, Letts PA, Krajewski SJ, Rance NE (2006) Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498(5):712–726
Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN (2002) Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143(11):4366–4374
Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE (2010) Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166(2):680–697
Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN (2010) Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22(1):1–12
Rance NE (2009) Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30(1):111–122
Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA (2010) Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 1364:116–128
Zhang L, Brass LF, Manning DR (2009) The Gq and G12 families of heterotrimeric G proteins report functional selectivity. Mol Pharmacol 75(1):235–241
Kelly MJ, Qiu J, Ronnekleiv OK (2003) Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci 1007:6–16
Sadja R, Alagem N, Reuveny E (2003) Gating of GIRK channels: details of an intricate, membrane-delimited signaling complex. Neuron 39(1):9–12
Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM (2008) Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149(9):4387–4395
Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K-I, Ichikawa M, Okamura H (2011) Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology 94(4):323–332
Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, Ajiki K, Ichikawa M, Okamura H, Maeda K-I, Tsukamura H (2011) Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol 23(10):863–870
Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ (2011) Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152(3):1001–1012
True C, Kirigiti M, Ciofi P, Grove KL, Smith MS (2011) Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol 23(1):52–64
Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE (1984) Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res 40:185–232
Flugge G, Oertel WH, Wuttke W (1986) Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology 43(1):1–5
Skinner DC, Herbison AE (1997) Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y, and beta-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology 138(6):2585–2595
Akesson TR, Micevych PE (1988) Estrogen concentration by substance P-immunoreactive neurons in the medial basal hypothalamus of the female rat. J Neurosci Res 19(4):412–419
Herbison AE (1994) Somatostatin-immunoreactive neurones in the hypothalamic ventromedial nucleus possess oestrogen receptors in the male and female rat. J Neuroendocrinol 6(3):323–328
Lehman MN, Karsch FJ (1993) Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133(2):887–895
Rance NE, Young WS III (1991) Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128(5):2239–2247
Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE (2000) Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141(11):4218–4225
Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA (2005) Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9):3686–3692
Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A (2006) Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 401(3):225–230
Smith JT, Clay CM, Caraty A, Clarke IJ (2007) KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148(3):1150–1157
Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM et al (2005) Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146(7):2976–2984
Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK (2009) TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41(3):354–358
Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A (2010) TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95(5):2287–2295
Yang JJ, Caligioni CS, Chan YM, Seminara SB (2012) Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153(3):1498–1508
Sandoval-Guzman T, Rance NE (2004) Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026(2):307–312
Wakabayashi Y, Yamamura T, Ohkura S, Homma T, Sakamoto K, Mori Y, Okamura H (2012) Senktide, a neurokinin B receptor agonist, stimulates pulsatile LH secretion through a mechanism mediated by the GnRH pulse generator in goats. In: Society for Neuroscience 42nd annual meeting (Neuroscience 2012), New Orleans, 2012, #90.07
Sakamoto K, Murata K, Wakabayashi Y, Yayou K, Ohkura S, Takeuchi Y, Mori Y, Okamura H (2012) Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev, in press
Whisnant CS, Goodman RL (1988) Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod 39(5):1032–1038
Whisnant CS, Goodman RL (1994) Effect of anterior hypothalamic deafferentation on the negative feedback of gonadal steroids on luteinizing hormone pulse frequency in the ewe. Domest Anim Endocrinol 11(2):151–159
Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN (2005) Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146(4):1835–1842
Brown CH, Ludwig M, Leng G (1998) kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J Neurosci 18(22):9480–9488
Iremonger KJ, Bains JS (2009) Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci 29(22):7349–7358
Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF Jr, Plant TM (2006) Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147(5):2122–2126
Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF Jr, Plant TM (2007) Effect of continuous intravenous administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology 148(7):3364–3370
Roa J, Vigo E, Garcia-Galiano D, Castellano JM, Navarro VM, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M (2008) Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab 294(6):E1088–E1096
Wakabayashi Y, Yamamura T, Ohkura S, Tanaka T, Kusaka M, Okamura H (2011) Effects of chronic administration of a metastin analog, TAK-683, on the GnRH pulse generator activity in ovariectomized goats. In: The Endocrine Society 93rd annual meeting & expo (ENDO 2011), Boston, 2011, #P2-264
Herbison AE, de Tassigny X, Doran J, Colledge WH (2010) Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151(1):312–321
Foradori CD, Amstalden M, Goodman RL, Lehman MN (2006) Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18(7):534–541
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K-I (2007) Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53(2):367–378
Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT (2009) Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 4(12):e8334
Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E (2008) An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149(8):4151–4157
d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH (2008) Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149(8):3926–3932
Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP (2009) Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29(12):3920–3929
de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E (2003) Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 100(19):10972–10976
Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK et al (2003) The GPR54 gene as a regulator of puberty. N Engl J Med 349(17):1614–1627
Oakley AE, Clifton DK, Steiner RA (2009) Kisspeptin signaling in the brain. Endocr Rev 30(6):713–743
Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL (2010) Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151(8):3836–3846
Ramaswamy S, Seminara SB, Plant TM (2011) Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94(3):237–245
Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M (2012) Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153(1):316–328
Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM (2012) Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 153(6):2756–2765
Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K-I, Tsukamura H (2011) Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 57(3):409–415
Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA (2004) Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80(4):264–272
Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE (2005) Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489(3):372–386
Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA (2011) Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152(11):4298–4309
Qiu J, Fang Y, Bosch MA, Ronnekleiv OK, Kelly MJ (2011) Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152(4):1503–1514
Levine JE (1999) Pulsatility in primates—a perspective from the placode. Endocrinology 140(3):1033–1035
Levine JE (2000) The hypothalamus as a major integrating center. In: Conn P, Freeman ME (eds) Neuroendocrinology in physiology and medicine. Humana Press, Totowa, NJ, pp 75–93
Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE (2012) Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 153(6):2800–2812
Gallo RV (1990) Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol 2(5):685–691
Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN (2004) Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145(6):2959–2967
Rance NE, Bruce TR (1994) Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60(4):337–345
Pillon D, Caraty A, Fabre-Nys C, Bruneau G (2003) Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15(8):749–753
Rometo AM, Krajewski SJ, Voytko ML, Rance NE (2007) Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92(7):2744–2750
Karsch FJ (1984) The hypothalamus and anterior pituitary gland. In: Austin CR, Short RV (eds) Reproduction in mammals: 3. Hormonal control of reproduction. Cambridge University Press, Cambridge
Goodman RL (1994) Neuroendocrine control of the ovine estrous cycle. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven Press, New York, pp 659–709
Sun J, Chu Z, Moenter SM (2010) Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 30(11):3912–3923
Cagampang FR, Maeda K-I, Tsukamura H, Ohkura S, Ota K (1991) Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J Endocrinol 129(3):321–328
Nagatani S, Tsukamura H, Maeda K-I (1994) Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology 135(3):870–875
Martin GB, Oldham CM, Cognie Y, Pearce DT (1986) The physiological responses of anovulatory ewes to the introduction of rams—a review. Livest Prod Sci 15:219–247
Delgadillo JA, Gelez H, Ungerfeld R, Hawken PA, Martin GB (2009) The ‘male effect’ in sheep and goats—revisiting the dogmas. Behav Brain Res 200(2):304–314
Murata K, Wakabayashi Y, Sakamoto K, Tanaka T, Takeuchi Y, Mori Y, Okamura H (2011) Effects of brief exposure of male pheromone on multiple-unit activity at close proximity to kisspeptin neurons in the goat arcuate nucleus. J Reprod Dev 57(2):197–202
Okamura H, Murata K, Sakamoto K, Wakabayashi Y, Ohkura S, Takeuchi Y, Mori Y (2010) Male effect pheromone tickles the gonadotrophin-releasing hormone pulse generator. J Neuroendocrinol 22(7):825–832
Veldhuis JD, Evans WS, Demers LM, Thorner MO, Wakat D, Rogol AD (1985) Altered neuroendocrine regulation of gonadotropin secretion in women distance runners. J Clin Endocrinol Metab 61(3):557–563
Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC (1984) Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. J Clin Endocrinol Metab 59(5):941–948
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Okamura, H., Tsukamura, H., Ohkura, S., Uenoyama, Y., Wakabayashi, Y., Maeda, Ki. (2013). Kisspeptin and GnRH Pulse Generation. In: Kauffman, A., Smith, J. (eds) Kisspeptin Signaling in Reproductive Biology. Advances in Experimental Medicine and Biology, vol 784. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6199-9_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6199-9_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6198-2
Online ISBN: 978-1-4614-6199-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)