Abstract
Intrapartum care uses electronic fetal heart rate monitoring (EFHRM) for over 50 years to indirectly assess fetal oxygenation. However, this approach has been associated with an increase in cesarean delivery rates and limited improvements in neonatal hypoxic outcome. To address these shortcomings, a novel transabdominal fetal pulse oximeter (TFO) is being developed to provide an objective measurement of fetal oxygenation. Previous studies have evaluated the performance of TFO on pregnant ewe. Building on the animal model, this study aims to determine whether TFO can successfully capture human fetal heart rate (FHR) signals during non-stress testing (NST) as a proof-of-concept. Eight ongoing pregnancies meeting specific inclusion criteria (18–40 years old, singleton, and at least 36 weeks' gestation) were enrolled with consent. Each study session was 15 to 20 min long. Reference maternal heart rate (MHR) and FHR were obtained using finger pulse oximetry and cardiotocography for subsequent comparison. The overall root-mean-square error was 9.7BPM for FHR and 4.4 for MHR, while the overall mean-absolute error was 7.6BPM for FHR and 1.8 for MHR. Bland–Altman analysis displayed a mean bias ± standard deviation between TFO and reference of -3.9 ± 8.9BPM, with limits of agreement ranging from -21.4 to 13.6 BPM. Both maternal and fetal heart rate measurements obtained from TFO exhibited a p-value < 0.001, showing significant correlation with the reference. This proof-of-concept study successfully demonstrates that TFO can accurately differentiate maternal and fetal heart signals in human subjects. This achievement marks the initial step towards enabling fetal oxygen saturation measurement in humans using TFO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electronic fetal heart rate monitoring (EFHRM), a surrogate for measuring fetal oxygenation, has been in routine obstetric care for over 50 years. In 1970, Paul and Hon reported a 75% reduction in primary cesarean births and decreased need for active stimulation in high-risk patients when EFHRM was employed [1]. Although initially successful, widespread adoption of EFHRM led to increased cesarean rates without substantial improvement in neonatal hypoxic outcome [2].
The National Institute of Health–Eunice Kennedy Shriver National Institute of Child Health and Human Development proposed a consensus nomenclature for fetal heart patterns, categorizing them into three groups (Category I, II, and III) to standardize interpretation and communication for the purpose of reducing cesareans for a “fetus-in-distress” [3]. A fetal heart rate (FHR) moderate variability pattern represents a nonhypoxic, nonacidotic fetus and the continued presence of moderate variability provides reassurance to continue labor with surveillance and evaluation [3, 4]. Despite the three-category approach, cesarean rates remained unchanged [5]. Additionally, many newborns with clinically relevant metabolic acidosis or hypoxic–ischemic encephalopathy fell under the category II FHR pattern, without significant decelerations [6, 7]. Significant fetal heart rate decelerations include severe variable, late, or recurrent prolonged decelerations, characterized by a width of 60 s and a drop of either 60 beats below the baseline or below 60 beats-per-minute (BPM) [8]. Although deterioration of baseline moderate variability may be a suggestion of decreasing fetal oxygen saturation, the change from baseline must correlate with the physiologic state of the pregnancy and the fetus [9]. To suggest possible pathogenic fetal desaturation (hypoxia), the baseline deterioration to minimal/absent variability should be persistent beyond the known physiologic fetal heart rate circadian cycles [9].
Although standard intervention for significant decelerations in category II may be promising, correlating category II with fetal oxygenation may be helpful to assess fetal acidemia risk [8]. Like EFHRM, pulse oximetry has become the de-facto method of assessing ex-utero blood oxygen saturation. Pulse oximetry estimates oxygen saturation by illuminating the skin and measuring changes in light absorption of oxyhemoglobin and deoxyhemoglobin using two light wavelengths: 660 nm (red) and 940 nm (infrared) [10, 11]. A transabdominal fetal oximeter (TFO) device was developed based on this principle to determine intrapartum fetal oxygen saturation (SpO2) in pregnant ewes [12]. Fetal hypoxia was induced in a term pregnant ewe by placing an aortic occlusion balloon below the maternal ewe kidneys, gradually decreasing blood flow to the uterus. The TFO reported fetal SpO2 strongly correlated with fetal arterial blood gas measurements. Using the same TFO device, we hope to translate the findings in human pregnancy. Fetal oxygen saturation measurement could increase the accuracy of fetal hypoxia detection with EFHRM. The first step is to extract and discriminate the fetal heart signal from the maternal heart signal to enable fetal oxygen saturation calculation.
This study aims to demonstrate and evaluate whether the TFO can successfully acquire the human fetal heart signal during routine antepartum non-stress testing (NST).
Materials and Methods
Study Population
This proof-of-concept (POC) study aims to evaluate the TFO device's ability to differentiate maternal and fetal heart signals in pregnancies undergoing NST at UC Davis Health's antenatal testing unit (ATU). Pregnancies referred to ATU at 34 weeks’ gestation with confirmed dating were approached for consent, with the following inclusion criteria: 18 + years old, singleton pregnancy, and 36 + weeks’ gestation at the time of TFO data acquisition with NST. The pregnancy was excluded for the following: inability to consent verbally, non-English speaker without access to professional interpreters for counseling and consent, presenting at the ATU with symptoms or signs concerning for rupture of membranes or labor, presence of fetal cardiac or intracranial defects that could impact the FHR pattern, severe fetal growth restriction (less than the Hadlock fifth percentile estimate of fetal weight), presenting to ATU with severe range blood pressure or other medical conditions requiring immediate evaluation in labor and delivery, allergies to adhesives or wrapping materials used for device placement. Approval was obtained from UC Davis Health Institutional Review Board for Human Subjects before study commencement.
Transabdominal Fetal Pulse Oximeter
The device, developed by the Laboratory for Embedded and Programmable Systems (LEPS, https://lepsucd.com/) in the UC Davis Department of Electrical and Computer Engineering, is a transabdominal prototype offering convenient access to fetal arterial blood oxygen saturation (fSpO2) via pulse oximetry. The prototype has been validated in simulations, benchtop experiments: pulse detection through bio-phantom materials that mimic tissue layers in pregnant humans, and in-vivo pulse separation in overlaid human organs [13, 14]. In-vivo validation was then accomplished by successfully capturing the fetal signal in pregnant sheep models [12].
The transabdominal fetal pulse oximetry system (TFO) includes an optical probe (optode), embedded optode control system, and real-time software (Fig. 1) [15]. The optode houses two adjustable high-power light emitting diodes (LEDs) at 740 nm/850 nm wavelengths and five photodetectors placed at varying distances to overcome light attenuation and patient demographic variability challenges [14]. Variations in patient anatomy and tissue composition is expected to impact TFO, as scattering and absorption parameters vary among tissue [16]. High scattering and low absorption are desirable for reflectance pulse oximetry. Figure 2 shows a simplified tissue model to visualize these challenges. Near detectors collect maternal-only signal from shallow tissue, while farther detectors capture fetal signal from deeper tissue. Simulation results showed the optode can sense FHR up to 5 cm depth [13, 17]. Skin temperature is monitored continuously by TFO, and LED power is adjusted such that the temperature remains within the safety limits (ISO 80601–2-61 standard), enabling long term safe use. The LEDs and photodetectors are soldered on a flexible printed circuit board (PCB) and placed inside the flexible black silicone housing. The flexible components conform to the maternal abdomen for optimal skin contact. The black housing blocks ambient light. The embedded optode control system adjusts LED parameters, amplifies/samples the sensed signal using a programmable gain amplifier and high-resolution analog-to- digital converter (ADC). The custom real-time software offers control, visualization, and data storage capabilities.
A simplified tissue model with representative light paths between near-infrared (NIR) light source and photodetectors [31]. Representative light paths showing further photodetectors from light source capture light that has propagated deeper into the maternal abdomen, reaching the fetus. However, intensity of light received is also decreased with higher source-detector distance
Standardized Antepartum Testing Procedure
After obtaining patient demographics and vital signs, patients were positioned supine, following UC Davis Health Standard Operating Procedure for NST. The patients’ skin colors were compared to a validated scale shown in Fig. 3 (Pantone Skin Tone Guide, Pantone, Carlstadt NJ). After determining the abdominal area where the device will be applied, the designated ATU nurse or one of the clinical investigators used a bedside SonoSite LX ultrasound machine (FujiFilm SonoSite Inc., Bothell WA) to determine placenta location, and measure sonographic distances in the sagittal and coronal planes. Figure 4 shows the placement of the monitoring transducer relative to the TFO. The following distances were obtained: maternal skin to uterine wall, uterine wall thickness, maternal skin to closest fetal part, and presence of fluid pocket. At each study episode, a dataset was collected for the entire NST duration. From the cardiotocograph device (Corometrics 250 CX series, GE Health Care, Wauwatosa WI), reference maternal and fetal heart rate values were obtained for a post hoc comparison with TFO readings. Non-electronic iformation and ultrasound images were documented on a study data collection sheet and kept with the attending clinical research coordinator. TFO data were saved on a portable computer attached to the study device and stored in the university laboratory.
Patient’s skin color measurement setup [21]. The patient’s skin is compared to a standard color tone using a validated skin color scale (Skin Tone Guide, Pantone, Carlstadt NJ) where 1 is lightest and 10 is darkest possible skin color classification
Concurrent placement of Transabdominal Fetal Pulse Oximeter (TFO) and Electronic Fetal Heart Rate Monitoring (EFHRM) during non-stress testing (NST) visit. Picture of measurement set up during the study showing the placement of the monitoring transducer relative to the TFO and highlighting different components of the TFO system
Fetal and Maternal Heart Rate Extraction
FHR is a precursor to computing oxygen saturation via pulse oximetry [10]. In this POC, we compared maternal and fetal heart rates (MHR, FHR) collected from a cardiotocograph to TFO data. TFO collects mixed PPG waveforms from various sources due to near-infrared (NIR) light traveling through maternal and fetal tissues. To isolate the fetal PPG waveform for oxygen saturation calculation, additional processing is required.
The steps involved in extracting the fetal signal from the mixed PPG signal (MHR, FHR and others) are: (1) bandpass-filtering the mixed-PPG to remove all noise signals with a frequency below reference MHR and above 270 BPM; (2) applying Recursive Least Squares (RLS) Adaptive Noise Cancellation (ANC) algorithm to reduce maternal signal contribution from the mixed-PPG [18, 19]; (3) analyzing ANC output in frequency domain using power spectral densities (PSDs); and (4) reporting FHR within the typical range of 110BPM–160 BPM as the frequency with the highest power in the PSD [20,21,22].
To extract the maternal signal, the following steps are applied to the PPG captured by nearest detector to the light source on TFO optode: (1) maternal signal is bandpass-filtered between 30 and 270 BPM to isolate the physiological signals of interest; (2) the filtered maternal PPG signal is analyzed in frequency domain by computing its PSD; and (4) the frequency between 60 BPM – 120 BPM (typical range for MHR) with highest power in the PSD is estimated as MHR [21, 22].
Statistical Analysis
Using MATLAB software version R2020a (Mathworks, Natick, MA), TFO's MHR and FHR estimates are compared to cardiotocograph device measurements. The accuracy is assessed using root-mean-square error (RMSE) (Eq. (1)) and mean-absolute-error (MAE) (Eq. (2)). Reference values outside the typical MHR and FHR ranges are excluded from error calculation.
The Bland–Altman plot is used to evaluate the agreement between TFO's FHR estimates and the reference values, identifying systematic biases and outliers.
Linear regression analysis is used for correlation between TFO and reference heart rate values, with outliers removed using the median method if they are more than three scaled median absolute deviation (MAD) (Eq. (3)) away from the median estimated heart rate value.
Results
Data from one visit was analyzed for each of the eight enrolled women. The first visit data was used for all but two subjects (A and H), whose second visit data was used due to accuracy issues with the cardiotocograph readings requiring multiple readjustments to TOCO sensor. Participant’s data was recorded for 15 to 20 min, with skin temperature monitored and kept below the 41 °C safety limit (ISO80601-2–61 standard). The TFO estimated FHR and MHR values every 30 s based on 1-min averages using the furthest detector for FHR and the nearest for MHR.
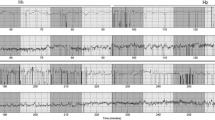
Figure 5 is an example of maternal, mixed, and noise-cancelled fetal signal PSDs over time (spectrograms) computed on 1-min-long windows with 30 s of overlap. The reference and estimated FHR and MHR are plotted on top. Applying ANC to the mixed signal reduced MHR power and its second harmonic, improving the distinction of the FHR peak. Canceling the second MHR harmonic is crucial as it can be mistaken for FHR in the 110–160 BPM range. The latter misidentification problem can also be present in cardiotocograph FHR measurements [23].
Example spectrogram from subject A [21]. The maternal heart rate (MHR), MHR 2nd Harmonic and fetal heart rate (FHR) are outlined. By cancelling the MHR 2nd Harmonic, the FHR signal is more evident
The study demographics are presented in Table 1. Participant G had oblique fetal presentation, while all other participants had cephalic fetal presentation. Participant E in Table 1 does not have results for MHR due to neglecting to connect the reference MHR sensor (finger pulse oximeter). The results show that MHR could be accurately estimated with an error below 2 BPM in 75% of participants. FHR could also be estimated with decent accuracy, with an error below 10 BPM in 75% of participants. The overall RMSE in eight patients was 9.7 BPM for FHR and 4.4 for MHR, while the overall MAE was 7.6 BPM for FHR and 1.8 for MHR. The FHR is distinctly separated from the MHR.
Fetal depth ranged from 1.72 to 4.46 cm from the abdominal surface where TFO was placed. Deeper fetuses did not necessarily result in lower FHR accuracy (see Table 1). Bad skin contact or motion artifacts, including movements from the fetus, mother, or external interventions, were the primary causes of large errors in FHR or MHR. The most significant motion artifacts occurred when readjusting the cardiotocograph sensor. Subject F's data shows bright horizontal lines in the spectrograms, indicating motion artifacts (Fig. 6).
Transabdominal Fetal Pulse Oximeter (TFO) Spectrograms from Subject F showing fetal heart rate (FHR) misidentification issue. Notice the wrong reference FHR after minute 16 and wrong TFO FHR due to misidentification and motion artifacts. Maternal heart rate (MHR) 2nd harmonic (in black) is misidentified as FHR
Another major artifact causing errors in FHR is the presence of MHR 2nd harmonic within the FHR range (110–160 BPM); leading to misidentification by TFO. There are also instances where reference cardiotocograph misidentifies FHR as the MHR 2nd harmonic, while TFO’s FHR remains accurate (Fig. 7). Between minutes 8–12 of subject B, the reference FHR values abruptly rise towards MHR 2nd harmonic values. However, after sensor readjustment, the cardiotocograph's FHR misidentification is resolved (Fig. 7). The application of ANC reduced the misidentification issue in TFO. However, if FHR is very close or identical to 2nd harmonic of MHR, then ANC degrades TFO’s FHR accuracy because it cancels the contribution of MHR 2nd harmonic.
Bland–Altman plot (Fig. 8) showed a mean bias standard deviation between TFO and referenced FHR measurements as -3.9 ± 8.9 BPM and the limits of agreement were -21.4 to 13.6 BPM. Visual inspection of the Bland–Altman plot shows the proposed method does not have a systemic bias relative to the reference method, since the bias between TFO and reference FHR values are randomly scattered around the mean bias.
Linear regression analysis (Fig. 9) showed a strong correlation between TFO and referenced MHR measurements (Pearson’s r = 0.91, p < 0.001 after outlier removal). TFO FHR estimates were moderately correlated with the reference (Pearson’s r = 0.34 after outlier removal), and a highly significant relationship was observed (p < 0.001).
Comment
Principal findings
This proof of concept demonstrates the feasibility of the TFO to identify maternal and fetal heart rates separately using an ANC algorithm. The results show that MHR could be accurately estimated, and FHR could be extracted with reasonable accuracy in human subjects with various skin colors and distances from the skin to the fetus.
Results in the context of what is known
Moderate baseline variability in intrapartum fetal heart tracing is an indirect reassurance of a nonhypoxic fetus, but any deterioration from this baseline pattern identified as minimal to absent baseline variability is an assumption of possible fetal hypoxia [3, 9]. Pulse oximetry allows direct estimation of oxygen saturation and if available with EFHRM would likely be able to determine if the deterioration of moderate baseline variability is indicative of fetal hypoxia versus a physiologic change in the quality of baseline fetal heart rate variability [10].
In a 2000 randomized controlled trial by Garite et al., a fetal oximetry device applied to the fetal cheek successfully assessed fetal oxygen saturation. The trial showed a reduction in cesarean delivery and timely intervention for fetal hypoxia, however, there was an increased number of operative deliveries for labor dystocia [24]. The device required application through the vagina, with the criteria of ruptured fetal membranes and the cervix being at least 2 cm dilated.
A 2014 Cochrane Systematic Review evaluated the effectiveness of fetal intrapartum invasive pulse oximetry in reducing operative deliveries [25]. The review included seven randomized controlled trials with a total of 8013 pregnancies. The findings showed that the combination of fetal pulse oximetry and CTG did not significantly impact the overall rates of caesarean sections. However, when applied in cases where there were existing concerns about the fetus’ well-being, fetal pulse oximetry led to a reduction in the number of caesarean sections performed specifically for addressing the fetus’ hypoxia risk [25].
Vintzileos et al. demonstrated a noninvasive transabdominal fetal pulse oximeter using continuous-wave NIR spectroscopy [26]. Similar to our POC study, the device was applied during NST in 6 women, and fetal oxygen saturation was calculated with a mean saturation of 61% correlated to an FHR range of 132–165 bpm. They observed wide variability in measurements, possibly due to saturation variations or technical errors. Although our TFO also utilizes continuous-wave NIR spectroscopy, the differences in our device include the specific LED wavelengths, number and type of photodetectors, their respective distance to light source, signal acquisition circuitry, and signal processing algorithms. In our study, the accuracy of TFO’s MHR and FHR estimates were computed against cardiotocograph device’s measurements. By using RMSE and MAE as accuracy metrics, accounting for outliers, and excluding reference values outside the typical MHR and FHR ranges, we considered all the extraneous factors that may influence the acquisition of the MHR and FHR. This approach allowed us to derive a distinct fetal signal separate from the maternal signal.
Clinical and research implications
Isolation of the fetal signal is most essential to accurately calculate the fetal oxygen saturation. In our ewe model validation, we had the benefit of obtaining ground truth oxygen saturation from the lamb arterial blood gas (ABG) drawn while segmentally inducing hypoxia. We have developed a machine learning based model for fetal oxygenation estimation from TFO readings [27, 28]. Ground truth oxygenation is needed for an initial calibration of the model. Once a model is calibrated, it can then be used to compute fetal oxygenation in patients without access to ground truth oxygenation.
The TFO fetal oxygen saturation highly correlated with the fetal ABG oxygen saturation across five ewes (Pearson’s r > 0.61 and p < 0.001), suggesting that the transcutaneous measurements are penetrating through the maternal abdomen sufficiently and are expressing the underlying fetal tissue physiology [27]. Unlike our ewe model, we have no truths to calibrate nor compare our human fetal oxygen saturation computations. The intent of this POC is to translate the findings from our ewe model and validate that our algorithm can distinctly separate fetal from maternal signal using the same TFO device.
Strengths and limitations
Unlike invasive fetal pulse oximetry, TFO faces limitations as the separation between the device and fetal arteries increases due to the presence of the myometrium, maternal abdominal wall, and amniotic fluid. As with Vintzileos et al., the strength of our study is the confirmation that noninvasive transabdominal fetal oximetry is feasible using NIR, even at depths of up to 5 cm in human fetuses. Further, we successfully translated our experience from the ewe model to humans, achieving accurate separation of the fetal signal from maternal signals and ambient noise. Accurate isolation of the fetal signal is the first step in estimating fetal oxygen saturation non-invasively. Although we did not calculate fetal oxygen saturation in this study, this decision was deliberate as we lacked a true reference for comparison. This POC study’s main purpose is to demonstrate the discrimination of the fetal signal above all other signals detected by NIR.
None of our subjects had adverse events from the device application. All subjects completed the data acquisition session. The only notable issue was the tolerable heat generated by the optode, which did not result in any skin irritation or burns. Unlike the transvaginal fetal pulse oximeter that requires vaginal insertion through a dilated cervix and rupturing of the fetal [26], our TFO device offers a non-invasive solution where the reflectance pulse oximeter is placed on the maternal abdomen.
TFO's ultimate use would be intrapartum. There exist limitations in the intrapartum setting in addition to those addressed in this antepartum POC. First, uterine contractions during labor introduce motion artifacts and may also cause a drop in fetal oxygen saturation in peripheral tissues confounding the TFO reading [29].
Second, due to the descent of the fetal head, maternal iliac vessel signals would introduce an additional source of error. Third, fetuses at term (> 39 weeks) have a lower baseline FHR due to the progressive maturation of the parasympathetic nervous system [30]. This may result in the smaller difference between the maternal and fetal heart rates, potentially increasing error. Nonetheless, we expect similar errors as seen in our antepartum study where small difference was observed between FHR and MHR 2nd harmonic. Hence, the discrimination of fetal signal remains essential during labor and additional limitations will be assessed in future intrapartum studies.
Conclusions
This proof of concept showed that transabdominal fetal pulse oximetry is possible given that the fetal heart signal was confidently separated from the maternal heart signal simultaneously eliminating the error caused by extraneous signals. The ability to isolate the fetal heart signal separate from maternal signal overcomes the first obstacle in developing a successful noninvasive method of detecting human fetal oxygen saturation. We are conducting the next step of validating our TFO device in the intrapartum setting at the same time we continue to refine our algorithm in the ewe model to improve the device prototype to reduce the error in detecting FHR.
References
Paul RH, Hon EH. A clinical fetal monitor. Obstet Gynecol. 1970;35(02):161–9.
Thacker SB, Stroup D, Chang M. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2001;(2):CD000063
Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(03):661–6.
Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(05):1181–93.
Caughey AB, Cahill AG, Guise JM, Rouse DJ, American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(03):179–93.
Cahill AG, Roehl KA, Odibo AO, Macones GA. Association and prediction of neonatal acidemia. Am J Obstet Gynecol. 2012;207(03):206.e1-206.e8.
Graham EM, Petersen SM, Christo DK, Fox HE. Intrapartum electronic fetal heart rate monitoring and the prevention of perinatal brain injury. Obstet Gynecol. 2006;108(3 Pt 1):656–66.
Shields LE, Wiesner S, Klein C, Pelletreau B, Hedriana HL. A Standardized Approach for Category II Fetal Heart Rate with Significant Decelerations: Maternal and Neonatal Outcomes. Am J Perinatol. 2018;35(14):1405–10. https://doi.org/10.1055/s-0038-1660459.
Jia YJ, Ghi T, Pereira S, Gracia Perez-Bonfils A, Chandraharan E. Pathophysiological interpretation of fetal heart rate tracings in clinical practice. Am J Obstet Gynecol. 2023;228(6):622–44. https://doi.org/10.1016/j.ajog.2022.05.023.
Jubran A. Pulse oximetry. Crit Care. 2015;19(1):272. https://doi.org/10.1186/s13054-015-0984-8.
Zourabian A, Siegel A, Chance B, Rumanian N, Rode M, Boas D. Transabdominal monitoring of fetal arterial blood oxygenation using pulse oximetry. J Biomed Opt. 2000;5:391–405.
Fong D, Yamashiro KJ, Johnson MA, Vali K, Galganski LA, Pivetti CD, Farmer DL, Hedriana HL, Ghiasi S. Validation of a Novel Transabdominal Fetal Oximeter in a Hypoxic Fetal Lamb Model. Reprod Sci. 2020;27(10):1960–6. https://doi.org/10.1007/s43032-020-00215-5.
Fong D, Knoesen A, Ghiasi S. Transabdominal fetal pulse oximetry: The case of fetal signal optimization, 2017 IEEE 19th International Conference on e-Health Networking, Applications and Services (Healthcom); 2017. pp. 1–6. https://doi.org/10.1109/HealthCom.2017.8210799.
Fong D, Knoesen A, Motamedi M, O’Neill T, Ghiasi S. Recovering the fetal signal in transabdominal fetal pulse oximetry. Smart Health. 2018;9:23–36. https://doi.org/10.1016/j.smhl.2018.07.011.
Fong D, Yamashiro K, Vali K, Galganski L, Thies J, Moeinzadeh R, Pivetti C, Knoesen A, Srinivasan V, Hedriana H, Farmer D, Johnson M, Ghiasi S. Design and in vivo evaluation of a non-invasive transabdominal fetal pulse oximeter. IEEE Trans Biomed Eng. 2021;68(1):256–66. https://doi.org/10.1109/TBME.2020.3000977.
Wisotzky EL, Uecker FC, Dommerich S, Hilsmann A, Eisert P, Arens P. Determination of optical properties of human tissues obtained from parotidectomy in the spectral range of 250 to 800 nm. J Biomed Optics. 2019;24(12):125001. https://doi.org/10.1117/1.JBO.24.12.125001.
Fong D, Srinivasan VJ, Vali K, Ghiasi S. Optode design space exploration for clinically-robust non-invasive fetal oximetry. ACM Trans Embed Comput Syst. 2019;18(5s):1–22. https://doi.org/10.1145/3358207
Kasap B, Vali K, Qian W, Chak WH, Vafi A, Saito N, Ghiasi S. Multi-detector heart rate extraction method for transabdominal fetal pulse oximetry. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Mexico. 2021. pp. 1072–1075. https://doi.org/10.1109/EMBC46164.2021.9630946
Kasap B, Vali K, Qian W, Saffarpour M, Ghiasi S. Kubai: Sensor fusion for non-invasive fetal heart rate tracking. IEEE Trans Biomed Eng. 2023;70(7):2193–202. https://doi.org/10.1109/TBME.2023.3238736.
Pildner von Steinburg S, Boulesteix AL, Lederer C, Grunow S, Schiermeier S, Hatzmann W, Schneider KT, Daumer M. What is the “normal” fetal heart rate? PeerJ. 2013;1:e82. https://doi.org/10.7717/peerj.82.
Kasap B, Vali K, Qian W, Hedriana HL, Wang A, Farmer DL, et al. Towards noninvasive accurate detection of intrapartum fetal hypoxic distress. 2021 IEEE 17th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Athens, Greece. 2021. pp. 1–4. https://doi.org/10.1109/bsn51625.2021.9507036.
Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S, et al. Trends of blood pressure and heart rate in normal pregnancies: A systematic review and meta-analysis. BMC Med. 2019;17:1. https://doi.org/10.1186/s12916-019-1399-1.
Murray ML. Maternal or fetal heart rate? avoiding intrapartum misidentification. J Obstet Gynecol Neonatal Nurs. 2004;33:93–104. https://doi.org/10.1177/0884217503261161.
Garite TJ, Dildy GA, McNamara H, Nageotte MP, Boehm FH, Dellinger EH, Knuppel RA, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049–58.
East CE, Begg L, Colditz PB, Lau R. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews. 2014;2014(1):CD004075
Vintzileos AM, Nioka S, Lake M, Li P, Luo Q, Chance B. Transabdominal fetal pulse oximetry with near-infrared spectroscopy. Am J Obstet Gynecol. 2005;192(1):129–33. https://doi.org/10.1016/j.ajog.2004.07.022.
Qian W, Vali K, Kasap B, et al. Continuous Transabdominal Fetal Pulse Oximetry (TFO) in Pregnant Ewe Models under Induced Fetal Hypoxia. Am J Obstet Gynecol. 2023;228(1):S242–3. https://doi.org/10.1016/j.ajog.2022.11.441.
Vali K, Kasap B, Qian W, et al. Non-invasive transabdominal assessment of In-Utero fetal oxygen saturation in a hypoxic lamb model. Am J Obstet Gynecol. 2021;224(2):S604. https://doi.org/10.1016/j.ajog.2020.12.999.
Wyatt JS. Cerebral oxygenation and haemo-dynamics in the foetus and newborn infant. Phil Trans R Soc Lond B. 1997;352(1354):697–700. https://doi.org/10.1098/rstb.1997.0051.
Amorim-Costa C, Costa-Santos C, Ayres-de-Campos D, Bernardes J. Longitudinal evaluation of computerized cardiotocographic parameters throughout pregnancy in normal fetuses: a prospective cohort study. Acta Obstet Gynecol Scand. 2016;95(10):1143–52.
Kasap B, Vali K, Qian W, Saffarpour M, Fowler R, Ghiasi S. Robust Fetal Heart Rate Tracking through Fetal Electrocardiography (ECG) and Photoplethysmography (PPG) Fusion. Annu Int Conf IEEE Eng Med Biol Soc. 2023Jul;2023:1–4. https://doi.org/10.1109/EMBC40787.2023.10341068.
Funding
This work was supported by the National Science Foundation (NSF) grant numbers IIS-1838939 (Drs. Ghiasi and Hedriana); the National Institutes of Health (NIH) grant number 5R21HD097467 (Dr. Ghiasi); UC Davis Clinical and Translational Science Center through NIH grant number UL1 TR001860; the National Center for Interventional Biophotonic Technologies (NCIBT) through a grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH grant number 1P41EB032840.
The funding agencies were not involved in study design; in collection, analysis, or interpretation of the data; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Approval was obtained from the Institutional Review Board of University of California Davis. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Patients signed informed consent regarding publishing their data and photographs.
Conflict of Interest
Soheil Ghiasi is a co-founder of Storx Technologies, an early-stage startup, that is spun off to advance UC Davis academic research on transabdominal fetal pulse oximeter towards impacting patient care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Abstract and findings were presented at the Society for Maternal–Fetal Medicine 43rd Annual Pregnancy Meeting, San Francisco CA, February 6–11, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasap, B., Vali, K., Qian, W. et al. Transcutaneous Discrimination of Fetal Heart Rate from Maternal Heart Rate: A Fetal Oximetry Proof-of-Concept. Reprod. Sci. 31, 2331–2341 (2024). https://doi.org/10.1007/s43032-024-01582-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-024-01582-z