Abstract
Polycystic ovary syndrome (PCOS) is an endocrine-metabolic disease affecting about 20–25% of women in reproductive age. Different mechanisms could contribute to the development of its typical clinical features (i.e. hirsutism, acne, oligo-amenorrhea, alopecia). Some genetic and epigenetic aspects and lifestyle changes seem to be involved in PCOS development. In this review, we shall summarize data from principal studies evaluating the impact of major genetic, epigenetic and environmental factors on the appearance of this female disorder. Literature review and analysis of the most relevant data until May 2020. Current data suggest the importance of genetics and epigenetics in the appearance of PCOS. Several genes, including those related to adrenal and ovarian steroidogenesis as well as those associated with hormonal response to gonadotrophins, androgens and insulin, have been demonstrated to be associated with PCOS. Besides, the phenomenon of methylation of genes and the presence of specific microRNA (miRNA) could take part in PCOS aetiology. Intrauterine exposure to androgens, glucocorticoids and/or some stressful conditions for foetus could contribute to the development of PCOS and other disorders observed in adolescence and later (e.g. premature adrenarche, atypical puberty, metabolic syndrome). Emerging studies report a theoretical role of endocrine disruptors, intestinal dysbiosis and Advanced Glycation End products (AGEs) in PCOS. PCOS is a polygenic and multifactorial hormonal and metabolic dysfunction. An appropriate knowledge of personal and/or family history, lifestyle and nutritional habits of PCOS patients has a great importance to early identify and manage this syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is a hormonal and metabolic disorder characterized by menstrual irregularities, hyperandrogenism (acne, amenorrhea, alopecia, etc.) and, sometimes, infertility [1, 2]. It is well recognized [3] that several hormonal alterations can favour clinical appearance of PCOS: for example, the increased serum levels of male hormones, like testosterone (T) and/or androstenedione (A), that is to say hyperandrogenemia, could produce skin signs like hirsutism and/or acne and alter lipid profile [3]. At the same time, the decreased insulin sensitivity associated with compensatory hyperinsulinemia may negatively influence glycaemic homeostasis and affect per se ovarian function [4, 5].
It must be underlined that the variable expressiveness of PCOS may partially depend on biological characteristics of each affected woman as well as on her lifestyle context and/or genetic predisposition [6]. Many authors pointed out the key role of environment, intrauterine and infancy history in the development of PCOS [7]. However, the actual involvement of genetic, epigenetic and environmental factors in this syndrome is still a matter of debate.
In this paper, we shall review the most important data about the relationship between PCOS and genetics, epigenetics and lifestyle. Our main purpose is to provide an updated narrative review of several interesting features of PCOS, other than hormonal and metabolic changes. Hereby, clinicians could approach and manage this complex syndrome with higher awareness, taking into account the importance of family history, intrauterine life and personal behaviour in the appearance of PCOS.
Methods
We searched in PubMed and Medline databases for major publications, including systematic reviews, meta-analyses and randomized controlled trials (RCTs) concerning genetic, epigenetic and environmental factors relating to PCOS. We included the most relevant data until May 2020. Our review excluded papers published in non-peer-reviewed journals.
Ethnicity
The prevalence of PCOS generally differs among countries and ethnic groups. For instance, Zhao et al. [8] by analysing the frequency of the syndrome diagnosed according to the National Institute of Health (NIH) criteria [9] showed that Mexican American and Caucasian subjects were affected by PCOS more often than South Chinese and Asian women. However, data about the impact of the ethnicity on PCOS remain inconclusive, because of the extreme heterogeneity of the available population studies, the differences regarding the chosen diagnostic criteria (e.g. NIH, Rotterdam) [1, 2] and the various cut-offs that were employed to define hirsutism, waist-to-hip ratio (WHR), body mass index (BMI) and insulin sensitivity [10].
Heritability
PCOS may be characterized by a familiar trait which could explain the evidence demonstrating a high prevalence (20–50%) of PCOS and its typical features [e.g. hyperandrogenemia, hirsutism, reproductive dysfunction, polycystic ovarian morphology (PCOM), level of serum Anti-mullerian hormone (AMH)] in female relatives of women with PCOS (mothers, sisters, haunts, grandmothers, post-pubertal daughters) [11, 12].
Furthermore, a high frequency of cardio-metabolic alterations—that is to say, glucose intolerance, hyperinsulinemia, dyslipidaemia and metabolic syndrome (MS)—was found in both male and female relatives of PCOS patients [13, 14].
Interestingly, Kahsar-Miller et al. [15], by studying a group of 93 PCOS patients (mean age= 27.5 ± 8.1 years; mean BMI= 33.0 ± 8.7 kg/cm2), found that 19 of 78 (24%) of their mothers and 16 of 50 (32%) of their sisters were affected by the same syndrome. More specifically, 5 of 78 (6%) of their mothers and 1 of 50 (2%) of their sisters had hirsutism [defined as either a Ferriman-Gallwey (FG) score ≥ 6 or a FG score ≥ 3 associated with a history of 12 or more sessions of electrology to any of the body areas scored].
More precisely, Legro et al. [16] demonstrated that the hyperandrogenic trait was common among some female first-degree relatives of women affected by PCOS, regardless of the clinical evidence of the complete syndrome according to the classical criteria. Another study [17] evaluating Dutch twin women [n=1332 monozygotic (MZ) (i.e. genetically identical) twins and n= 1873 dizygotic (DZ) twins/singleton sisters of twins] showed that correlations for MZ twins were more than twice than those observed for their DZ sisters as for oligo-amenorrhea (less than nine menstrual cycles in a year) [r= 0.67 95% confidence interval (CI): 0.49–0.80 vs r= 0.07 (95% CI: −0.19–0.34)], acne [r= 0.78 (95% CI: 0.69–0.84) vs r= 0.44 (95% CI: 0.30–0.56)], hirsutism [r= 0.86 (95% CI: 0.75–0.92) vs r= 0.28 (95% CI: 0.05–0.50)] and diagnosis of PCOS (defined as less than nine menstrual cycles per year associated with acne or hirsutism) [r= 0.71 (95% CI 0.43–0.88) vs r= 0.38 (95% CI: 0.00–0.66)] [17].
At the first sight, these data would seem to highlight the possible deep implication of genetic factors in the appearance of PCOS and to suggest that a positive family history might play a critical role in the diagnosis and management of this syndrome.
Nevertheless, according to these same evidences [11, 17], it could be supposed that different mechanisms might contribute to the heritability of one or more aspects of this syndrome and that a possible interplay between, on one hand, a genetic predisposition and, on the other hand, an exposure to common environmental factors and/or epigenetic alterations might exist. For instance, the higher prevalence of insulin resistance (IR) and type 2 diabetes mellitus (T2DM) reported in the parents and siblings of PCOS patients compared with controls [14] could result partly from a genetically determined condition and partly from the familiar similarities in terms of lifestyle/dietary habits, ethnicity and environment that could, in turn, affect epigenetics (See also sections “Genetics” and “Epigenetics and intrauterine environment”). In other words, the familial aggregation of PCOS and its associated features indicates the importance of genetic factors in the aetiology of this disease [14]. However, different phenotypes can be present in the same family due to the variability of expression and/or penetrance of the same gene and the potential impact of other concomitant factors.
Genetics
Different genes seem to be involved in PCOS aetiology [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] (Table 1). In particular, some authors hypothesized that the alterations of the expression of some specific genes associated with adrenal and ovarian steroidogenesis may contribute to hyperandrogenism [e.g. CYP11A1 (coding for P450 cholesterol side-chain-cleavage, P450scc), CYP17A1 (coding for 17α-hydroxylase and 17, 20-lyase), CYP21 (coding for 21-hydroxylase)] [19,20,21,22].
According to some studies [23, 24], the gene DENND1A—normally found in cells of ovarian theca and adrenal glands—and, specifically, its variant 2 maybe upregulated in theca cells of PCOS women, favouring androgen excess [25,26,27].
Dapas et al. [25], by performing a whole-genome sequencing on DNA of 261 women from 62 families (age range: 14–63 years) with one or more daughters with PCOS, found that 32 rare variants (2 coding, 30 noncoding) in the DENND1A were significantly associated with the reproductive and metabolic features of PCOS [Nominal P (Pn) = 5.31×10−5, Corrected P (Pc )= 0.039] and subjects with one or more of these DENND1A variants had significantly higher Luteinizing Hormone/Follicle Stimulating Hormone (LH/FSH) ratios (Pc = 0.0036). Additionally, the hyperandrogenic phenotype of PCOS (reproductive-age women with hyperandrogenemia and regular menses) with one or more DENND1A variants had significantly higher LH/FSH ratios than PCOS/HA phenotype women without DENND1A variants (Pc = 0.0180). Besides, in an interesting manner, even if unaffected by PCOS, women with one or more DENND1A variants tended to higher LH/FSH ratios than those of unaffected women without DENND1A variants (Pc = 0.1758).
On the other side, the co-presence of DENND1A.V2 in human adrenal gland could partly explain the possible coexistence of ovarian and adrenal steroidogenic abnormalities, clinically reported in approximately 25% of women with PCOS [19].
Other genes that are putatively linked to PCOS development maybe those related to the effects of steroid hormones [28]—in the case of genes coding for androgen receptor (AR), Sex Hormone-Binding Globulin (SHBG) and AMH—and/or those related to the regulation of the gonadotrophins [26,27,28,29,30] (Table 1). Specifically, some interesting data suggested the possible implications of the polymorphisms of both Follicle Stimulating Hormone Receptor (FSHR) and Luteinizing Hormone Receptor (LHR) in the development of PCOS [30,31,32,33,34,35,36] (Table 1).
Although the FSHR variants resulted the most studied (e.g. exon 10: rs6165: T307A and rs6166: N680S), a clear correlation between genotype and FSH levels is still lacking. Besides, many single nucleotide polymorphisms (SNPs) in the LHR or in FSH-β gene could also influence the phenotype of PCOS [31,32,33,34].
Other authors focused on genes involved in insulin activity and secretion to clarify the appearance of the metabolic traits of PCOS [37] (Table 1). The phenomenon of decreased insulin sensitivity could be explained by different mechanisms: (a) presence of anti-insulin antibodies; (b) impairments of insulin receptors (INSRs); (c) presence of several post-receptor defects, etc. [38]. Overall, all these abnormalities could be due to one or more specific genetic alterations that sometimes may coexist in the same subject and may trigger IR in adolescence and/or adult life, concurrently with other lifestyle and environmental factors [38]. Different SNPs in the gene coding for of INSR (e.g. rs2059807 and rs1799817) have been associated with PCOS through genome-wide association studies (GWAS) [39]. Nevertheless, these data are still controversial and the effective role of IR in the biology of theca cells remains unknown.
Furthermore, other genes related to energy homeostasis and/or chronic inflammation could influence PCOS development [19, 40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. In particular, the SNP rs9939609 of FTO (Fat Mass Obesity) gene appeared significantly higher in affected women as compared to healthy women and seemed to be particularly associated with obesity and T2DM [19] (Table 1). More specifically, an association study on 386 patients (mean age: 28.0 ± 6.5 SD years) with PCOS (defined by the Rotterdam-criteria) by Tan et al. [43] found that the impact of the FTO SNPs on BMI is larger in PCOS patients than in the general population, with an average effect of the FTO risk allele of 0.46 kg/m2 (95% CI 0.17–0.75 kg/m2). According to these results, it can be argued that the variants of FTO could exert a significant pathogenic role mostly in PCOS phenotype characterized by stronger metabolic alterations. However, it should be considered that, even if genetic variations of FTO resulted to be particularly related to the metabolic abnormalities of PCOS and, thus, might have substantial implications in the aetiology of this disorder, both obesity and/or IR remain markedly influenced by other endogenous and exogenous determinants [14].
Hence, although these findings appear promising, none of these genes resulted to be exclusively associated with the appearance of this syndrome, as expected for a multifactorial disease.
Epigenetics and Intrauterine Environment
The role of epigenetics regarding PCOS is intriguing [56]. Epigenetics refers to the modulations of the expression of specific genes in absence of any effective change of the sequence of DNA, due to the following mechanisms: (a) methylation [i.e. the addition of methyl (-CH3) groups to the fifth carbon atom of the pyrimidine ring of a cytosine followed by a guanine, called CpG dinucleotides (CpGs)] [57, 58]; (b) presence of microRNAs (miRNAs); (c) post-translational modifications of the histones [35]. Although epigenetic phenomena do not affect genotype, they may be transmissible and may influence phenotype.
More precisely, the DNA methylation generally induces the inactivation and/or dysregulation of transcription of a particular gene [57], producing different final effects depending on whether the expression of such a gene is favourable or deleterious for a specific condition [56] (Table 2). Interestingly, the process of methylation of several genes associated with reproductive function, ovarian steroidogenesis [e.g. genes encoding for aromatase, peroxisome proliferator-activated receptor gamma 1 (PPARγ1)], glucose and lipid metabolism, adipose tissue activity, inflammation and regulation of immune response has been proposed to be potentially involved in the development of PCOS [56, 59,59,60,62] (Table 2). In particular, a recent study by Echiburù et al. [61] analysed DNA methylation in the promoter regions of genes encoding for leptin (LEP), leptin receptor (LEPR), adiponectin (ADIPOQ), adiponectin receptor 1 and 2 (ADIPOR1 and ADIPOR2), AMH and AR in 24 infants (2–3 months old) of women affected by PCOS (12 treated with metformin during pregnancy) and 24 newborns of non-PCOS subjects. The authors found that the daughters of women with PCOS showed differences in 1 CpG site located in the promoter region of LEPR and 2 in LEP as well as 1 in ADIPOR2 and 2 in AR. Furthermore, in the Chr1-65419664 site of the LEPR promoter, the proportion of methylation was significantly higher in infants who were born from non-treated PCOS and from treated with metformin PCOS compared to those who were born from healthy subjects (p= 0.016 and p= 0.037, respectively). Moreover, the ChrX-67543969 and ChrX-67544981 sites of the AR promoter were less methylated in PCOS compared to controls (p = 0.005 and p = 0.049, respectively), suggesting an increased expression of ARs in affected subjects [61].
Interestingly, other differentially methylated genes (DMGs) which predominantly contribute to hyperandrogenism and oocyte development defects, for instance aldo-keto reductase family 1 member C3 (AKR1C3), resistin (RETN), calcium-sensing receptor (CASR), growth hormone releasing hormone receptor (GHRHR) and tumour necrosis factor (TNF), were proposed as novel epigenetic candidates in mediating ovarian dysfunction by Sagvekar et al. [63].
Although, according to these data, a critical role of DNA methylation in hormonal function and signalling in PCOS appears plausible, current evidence needs to be furtherly investigated and confirmed in larger human studies.
The miRNAs are short (20–24 nucleotides) non-coding sequences of RNA that can negatively regulate the expression of genes at post-transcriptional level [64] (Table 3). It has been hypothesized that a great number of miRNAs that are abnormally expressed in ovarian cells, adipose tissue, follicular fluid and peripheral blood leukocytes of women with PCOS might be associated with the occurrence of this syndrome [64,64,65,66,67,68,70] (Table 3).
However, the effective role of miRNAs in clinical practice as possible biomarkers of PCOS remains questionable and limited. This phenomenon is likely due to the heterogeneity of the current available studies with respect to the investigated tissues and to the characteristics of study population [64]. At the same time, in some cases, more than a single miRNA is associated with the same potential regulatory role, suggesting that an interaction among several miRNAs may lead to a similar final functional consequence [67, 68]. Additionally, it must be underlined that the changes of expression of miRNAs (downregulation or upregulation) are usually related to a specific tract of the syndrome rather than to its complete hormonal and metabolic milieu [67, 68].
For instance, Chuang et al. [68], by studying the samples of subcutaneous abdominal adipose tissue from 33 women [15 without IR according to the homeostasis model assessment (HOMA-IR) (HOMA-IR < 2.5) (7 without and 8 with PCOS) and 18 with IR (HOMA-IR ≥ 2.5) (8 without and 10 with PCOS)], reported that miR-223 was significantly increased among all insulin-resistant women (p = 0.0004) while any significant difference was detected in miRNA-223 expression with regard to PCOS status. Comparing all four subgroups (7 subjects without PCOS and without IR, 8 without PCOS but with IR, 8 with PCOS but without IR and 10 with both PCOS and IR), miRNA-223 was only significantly overexpressed in the two groups of women with IR, compared to the subjects without PCOS and without IR (p < 0.01). These results seemed to confirm other evidence [67] according to which the overexpression of some miRNAs (e.g. miRNA-93, miRNA223) could be implicated in the metabolic dysfunction of PCOS more specifically.
The intrauterine exposure to androgens and/or to glucocorticoids is another important condition that may partially explain the tendency to develop the typical features of PCOS in adolescence and adulthood [71].
Barnes et al. [72] found ovarian hyperandrogenism in 8 women affected by congenital adrenal virilizing disorders (7 with well-controlled classic 21-hydroxylase deficiency and 1 with adrenal carcinoma removed at ~ 2 years). The authors hypothesized that this finding could be due to their intrauterine exposure to high levels of androgens that interfered with the hypothalamus-pituitary-gonads axis later, when they became girls.
According to the emerging data [73], female foetal hyper-exposure to androgens might influence the expression of some genes involved in ovarian steroidogenesis, insulin activity and pulsatility of GnRH [73]. Possibly, this mechanism could be partially due to the high level of AMH found in PCOS patients which can inhibit the activity of placental aromatase, an enzyme that normally transforms androgens into oestrogens thus preserving foetus from a potential hyper-exposure to androgens in utero [74, 75].
The consequent increase of androgens could exert a sort of “programming” at ovarian level and predispose the exposed female foetus to hyperandrogenism/hyperandrogenemia during the lifetime functional phases of ovary. Nevertheless, in this regard, the available data remain inconclusive [76].
On the other hand, prenatal androgen excess could be related to the hyperandrogenism/hyperandrogenemia of mothers affected by PCOS per se as well as to a genetic predisposition of the foetus on its own [27, 77].
In fact, as discussed above (see also the section “Genetics”) [19, 26], different genes involved in the activity and signalling of steroid hormones could play a key role in the appearance of PCOS, especially the AR gene, as showed also in animal models [27, 78]. More precisely, the genetic polymorphisms in exon 1 of the AR gene seem to influence AR activity and, according to in vivo data, CAG repeat polymorphism—encoding for an AR with increased activity—may play a critical role in the pathogenesis of PCOS. However, the studies regarding the association between the CAG repeat polymorphism of the AR gene and PCOS have still conflicting results [79].
As for mothers with PCOS, many authors, like Milaqueo et al. [80], hypothesized that changes in the activities of some placental enzymes—such as lower activity of placental aromatase and/or higher activity of 3-βhydroxysteroid-dehydrogenase—could contribute to the increased serum androgen levels of pregnant PCOS women. In light of these observations, the impairment of the activity of placental aromatase—that should be a theoretical barrier to preserve child from androgen excess due to the maternal hormonal status, as previously discussed [67, 81]—may assume an important significance. Anyway, these findings need to be clarified.
It has been suggested that PCOS might be a long-term consequence of an adaptive phenomenon occurring during the intrauterine life [27].
According to this hypothesis, a condition of nutritional restriction and/or foetal hypoxia during pregnancy might be perceived by the foetus as a potent “stressor”, producing a centralization of blood flow towards the principal vital organs (i.e. brain, heart, adrenal glands) in order to preserve their essential functions (“Thrifty phenotype hypothesis”) [82]. This process might facilitate a glucocorticoid excess and induce reproductive and/or metabolic disorders in female children during their perinatal period and later [83]. In an interesting manner, female intrauterine growth retardation (IUGR) was often associated with premature pubarche, ovarian hyperandrogenism and hyperinsulinemia [84]; moreover, Small for gestational age (SGA) female newborns were more often affected by PCOS than healthy children [84]. Hence, PCOS maybe the final stage of a hypothetical endocrine-metabolic sequence arising from prenatal onset and likely evolving during the life course of the same subject.
Conversely, a retrospective study by Fulghesu et al. [85] enrolling 170 PCOS adolescent patients compared to 75 healthy controls (age range: 12–19 years) did not report any significant difference in birth weight between the two groups (p= 0.73) and only 15 girls with PCOS were been SGA. However, PCOS SGA adolescents in comparison to the control group had basal insulin levels (15.93 ± 7.16 μU/mL vs. 10.97 ± 5.79 μU/mL), and HOMA-IR values (3.2 ± 1.54 vs. 2.19 ± 1.28) significantly higher (p< 0.01 and p< 0.02, respectively). Besides, a trend towards higher total levels of T in SGA PCOS girls compared to appropriate for gestational age (AGA) PCOS girls was observed (p= 0.09).
At the same time, Legro et al. [86], by studying the possible association between birthweight and metabolic and reproductive abnormalities in first-degree relatives (age range: 20–50 years) of women with PCOS (n= 1038 subjects; n=845 women, n=193 men) compared to a healthy control group (n=168 subjects; n=86 women, n=82 men), did not find any significant differences in birthweight between PCOS family members and controls. Moreover, within PCOS families, no association between any reproductive parameters, such as menstrual irregularities or levels of T, or metabolic endpoints [Fasting glucose and insulin, HOMA, 75-g oral glucose tolerance test (OGTT), cholesterol, etc.] and birthweight was reported.
These data maybe conceivably explained by the fact that both hyperandrogenemia and/or hyperinsulinemia correlate with the birthweight only partially but are more complex conditions that may depend on various factors, sometimes not yet understood.
Extrauterine Environment
IR associated with compensatory hyperinsulinemia may play a crucial role in the intriguing relationship between intrauterine and extrauterine aspects implicated into the development of PCOS [87]. It is well known that a high percentage of PCOS (50–70%) can be affected by IR, even regardless of body weight [87]. This data could be explained by the fact that IR can arise from a genetic predisposition [37]—as discussed above—but, at the same time, it can be triggered and/or induced by some inadequate nutritional and/or lifestyle habits [5]; in both cases, the endocrine-metabolic features of PCOS could occur in adult life as well as in childhood and adolescence [87]. Additionally, this cross-talk between the exogenous factors (i.e. diet, lifestyle) and some predisposing intrauterine conditions can also promote other disorders (e.g. premature adrenarche, atypical puberty and metabolic syndrome) [27]. On the other side, IR could negatively affect pregnancy per se, since this metabolic dysfunction has been associated with gestational diabetes, preterm labour, preeclampsia and intrauterine growth restriction (IUGR) [88].
Recent evidence highlighted how the Advanced Glycation End Products (AGEs) could take part in the pathophysiology of PCOS and produce negative effects on reproductive health. Generally, AGEs are endogenous consequences of the alterations of glucose metabolism commonly observed in diabetics [89]. However, AGEs maybe also linked to the dietary habits: in fact, they derive from some post-translational modifications forming permanent rearrangements through a ubiquitous reaction (i.e. Maillard reaction) during the cooking or processing of foods under high-temperature conditions [90]. In particular, Western-type diet, full of saturated fats and animal-derived products, usually cooked for many time at high temperature, are strong sources of AGEs. Various mechanisms of interference of AGEs were proposed for PCOS [91, 92]: (a) promotion of adipogenesis and IR; (b) direct systemic pro-inflammatory effects; (c) deposition in theca cells and/or abnormalities regarding glucose signalling and uptake in granulosa cells [91, 92]. Furthermore, AGEs might mimic different hormones and/or regulate/modify their activities. Lin et al. [93] showed that, in human ovarian granulosa cell cultures, AGEs inhibited cellular proliferation and secretion of progesterone possibly through the downregulation of LHR. Many data reported that a rich AGE diet in female subjects directly correlated with high levels of androgens, AMH, insulin and A, promoting ovarian dysfunction and/or infertility [90, 94]. Diamanti-Kandarakis et al. [95] showed that women with all the characteristics of PCOS according to the Rotterdam criteria had higher levels of AGEs compared to those with isolated hyperandrogenemia or anovulation or PCOS ovarian morphology at ultrasound or controls (p < 0.001). Moreover, Tantalaki et al. [96] reported in 23 patients with PCOS (mean age: 23.4±5.7 years; BMI: 26±5.7 kg/ m2)—invited to complete a 6-month period (3 phases of 2 months each) alternating a hypocaloric diet with ad libitum AGEs content (Hypo), an isocaloric diet with high AGEs (HA) and an isocaloric diet with low AGEs (LA)—that AGEs, T, oxidative stress, insulin and HOMA-IR index were significantly increased on the HA isocaloric diet (1869.6±114.6 kcals/day) with high AGEs (16±1 x 106 U/day) and decreased on the LA isocaloric diet (1869.6±114.6 kcals/day) with low AGEs (5.7±0.3 x 106 U/day) (p< 0.05). This study confirmed that the dietary intake of AGEs could deeply influence the hormonal and metabolic profile of PCOS, suggesting that women affected by this syndrome could benefit from a diet with low AGEs [97]. Thus, a balanced and low in saturated fat diet should be an advisable choice for the management of PCOS [97]. However, it is important to underline that, although AGEs seem to be involved in other conditions potentially associated with PCOS (e.g. atherosclerosis, aging and carcinogenesis), these processes could be only partially controlled and/or delayed through a dietary intervention [98].
The dysbiosis of gut microbiota may be involved in the development of several clinical disorders (e.g. obesity, IR, autoimmune diseases) including PCOS [99,99,101]. Many data in animal models showed that prenatal androgen exposure could be associated with hypertension and specific alterations of gut microbiota [102]. Interestingly, Torres et al. [103] studied the faecal microbial diversity profiles of healthy subjects (n = 48 mean age: 29.4 ± 4.9 years), women with only PCOM (n = 42 mean age: 29.8 ± 5.3 years) and PCOS women according to all three Rotterdam criteria (n = 73 mean age: 27.4 ± 4.9 years), by analysing 16S ribosomal RNA gene sequence. The authors reported a lower α biodiversity (a parameter estimating the within-sample biodiversity) in women affected by PCOS compared to healthy subjects for observed sequence variants (SVs) (p = 0.04) and phylogenetic diversity (PD) (p = 0.02). Besides, serum total T levels and hirsutism showed negative correlations with SVs (p = 0.006 and p = 0.02, respectively) and PD (p = 0.05 and p = 0.03, respectively), suggesting a powerful association between the severity of the intestinal dysbiosis and this syndrome. However, it is important to notice that the composition of microbiota may be influenced by various aspects (e.g. sex, hormonal concentrations, weight, dietary and lifestyle factors) that could greatly differ among PCOS patients. Consequently, it remains difficult to prove the effective involvement of gut microbiota in the aetiology and management of PCOS [104].
Nowadays, there is a growing interest about the relationship between endocrine disruptors and PCOS aetiology [105]. A cross-sectional study of 71 PCOS (according to NIH criteria) [9] and 100 normal women, age- and BMI-matched by Kandaraki et al., showed higher level of bisphenol A (BPA)—a widespread environmental pollutant—in both obese and lean subjects affected by PCOS compared to their control healthy counterparts (respectively, p < 0.05 and p < 0.001) [106]. Besides, in both PCOS and controls, significant positive correlations between BPA with T (p < 0.05) and BPA and A (p < 0.05) were observed. Several mechanisms could explain the deleterious impact of BPA on fertility: for instance, the interference with androgen and progesterone receptors, the enhancement of steroidogenesis, the decreased metabolism of T [107]. Unfortunately, this evidence confirmed the potential alarming effects of some environmental contaminants on reproductive function [108] and general health that nowadays cannot be prevented in a satisfactory manner, although ensuring a healthy lifestyle and diet could be a useful support, even in such case.
In fact, according to the principal recommendations regarding PCOS [109] along with medications, choosing a mild-to-moderate physical activity (from 150–180 to 210 min/week) associated with a well-balanced, poor of saturated fat acids and rich of grains diet, avoiding smoking and alcohol consumption and maintaining sleeping hygiene should be advisable to approach this complex syndrome [6].
Conclusions
PCOS is a very challenging endocrine-metabolic disorder, since various aspects need to be considered to frame and manage its clinical features, as in the case of other multifactorial diseases. This is an updated narrative review discussing the most recent evidence about the role of genetics, epigenetics and environment in the development and maintenance of this syndrome in young adult life.
According to the available data, both heritability and genetics can have an important role in the appearance of PCOS [11, 12, 19]. In fact, on the one hand, a familiar trait of susceptibility to hyperadrogenism can be found among affected subjects, particularly among some female first-degree relatives of PCOS women, even if in absence of a clear and complete clinical evidence of the syndrome according to the classical diagnostic criteria [16]. On the other hand, different genes, such as those associated with adrenal and ovarian steroidogenesis, ARs, gonadotrophin and insulin activity and inflammatory response, were proposed to be involved in the aetiology of this syndrome [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Nevertheless, robust evidence demonstrating the predominance of a specific gene for PCOS is still lacking.
Many epigenetic modifications (e.g. the methylation of specific genes and/or the presence of miRNAs) [57, 64,64,65,66,67,68,70] seem to influence the phenotype of PCOS patients. Furthermore, medical history of mothers before and during pregnancy (e.g. a previous diagnosis of PCOS, hyperinsulinemia, diabetes, pre-eclampsia) and/or some intrauterine events inducing a “foetal distress”, could affect reproductive health of female foetuses during adolescence and adult life [80,80,82].
As for exogenous factors, although the role of dysbiosis of gut microbioma, endocrine disruptors and AGEs in the development of PCOS needs further elucidations [97, 102, 103, 105], a healthy lifestyle associated with a well-balanced, poor of saturated fat acids and rich of grains diet associated with a medical tailored treatment could sustain the metabolic and hormonal management of PCOS women [109].
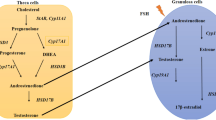
Taken together all these findings, clinicians should manage signs and symptoms of PCOS and prevent its possible long-term consequences more consciously (Fig. 1).
An aware knowledge of all the mechanisms at the basis of PCOS development could favour the promotion, achievement and maintenance of well-being in all affected subjects.
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. Journal of Clinical Endocrinology and Metabolism. 2004;89(6):2745–9.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta pii. 2019;S0009-8981(19):32118–7.
Macut D, Bjekić-Macut J, Rahelić D, Doknić M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163–70.
Moghetti P. Insulin Resistance and polycystic ovary syndrome. Curr Pharm Des. 2016;22(36):5526–34.
Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36.
Barber TM, Hanson P, Weickert MO, Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. 2019;13:1179558119874042.
Zhao Y, Qiao J. Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids. 2013;78(8):755–60.
Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertil Steril. 2005;83(5):1343–6.
Belenkaia LV, Lazareva LM, Walker W, Lizneva DV, Suturina LV. Criteria, phenotypes and prevalence of polycystic ovary syndrome. Minerva Ginecol. 2019;71(3):211–23.
Crosignani PG, Nicolosi AE. Polycystic ovarian disease: heritability and heterogeneity. Hum Reprod Update. 2001;7(1):3–7.
Diamanti-Kandarakis E, Alexandraki K, Bergiele A, Kandarakis H, Mastorakos G, Aessopos A. Presence of metabolic risk factors in non-obese PCOS sisters: evidence of heritability of insulin resistance. J Endocrinol Invest. 2004;27(10):931–6.
Vipin VP, Dabadghao P, Shukla M, Kapoor A, Raghuvanshi AS, Ramesh V. Cardiovascular disease risk in first-degree relatives of women with polycystic ovary syndrome. Fertil Steril. 2016;105(5):1338–44.
Yilmaz B, Vellanki P, Ata B, Yildiz BO. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2018;110(3):523–33.
Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75(1):53–8.
Legro RS, Driscoll D, Strauss JF 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95(25):14956–60.
Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–4.
Hiam D, Moreno-Asso A, Teede HJ, Laven JSE, Stepto NK, Moran LJ, et al. The genetics of polycystic ovary syndrome: an overview of candidate gene systematic reviews and genome-wide association studies. J Clin Med. 2019;8(10).
Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249–60.
Gharani N, Waterworth DM, Batty S, White D, Gilling-Smith C, Conway GS, et al. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6(3):397–402.
Gambineri A, Vicennati V, Genghini S, Tomassoni F, Pagotto U, Pasquali R, et al. Genetic variation in 11beta-hydroxysteroid dehydrogenase type 1 predicts adrenal hyperandrogenism among lean women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(6):2295–302.
Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF 3rd, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–11.
Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, et al. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016;434:25–35.
McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–27.
Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104:3835–50. https://doi.org/10.1210/jc.2018-02496.
Walters KA, Rodriguez Paris V, Aflatounian A, Handelsman DJ. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242(2):R23–50.
Gur EB, Karadeniz M, Turan GA. Fetal programming of polycystic ovary syndrome. World J Diabetes. 2015;6(7):936–42.
Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464.
Peng Y, Zhang W, Yang P, Tian Y, Su S, Zhang C, et al. ERBB4 confers risk for polycystic ovary syndrome in Han Chinese. Sci Rep. 2017;7:42000.
Xia JY, Tian W, Yin GH, Yan H. Association of Rs13405728, Rs12478601, and Rs2479106 single nucleotide polymorphisms and in vitro fertilization and embryo transfer efficacy in patients with polycystic ovarian syndrome: a case control genome-wide association study. Kaohsiung J Med Sci. 2019;35(1):49–55.
Laven JSE. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front Endocrinol (Lausanne). 2019;12(10):23.
Tong Y, Liao WX, Roy AC, Ng SC. Association of AccI polymorphism in the follicle-stimulating hormone beta gene with polycystic ovary syndrome. Fertil Steril. 2000;74(6):1233–6.
Tian Y, Zhao H, Chen H, Peng Y, Cui L, Du Y, et al. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in Han Chinese women. J Clin Endocrinol Metab. 2016;101(5):2178–84.
Deswal R, Nanda S, Dang AS. Association of luteinizing hormone and LH receptor gene polymorphism with susceptibility of Polycystic ovary syndrome. Syst Biol Reprod Med. 2019;65(5):400–8.
Zhang Y, Ho K, Keaton JM, Hartzel DN, Day F, Justice AE, et al. A genome-wide association study of polycystic ovary syndrome identified from electronic health records. Am J Obstet Gynecol. 2020;223(4):559.e1–559.e21.
Li T, Zhao H, Zhao X, Zhang B, Cui L, Shi Y, et al. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49(4):254–7.
Moorthy JD, Jain PR, Ramamoorthy T, Ayyappan R, Balasundaram U. Association of GWAS identified INSR variants (rs2059807 & rs1799817) with polycystic ovarian syndrome in Indian women. Int J Biol Macromol. 2019;144:663–70. https://doi.org/10.1016/j.ijbiomac.2019.10.235.
Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940.
Dakshinamoorthy J, Jain PR, Ramamoorthy T, Ayyappan R, Balasundaram U. Association of GWAS identified INSR variants (rs2059807 & rs1799817) with polycystic ovarian syndrome in Indian women. Int J Biol Macromol. 2020;144:663–70.
Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R. Variants in the 5alpha-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab. 2006;91(10):4085–91.
Graupp M, Wehr E, Schweighofer N, Pieber TR, Obermayer-Pietsch B. Association of genetic variants in the two isoforms of 5α-reductase, SRD5A1 and SRD5A2, in lean patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):175–9.
Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58(10):903–15.
Tan S, Scherag A, Janssen OE, Hahn S, Lahner H, Dietz T, et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS). BMC Med Genet. 2010;11:12.
Branavan U, Wijesundera S, Chandrasekaran V, Arambepola C, Wijeyaratne C. In depth analysis of the association of FTO SNP (rs9939609) with the expression of classical phenotype of PCOS: a Sri Lankan study. BMC Med Genet. 2020;21(1):30.
Cui L, Zhao H, Zhang B, Qu Z, Liu J, Liang X, et al. Genotype-phenotype correlations of PCOS susceptibility SNPs identified by GWAS in a large cohort of Han Chinese women. Hum Reprod. 2013;28(2):538–44.
Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012;49(2):90–5.
Chen L, Hu LM, Wang YF, Yang HY, Huang XY, Zhou W, et al. Genome-wide association study for SNPs associated with PCOS in human patients. Exp Ther Med. 2017;14(5):4896–900.
Tian Y, Li J, Su S, Cao Y, Wang Z, Zhao S, et al. PCOS-GWAS susceptibility variants in THADA, INSR, TOX3, and DENND1A are associated with metabolic syndrome or insulin resistance in women with PCOS. Front Endocrinol (Lausanne). 2020;11:274.
Pau CT, Mosbruger T, Saxena R, Welt CK. Phenotype and tissue expression as a function of genetic risk in polycystic ovary syndrome. PLoS One. 2017;12(1):e0168870.
Sun Y, Yuan Y, Yang H, Li J, Feng T, Ouyang Y, et al. Association between common genetic variants and polycystic ovary syndrome risk in a Chinese Han population. J Clin Res Pediatr Endocrinol. 2016;8(4):405–10.
Yu J, Ding C, Guan S, Wang C. Association of single nucleotide polymorphisms in the RAB5B gene 3’UTR region with polycystic ovary syndrome in Chinese Han women. Biosci Rep. 2019;39(5):BSR20190292.
Jiao X, Chen W, Zhang J, Wang W, Song J, Chen D, et al. Variant alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a Chinese population: a case-control study. Front Endocrinol (Lausanne). 2018;9:504.
Li M, Zhao H, Zhao SG, Wei DM, Zhao YR, Huang T, et al. The HMGA2-IMP2 pathway promotes granulosa cell proliferation in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(4):1049–59.
Zhai J, Liu J, Cheng X, Li S, Hong Y, Sun K, et al. Zinc finger gene 217 (ZNF217) promoted ovarian hyperstimulation syndrome (OHSS) through regulating E2 synthesis and inhibiting thrombospondin-1 (TSP-1). Sci Rep. 2017;7(1):3245.
Zhai J, Li S, Cheng X, Chen ZJ, Li W, Du Y. A candidate pathogenic gene, zinc finger gene 217 (ZNF217), may contribute to polycystic ovary syndrome through prostaglandin E2. Acta Obstet Gynecol Scand. 2020;99(1):119–26.
VÁzquez-martÍnez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón MA. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction. 2019;158:R27–40. https://doi.org/10.1530/REP-18-0449.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13(7):484–92.
Concha CF, Sir PT, Recabarren SE, Pérez BF. Epigenetics of polycystic ovary syndrome. Rev Med Chil. 2017;145(7):907–15.
Pan JX, Tan YJ, Wang FF, Hou NN, Xiang YQ, Zhang JY, Liu Y, Qu F, Meng Q, Xu J, Sheng JZ, Huang HF. Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: a new insight into its pathogenesis. Clin Epigenetics. 2018;10:6. https://doi.org/10.1186/s13148-018-0442-y.
Hiam D, Simar D, Laker R, Altıntaş A, Gibson-Helm M, Fletcher E, Moreno-Asso A, Trewin AJ, Barres R, Stepto NK. Epigenetic reprogramming of immune cells in women with PCOS impact genes controlling reproductive function. J Clin Endocrinol Metab. 2019;104(12):6155–70.
Echiburú B, Milagro F, Crisosto N, Pérez-Bravo F, Flores C, Arpón A, et al. DNA Methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics. 2020;1-17.
Wroblewski A, Strycharz J, Swiderska E, Drewniak K, Drzewoski J, Szemraj J, et al. Molecular insight into the interaction between epigenetics and leptin in metabolic disorders. Nutrients. 2019;11(8).
Sagvekar P, Kumar P, Mangoli V, Desai S, Mukherjee S. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin Epigenetics. 2019;11(1):61.
Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene. 2019;706:91–6.
Huang X, Liu C, Hao C, Tang Q, Liu R, Lin S, et al. Identification of altered microRnas in cumulus cells of PCOS patients: microRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2016;151(6):643–55.
Yao L, Li M, Hu J, Wang W, Gao M. MiRNA-335-5p negatively regulates granulosa cell proliferation via SGK3 in PCOS. Reproduction. 2018;156(5):439–49.
Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–86.
Chuang TY, Wu HL, Chen CC, Gamboa GM, Layman LC, Diamond MP, et al. MicroRNA-223 expression is upregulated in insulin resistant human adipose tissue. J Diabetes Res. 2015;2015:1–8. https://doi.org/10.1155/2015/943659.
McAllister JM, Han AX, Modi BP, Teves ME, Mavodza GR, Anderson ZL, et al. miRNA profiling reveals miRNA-130b-3p mediates DENND1A variant 2 expression and androgen biosynthesis. Endocrinology. 2019;160(8):1964–81.
Rashad NM, Ateya MA, Saraya YS, Elnagar WM, Helal KF, Lashin ME, et al. Association of miRNA - 320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome ovarian. Res. 2019;12(1):39.
Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23(4):421–32.
Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328–33.
Raperport C, Homburg R. The source of polycystic ovarian syndrome. Clin Med Insights Reprod Health. 2019;13:117955811987146. https://doi.org/10.1177/1179558119871467.
Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–46.
Tadaion Far F, Jahanian Sadatmahalleh S, Ziaei S, Kazemnejad A. Comparison of the umbilical cord Blood’s anti-Mullerian hormone level in the newborns of mothers with polycystic ovary syndrome (PCOS) and healthy mothers. J Ovarian Res. 2019;12(1):111.
Köninger A, Kampmeier A, Schmidt B, Frank M, Strowitzki T, Kimmig R, et al. Trends in anti-Müllerian hormone concentrations across different stages of pregnancy in women with polycystic ovary syndrome. Reprod Biomed Online. 2018;37(3):367–74.
Xita N, Tsatsoulis A. Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91(5):1660–6.
Abbott DH, Kraynak M, Dumesic DA, Levine JE. In utero androgen excess: a developmental commonality preceding polycystic ovary syndrome? Front Horm Res. 2019;53:1–17.
Baculescu N. The role of androgen receptor activity mediated by the CAG repeat polymorphism in the pathogenesis of PCOS. J Med Life. 2013;6(1):18–25.
Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–5.
Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303(11):E1373–85.
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–29.
de Melo AS, Dias SV, Cavalli Rde C, Cardoso VC, Bettiol H, Barbieri MA, et al. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150(1):R11–24.
Ibáñez L, de Zegher F, Potau N. Premature pubarche, ovarian hyperandrogenism, hyperinsulinism and the polycystic ovary syndrome: from a complex constellation to a simple sequence of prenatal onset. J Endocrinol Invest. 1998;21(9):558–66.
Fulghesu AM, Manca R, Loi S, Fruzzetti F. Insulin resistance and hyperandrogenism have no substantive association with birth weight in adolescents with polycystic ovary syndrome. Fertil Steril. 2015;103(3):808–14.
Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab. 2010;95(2):789–99.
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26(7):883–96.
Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long-term adverse consequences for mother and child. BMJ. 2017;356:j1.
Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24(1):59.
Gill V, Kumar V, Singh K, Kumar A, Kim JJ. Advanced glycation end products (AGEs) may be a link between modern diet and health. Biomolecules. 2019;9. https://doi.org/10.3390/biom9120888.
Merhi Z, Kandaraki EA, Diamanti-Kandarakis E. Implications and future perspectives of AGEs in PCOS pathophysiology. Trends Endocrinol Metab. 2019;30(3):150–62.
Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod. 2014;29(1):135–45.
Lin PH, Chang CC, Wu KH, Shih CK, Chiang W, Chen HY, et al. Dietary glycotoxins, advanced glycation end products, inhibit cell proliferation and progesterone secretion in ovarian granulosa cells and mimic PCOS-like symptoms. Biomolecules. 2019;9. https://doi.org/10.3390/biom9080327.
Garg D, Merhi Z. Relationship between advanced glycation end products and steroidogenesis in PCOS. Reprod Biol Endocrinol. 2016;14(1):71.
Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, et al. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS) Clin. Endocrinol. 2008;69:634–41.
Tantalaki E, Piperi C, Livadas S, Kollias A, Adamopoulos C, Koulouri A, et al. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones. 2014;13:65–73.
Faghfoori Z, Fazelian S, Shadnoush M, Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study diabetes. Metab Syndr. 2017;11(Suppl 1):S429–32.
Rutkowska AZ, Diamanti-Kandarakis E. Do advanced glycation end products (AGEs) contribute to the comorbidities of polycystic ovary syndrome (PCOS)? Curr Pharm Des. 2016;22(36):5558–71.
Crunkhorn S. Role of the gut microbiota in PCOS. Nat Rev Drug Discov. 2019;18(9):668.
Silva MSB, Giacobini P. Don’t trust your gut: when gut microbiota disrupt fertility. Cell Metab. 2019;30(4):616–8.
Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019;170(1):43–52.
Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400–21.
Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–11.
Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–62.
Hossein Rashidi B, Amanlou M, Behrouzi Lak T, Ghazizadeh M, Haghollahi F, Bagheri M, et al. The association between bisphenol A and polycystic ovarian syndrome: a case-control study. Acta Med Iran. 2017;55(12):759–64.
Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480–4.
Palioura E, Kandaraki E, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome: a focus on Bisphenol A and its potential pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17(3):137–44.
Hu Y, Wen S, Yuan D, Peng L, Zeng R, Yang Z, et al. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol. 2018;34(5):370–7.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. on behalf of the International PCOS Network Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human Reproduction. 2018;33(9):1602–18.
Availability of Data and Material
Not applicable
Code Availability
Not applicable
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this article
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bruni, V., Capozzi, A. & Lello, S. The Role of Genetics, Epigenetics and Lifestyle in Polycystic Ovary Syndrome Development: the State of the Art. Reprod. Sci. 29, 668–679 (2022). https://doi.org/10.1007/s43032-021-00515-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00515-4