Abstract
Current intrapartum fetal oxygen saturation (SaO2) monitoring methodologies are limited, mostly consisting of fetal heart rate monitoring which is a poor predictor of fetal hypoxia. A newly developed transabdominal fetal oximeter (TFO) may be able to determine fetal SaO2 non-invasively. This study is to validate a novel TFO in determining fetal SaO2 in a hypoxic fetal lamb model. Fetal hypoxia was induced in at-term pregnant ewe by placing an aortic occlusion balloon infrarenally and inflating it in a stepwise fashion to decrease blood flow to the uterine artery. The inflation was held at each step for 10 min, and fetal arterial blood gases (ABGs) were intermittently recorded from the fetal carotid artery. The balloon catheter was deflated when fetal SaO2 fell below 15%, and the fetus was recovered. A total of three desaturation experiments were performed. The average fetal SpO2 reported by the TFO was derived at each hypoxic level and correlated with the ABG measures. Fetal SaO2 from the ABGs ranged from 10.5 to 66%. The TFO SpO2 correlated with the ABG fetal SaO2 (r-squared = 0.856) with no significant differences (p > 0.5). The fetal SpO2 measurements from TFO were significantly different than the maternal SpO2 (p < 0.01), which suggests that the transcutaneous measurements are penetrating through the maternal abdomen sufficiently and are expressing the underlying fetal tissue physiology. The recently developed TFO system was able to non-invasively report the fetal SpO2, which showed strong correlation with ABG measures and showed no significant differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, intrapartum assessment of fetal well-being is primarily accomplished through fetal heart rate monitoring, which performs real-time continuous assessment of the fetal heart rate (FHR) and displayed on either paper or digital imagery. It is thought that fetal bradycardia after a contraction may be indicative of fetal asphyxia, and that surgical intervention could be needed [1]. Since the introduction of FHR monitoring as an intrapartum standard of care, the rates of cesarean deliveries (CDs) increased significantly, while the rates of adverse fetal outcomes associated with hypoxia remain unchanged [2,3,4]. The underlying problem is that many factors can cause a non-reassuring FHR tracing, some of which are normal physiological responses and may not necessitate intervention. For the detection of fetal hypoxia, fetal heart rate monitoring has high sensitivity, but low specificity. Clinically, this is manifested as a high rate of false positives, degrading patient outcomes and creating a malpractice nightmare [4,5,6,7]. Furthermore, the large variability of inter- and intra-observer interpretation of previously recorded indeterminate FHR traces clearly motivates the need for a more objective measure of fetal well-being [8,9,10,11].
Previously, transvaginal fetal pulse oximetry was evaluated in a multicenter randomized control trial, which showed that providing fetal oxygen saturation can improve the confidence of intrapartum fetal well-being when a non-reassuring FHR trace is present [12]. This semi-invasive method was performed by inserting a reflectance-based pulse oximeter through the birth canal to contact the presenting fetal tissues, thus constraining the measurements after the uterine membrane is ruptured. The study showed that while there was a reduction in CD rates due to non-reassuring FHR traces, the overall CD rate remained the same due to an increase in CDs due to dystocia. As such, the American College of Obstetricians and Gynecologists published an opinion that additional studies were needed before endorsing the utility of the device [13]. Although a follow-up study showed that low fetal SpO2 may be an indication for CD due to dystocia, the device was withdrawn from market, hampering any further investigations involving fetal oximetry [14, 15].
Fully non-invasive, transabdominal fetal pulse oximetry could potentially solve this problem by providing fetal oxygen saturation in a convenient manner while also enabling the possibility for antepartum assessment. Using a reflectance-based optical patch, photons are sent through the maternal abdomen and fetus to non-invasively estimate fetal oxygen saturation [16]. Some of the photons that reach the fetus propagate back toward the skin surface, where they are captured by a photodetector, and subsequently analyzed to extract fetal oxygen saturation. To investigate this approach, we recently designed and developed a novel transabdominal fetal pulse oximetry (TFO) system [17].
The purpose of this preliminary study was to evaluate the feasibility of our transabdominal fetal pulse oximeter to capture relevant fetal oxygen saturation values in a hypoxic fetal sheep in utero.
Materials and Methods
We designed and built a transabdominal fetal pulse oximeter to provide a noninvasive measure of fetal oxygen saturation (SpO2) (Fig. 1) [18,19,20]. This system introduces photons into the tissue using near-infrared emitters, which allows for better optical penetration depth. After propagating through the tissue, some of the light is captured by photodetectors located on the optical probe (optode), where the radiant power from incident photons is transformed into electrical current through the photoelectric effect. This electrical current is then amplified and converted into a voltage signal, which is subsequently digitized and recorded through our embedded optode control system. The measurements are simultaneously streamed to a laptop running custom-written software, where they are visually presented for real-time feedback and recorded for further analysis.
At its core, oxygen saturation is derived from calibrated light intensity measurements, where conventional pulse oximetry calculations are used to analyze the changes in light intensity caused by cardiac-induced arterial pulsations [21]. Fundamentally, this is described through the modified Beer–Lambert law (MBLL) which relates the temporal change in transcutaneous light intensity to changes in tissue composition [22]. Since oxy- and deoxyhemoglobin have different absorption properties at red and near-infrared light, their relative contribution in arterial blood can be identified by analyzing the MBLL at each wavelength [23]. In TFO, only a portion of the photons captured by photodetectors at the mother’s abdomen travel through fetal tissue, resulting in a light intensity signal that contains both maternal and fetal information. By analyzing the fetal contribution to the measured light intensity, the fetal SpO2 can be extracted from the transcutaneous measurements through signal processing [20].
To evaluate our TFO measurements on a known range of fetal oxygenation, we employed an in utero hypoxic fetal sheep model. The study was approved by the UC Davis Institutional Animal Care and Use Committee (IACUC) and care was in compliance with the Guide for the Care and Use of Laboratory Animals. An aortic occlusion balloon catheter was inserted through the common femoral artery and positioned below the renal arteries of an anesthetized pregnant ewe at term (136 days of gestation). The position of the balloon was confirmed via fluoroscopy. The fetal head was exposed, and a fetal carotid arterial line was placed for blood sampling and continuous hemodynamic monitoring. After replacing the lost amniotic fluid with warm saline, the uterus was closed around the fetal neck and one ear of the lamb was sutured to the ewe’s abdominal wall to create a deterministic location for the fetal head for transabdominal measurement. The laparotomy was then closed, leaving one fetal arm exposed for conventional pulse oximetry monitoring. Another pulse oximeter was placed on the ewe at the laparotomy site to monitor the ewe’s oxygen saturation at the abdomen. The TFO optical probe was then placed on the ewe’s abdomen just above the underlying fetus, which captured continuous measurements throughout the experiments. A high-level illustration of the hypoxic fetal sheep model setup can be seen in Fig. 2.
To induce varying levels of fetal hypoxia, the balloon catheter was inflated in a stepwise fashion to reduce the ewe’s mean arterial pressure by 5–10 mmHg and decrease blood flow to the uterine artery. At each step, the pressure was held for 10 min, where fetal arterial blood gases (ABG) were recorded at 2.5-, 5-, and 10-min intervals to derive fetal SaO2. ABG-derived fetal SaO2 values are considered to be the gold standard measurement modality. The stepwise inflation continued until two consecutive measurements of fetal SaO2 fell below 15%, at which point the balloon catheter was deflated, allowing the fetus to recover. A total of three hypoxic desaturation experiments (normoxia-hypoxia-recovery) were performed. The mean fetal oxygen saturation from the TFO (SpO2) was derived at each hypoxic level and compared against the mean ABG-derived fetal SaO2.
Results
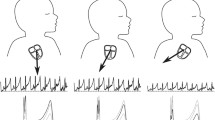
Figure 3 shows a snippet of the light intensity signals captured by TFO in the time-domain (a) and their power spectrum in the frequency domain (a). Strong peaks in the frequency spectra correspond to changes in the measured light intensity caused by the ewe and fetal lamb’s physiology. Since these physiological signals are not pure sine waves, they cause harmonics to appear at integer multiples of the fundamental frequencies. These measurements were analyzed using the modified Beer–Lambert law at both wavelengths to derive the transabdominal, fetal oxygen saturation (TFO SpO2).
Time-series measurements and Power-spectra of the transcutaneous light intensity signal. a Light intensity signal captured by our system in the time-domain and b corresponding power spectra in the frequency-domain at emitters 1 (blue) and 2 (orange) during the first hypoxic experiment (R1.3). Strong peaks in the power spectra correspond to annotated physiological expressions (respiratory and heart rate or RR and HR) seen in the light intensity at corresponding fundamental frequencies (solid arrow) and their harmonics (dotted arrow)
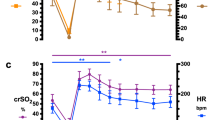
At each hypoxic level, the fetal oxygen saturation was recorded from arterial blood gasses (ABG SaO2), conventional pulse oximetry at the exposed fetal arm (PO SpO2), and non-invasive TFO measurements (TFO SpO2). These fetal measurements are shown alongside the maternal ewe’s oxygen saturation in Fig. 4. Fetal oxygen saturation from ABGs ranged from 10.5 to 66%. The TFO SpO2 ranged from 12.2 to 74.4% and had a positive correlation with the ABG fetal SaO2 (coefficient of determination, r-squared = 0.856) with a slope of relation of 1.22 and no significant differences (p > 0.5). The mean absolute error between the noninvasive TFO SpO2 and the invasive, gold standard ABG measurements was 5.63% ± 3.10% (SD). In comparison, the conventional fetal pulse oximeter (placed on the exposed fetal arm) resulted with a mean absolute error of 15% ± 2.77% (SD). It should be noted that conventional pulse oximeters often exhibit higher error values for SaO2 levels below 70%, possibly due to their empirical evaluation on healthy adults.
Arterial oxygen saturation during fetal lamb hypoxia. a Mean oxygen saturation at each hypoxic level measured through fetal arterial blood gasses (ABG) (square-filled, black), transabdominal fetal oximetry (TFO) (circle-filled, red), and conventional pulse oximetry on the fetal lamb (up-triangle-open, blue) and ewe (down-triangle-open, green) and b regression curve (dotted) of the fetal oxygen saturation derived from TFO and ABG. *Note that TFO measurements at R2.0 were not captured due to a technical error during the experiment
Since tissue is an opaque and highly scattering optical medium, one concern is that the TFO measurements may not penetrate the superficial (maternal) tissues well enough to reach the deeper fetal tissues, thus causing the measurements to reflect maternal characteristics. Using a paired t test, we determined that the TFO-derived fetal SpO2 measurements are significantly different than the maternal ewe’s SpO2 (p < 0.01), suggesting that the transcutaneous measurements are penetrating through the maternal abdomen sufficiently and is expressing the physiology seen in the underlying fetal tissues.
Comments
Principal Findings
This study demonstrates the feasibility of our TFO system to noninvasively measure the fetal oxygen saturation on a hypoxic fetal lamb in utero. To investigate the ability of noninvasive TFO to identify critically hypoxic fetuses, we considered a wide range of relevant fetal oxygenation values (10.5% to 66%), resulting with a mean absolute error of 5.63%. These transabdominal measurements showed strong correlation with invasive, gold standard SaO2 measurements from ABG, highlighting the TFO system’s ability to non-invasively measure fetal oxygen saturation.
Clinical Implications
An outcome from the high false positive rates of FHR monitoring is an increase in cesarean deliveries (CDs), while the rates of most adverse fetal health outcomes remain the same [4]. Currently, one in three children is born via CD in the USA, which exceeds the recommended range suggested by the World Health Organization [3, 24]. In addition to a mediocre inter- and intra-observer agreement of abnormal FHR traces, a significant proportion of CDs are performed partly in response to a non-reassuring FHR trace, highlighting that a more objective metric of fetal well-being is needed [11, 25]. As shown through transvaginal fetal pulse oximetry, fetal SpO2 may provide a more objective metric of fetal well-being [12]. In one study, researchers showed that fetal SpO2 of less than 30% for at least 10 min has good predictive value for detecting fetal acidosis [26]. However, the transvaginal fetal pulse oximeter used in these studies is no longer commercially available, hampering further investigation of the clinical utility of fetal SpO2 [15].
The recently developed transabdominal fetal pulse oximetry (TFO) system may provide a solution to this issue. Given the non-invasive nature of these measurements, TFO could potentially provide fetal SpO2 during both intrapartum and antepartum periods, though measurements during the antepartum period may be more challenging to acquire due to the increased distance between the optical probe and fetal tissue. As an assessment tool, this new monitoring technology could enable future studies that evaluate the effect that fetal oxygenation may have on fetal outcomes during various periods of late pregnancy. As a step toward this goal, we designed and developed the TFO system used in this work to provide physicians with a noninvasive measure of fetal SpO2 [18,19,20]. This study provides initial results acquired on a relevant biological model, with varying levels of fetal hypoxia, using our TFO system.
Strengths and Limitations
One of the strengths of the TFO system is that it is able to measure fetal oxygen saturation in a noninvasive manner, which is currently unavailable in obstetric practice. In comparison, transvaginal measures of fetal SpO2 required ruptured uterine membranes and a dilated cervix of at least 2 cm before application. Another benefit is that the study encompasses a wide range of fetal SpO2 values, improving our confidence in the device’s ability to detect critically hypoxic fetuses. By reducing blood flow to the uterine artery through partial aortic occlusion, controllable, repeatable induction of fetal hypoxia was accomplished while also minimizing the potential for desaturating the pregnant ewe’s abdominal tissue directly under the optical probe and reducing the risk of sudden fetal abortion through umbilical cord occlusion. Previous investigations involving noninvasive measures of fetal SpO2 used sophisticated optical instruments (photo-multiplier tubes), which can be difficult to translate toward widespread clinical-use due to their bulkiness, high voltage, oversensitivity, and cost [27, 28]. Alternatively, this system utilizes less-expensive, commodity optical components, which lend themselves well toward eventual clinical translation [18,19,20].
A weakness of this preliminary study is its limited measurement size. While repeated measures were employed in this study, including more hypoxic fetal sheep can provide insight on the operating limits of the TFO system. In addition, to ensure that the proper placement of the optical probe was maintained throughout the experiments, a deterministic fetal geometry was created by suturing one ear of the fetal lamb to the underside of the ewe’s abdomen. While others have also used this approach to acquire transabdominal measurements in sheep [29], it is less representative of a realistic antepartum scenario, where the effects of spontaneous fetal movement are unknown. An implication of an unrestricted fetus to consider in future studies is that fetal movement may cause the transabdominal fetal signal to occasionally be lost and may require the optode to be readjusted. Note that this issue is also prevalent in FHR monitoring, where ultrasound transducers occasionally need adjustment as the fetus moves. Nonetheless, these proof-of-concept measurements highlight the feasibility to perform TFO on a relevant animal model as an important step toward clinical studies.
Research Implications
In this proof-of-concept study, we induced varying levels of fetal hypoxia in an anesthetized pregnant ewe at term to non-invasively measure fetal SpO2 using a TFO system. Prospective studies include increasing the number of hypoxic fetal lambs investigated, as well as fully placing the fetal lamb back into the uterus, to investigate the operating limits of the TFO system. In addition, sampling arterial blood from the fetus at various locations can help evaluate the optimal placement of the optode to investigate the fetal SpO2 from a particular region of tissue, like the fetal brain to help prevent issues like anoxic brain injury. Furthermore, the increased blood flow and change in fetal position during active labor may affect the system’s ability to measure fetal SpO2. Evaluating TFO during active labor may provide additional insight on the operating limits of the technology. In addition, a study to evaluate its usage on pregnant women in the clinic is needed, of which these animal studies provide a necessary step toward this goal.
References
Schifrin BS. Fetal heart rate monitoring during labor. JAMA. 1972;222(2):196–202. https://doi.org/10.1001/jama.1972.03210020046012.
Nelson KB, Sartwelle TP, Rouse DJ. Electronic fetal monitoring, cerebral palsy, and caesarean section: assumptions versus evidence. BMJ. 2016;355:i6405.
Martin JA, Hamilton BE, Osterman JK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018;67(8):1–50.
Alfirevic Z, Devane D, Gyte GML, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017;(2). https://doi.org/10.1002/14651858.CD006066.pub3.
Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334(10):613–9.
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114(1):192–202. https://doi.org/10.1097/AOG.0b013e3181aef106.
Garite TJ. The search for an adequate back-up test for intrapartum fetal heart rate monitoring. Am J Obstet Gynecol. 2013;208(3):163–4.
Nielsen PV, Stigsby B, Nickelsen C, Nim J. Intra- and inter-observer variability in the assessment of intrapartum cardiotocograms. Acta Obstet Gynecol Scand. 1987;66(5):421–4.
Chauhan SP, Klauser CK, Woodring TC, Sanderson M, Magann EF, Morrison JC. Intrapartum nonreassuring fetal heart rate tracing and prediction of adverse outcomes: interobserver variability. Am J Obstet Gynecol. 2008;199(6):623.e1–5. https://doi.org/10.1016/j.ajog.2008.06.027.
Figueras F, Albela S, Bonino S, et al. Visual analysis of antepartum fetal heart rate tracings: inter- and intra-observer agreement and impact of knowledge of neonatal outcome. J Perinat Med. 2005;33(3):241–5. https://doi.org/10.1016/j.ajog.2008.06.027.
Sabiani L, Le Du R, Loundou A, et al. Intra- and interobserver agreement among obstetric experts in court regarding the review of abnormal fetal heart rate tracings and obstetrical management. Am J Obstet Gynecol. 2015;213(5):856e.1–8. https://doi.org/10.1016/j.ajog.2015.08.066.
Garite TJ, Dildy GA, McNamara H, Nageotte MP, Boehm FH, Dellinger EH, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183(5):1049–58. https://doi.org/10.1067/mob.2000.110632.
American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. ACOG Committee opinion number 258. Obstet Gynecol. 2001;98(3):523–4. https://doi.org/10.1016/s0029-7844(01)01557-5.
Porreco RP, Boehm FH, Dildy GA, Miller HS, Wickstrom EA, Garite TJ, et al. Dystocia in nulliparous patients monitored with fetal pulse oximetry. Am J Obstet Gynecol. 2004;190(1):113–7. https://doi.org/10.1016/s0002-9378(03)00855-x.
East CE, Chan FY, Colditz PB, Begg LM. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database Syst Rev. 2007;2:CD004075. https://doi.org/10.1002/14651858.CD004075.pub3.
Zourabian A, Siegel A, Chance B, Ramanujam N, Rode M, Boas DA. Trans-abdominal monitoring of fetal arterial blood oxygenation using pulse oximetry. J Biomed Opt. 2000;5(4):391–405.
Ghiasi S, Fong D. Inventors; University of California, Davis, assignee. Robust, clinical-grade transabdominal fetal pulse oximetry. US Patent Application 16/347,532 (pending). November 21, 2016.
Fong D, Knoesen A, Ghiasi S. Transabdominal fetal pulse oximetry: the case of fetal signal optimization. Proceedings of the IEEE 19th International Conference on e-Health Networking, Applications and Services (Healthcom). 2017;1–6. https://doi.org/10.1109/HealthCom.2017.8210799.
Fong DD, Knoesen A, Motamedi M, O’Neill T, Ghiasi S. Recovering the fetal signal in transabdominal fetal pulse oximetry. Smart Health. 2018;(9–10):23–36. https://doi.org/10.1016/j.smhl.2018.07.011.
Fong DD, Srinivasan VJ, Vali K, Ghiasi S. Optode design space exploration for clinically-robust non-invasive fetal Oximetry. ACM Trans Embed Comput Syst. 2019;18(5s):63.1–22. https://doi.org/10.1145/3358207.
Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107(6):789–99. https://doi.org/10.1016/j.rmed.2013.02.004.
Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33(12):1433–42.
Webster JG. Design of Pulse Oximeters. Boca Raton: CRC Press; 1997.
Appropriate technology for birth - World Health Organization. Lancet. 1985;2(8452):436–7.
Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29–38. https://doi.org/10.1097/AOG.0b013e31821e5f65.
Kühnert M, Seelbach-Göebel B, Butterwegge M. Predictive agreement between the fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178(2):330–5.
Vintzileos AM, Nioka S, Lake M, Li P, Luo Q, Chance B. Transabdominal fetal pulse oximetry with near-infrared spectroscopy. Am J Obstet Gynecol. 2005;192(1):129–33. https://doi.org/10.1016/j.ajog.2004.07.022.
Nioka S, Izzetoglu M, Mawn T, Nijland MJ, Boas D, Chance B. Fetal transabdominal pulse oximeter studies using a hypoxic sheep model. J Matern Fetal Neonatal Med. 2005;17(6):393–9.
Choe R, Durduran T, Guoqiang Y, et al. Transabdominal near infrared oximetry of hypoxic stress in fetal sheep brain in utero. PNAS. 2003;100(22):12950–4.
Funding
This study is funded by the National Science Foundation, Grant No. IIS-1838939.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflict of interest.
Ethics Approval
Animal work was approved by UC Davis Institutional Animal Care and Use Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fong, D.D., Yamashiro, K.J., Johnson, M.A. et al. Validation of a Novel Transabdominal Fetal Oximeter in a Hypoxic Fetal Lamb Model. Reprod. Sci. 27, 1960–1966 (2020). https://doi.org/10.1007/s43032-020-00215-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00215-5