Abstract
Oxytocin-dependent mechanisms are hypothesized to contribute to painful menses, but clinical trials of oxytocin antagonists for dysmenorrhea have had divergent outcomes. In contrast, broader studies have shown that increased systemic oxytocin concentrations are associated with increased pain tolerance and improved psychosocial function. We sought to confirm whether increased serum oxytocin concentrations are associated with menstrual pain and other psychosocial factors. Women with a history of primary dysmenorrhea (n = 19), secondary dysmenorrhea (n = 12), and healthy controls (n = 15) completed pain and psychosocial questionnaires, provided a medical history, and rated their pain during the first 48 h of menses. Serum samples were collected during menses to measure oxytocin concentrations. Oxytocin was significantly lower in participants with a history of primary (704 ± 33 pg/mL; p < 0.001) or secondary (711 ± 66 pg/mL; p < 0.01) dysmenorrhea compared to healthy controls (967 ± 53 pg/mL). Menstrual pain over the past 3 months (r = −0.58; p < 0.001) and during the study visit (r = −0.45; p = 0.002) was negatively correlated with oxytocin concentrations. Pain catastrophizing (r = −0.39), pain behavior (r = −0.32), and pain interference (r = −0.31) were also negatively correlated with oxytocin levels (p’s < 0.05). Oxytocin was not significantly correlated with psychosocial factors. Contrary to our hypothesis, women with a history of primary or secondary dysmenorrhea had lower oxytocin concentrations during menses when compared to healthy controls. Lower circulating oxytocin concentrations were also associated with worse menstrual pain and pain-related behavior. When considering the existing literature, low circulating oxytocin may be a sign of dysfunctional endogenous pain modulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxytocin is well-known for its critical role in social bonding, lactation, and parturition (for review see [1]). Although the hormone acts as a potent uterotonic agent, oxytocin’s role in menstrual pain is disputed. Numerous studies document that serum oxytocin concentrations increase before labor and stimulate uterine contractility during pregnancy (for review see [2]). Oxytocin is also capable of stimulating uterine contractility in nonpregnant women [3]. Given that uterine contractions are posited to be responsible for cramping pain [4], an oxytocin-dependent mechanism could drive menstrual pain in women with dysmenorrhea.
In the last decade, only one small pilot study reported that dysmenorrheic women have higher plasma oxytocin concentrations at the start of menses when compared to eumenorrheic women [5]. The authors suggest that high oxytocin in plasma may be due to low oxytocin receptor expression previously observed in the endometrium of dysmenorrheic women [6]. In other pain conditions, however, oxytocin appears to have a differing role. Other clinical pain studies reported that higher endogenous serum oxytocin concentrations were associated with increased pain tolerance [7,8,9]. Determining if circulating oxytocin mediates dysmenorrhea would improve our pathophysiological understanding of this condition and help determine if oxytocin-based therapies would be useful. For example, earlier work suggested that the oxytocin receptor antagonist, atosiban, could alleviate menstrual pain [10], but a subsequent trial failed [11].
Consistent with oxytocin’s established role in uterine contractility and Liedman’s findings, we hypothesized that serum oxytocin concentrations would be higher in dysmenorrheic women and would be associated with greater menstrual pain. To test this hypothesis, dysmenorrheic participants and healthy controls rated their menstrual pain and provided blood samples to measure serum concentrations. To establish whether circulating oxytocin is associated with menstrual pain due to structural causes, we also studied women with secondary dysmenorrhea. Given the neuropeptide’s importance in social behavior [12], we also evaluated the association between oxytocin and psychosocial variables.
Materials and Methods
Participant Recruitment
The data in this manuscript is combined from two separate studies that similarly collect blood during menses, but focused on MRI results [4] or abdominal EMG activity [13]. Participants with dysmenorrhea and healthy controls (ages 18–45) were prospectively recruited from physician referral or from local advertisements during the abdominal EMG study between January 2015 and February 2017 and between October 2017 and August 2018, during MRI study. The NorthShore Institutional Review Board approved both of these HIPAA-compliant studies. In both studies, MRI or ultrasonography (US) was used to investigate the mechanisms underlying dysmenorrhea.
Participants with dysmenorrhea were required to have menstrual pain of a 4 or higher on a numeric rating scale (NRS; 0: “no pain at all” to 10: “worst pain imaginable” [14]) when not taking analgesics. Healthy controls were required to have menstrual pain of 3 or less on an NRS without medication. The level of menstrual pain for both groups was confirmed with a daily diary as described below. Exclusion criteria for the study included a history of pelvic or abdominal malignancies, irregular menses, a pregnancy within prior 6 months, breastfeeding, an active genitourinary infection in the previous 4 weeks, body mass index > 40, an unwillingness to stop taking NSAIDs 8–12 h before the start of the study visit, a reluctance to have a withdrawal bleed on continuous oral contraceptives, an inability to read/comprehend a consent form in English, or having a known standard MRI contraindication.
All participants were scheduled for a menses and a non-menses visit. In between these visits, participants were asked to report their menstrual pain using the NRS via online menstrual diaries [15].
Assessment Visit
Participants were scheduled for a visit during the first 48 h of menstrual bleeding (menses) and again during the peri-ovulatory phase of their menstrual cycle (non-menses). Participants were instructed to abstain from taking short-acting analgesic medications at least 8 h before the visit, or 12 h for longer acting analgesics. The data presented here was collected during the menses visit.
Upon arrival, participants gave their written informed consent and were asked to rate their baseline and maximum menstrual pain using an NRS scale before the MRI/US. Baseline pain referred to the average intensity of pain the participant experienced in the last 20 min, while maximum menstrual pain referred to the highest intensity of pain experienced in the same timeframe. In this study, we used maximum menstrual pain as the primary measure of menstrual pain at the visit. After the MRI/US scan, participants filled out questionnaires to obtain complete medical, surgical, psychological, gynecological, and obstetrical history. Included in these measures was a self-rating of menstrual pain over the past 3 months with and without analgesics on a 0–100 mm visual analog scale (VAS). We verified that participants reporting a history of endometriosis had prior surgical confirmation via their medical records or their medical history questionnaire. Additionally, other potential contributing anatomical factors (e.g., adenomyosis and leiomyoma) were confirmed in participants that underwent MRI.
Blood Collection and Serum Retrieval
Blood samples were collected during the first 48 h of menses by a research nurse using BD Vacutainer serum tubes (BD and Co., Franklin Lakes, NJ) and stayed at room temperature for 30–60 min to allow the blood to clot. Blood samples were then centrifuged at 3000x g for 5–10 min at 0 °C to retrieve the serum supernatant. The serum was immediately transferred into cryogenic vials (Corning Inc., Corning NY) and stored at −20 °C until needed.
Serum Oxytocin Quantification
Serum oxytocin was measured using the validated enzyme-linked immunosorbent assay [16,17,18] (Enzo Life Sciences, Inc., Farmingdale NY). Serum samples were diluted 1:8 and analyzed in duplicate per manufacturer’s protocols. Samples were excluded if their coefficients of variance were greater than 20%. Oxytocin concentrations from all participants ranged from 434 pg/mL to 1324 pg/mL, with a mean of 788 ± 31 pg/mL. Inter- and intra-assay coefficients of variation were 9.6% and 2.0%, respectively.
Statistical Analyses
A post hoc power analysis (α = 0.05) confirmed we had a 99% power for evaluating the significance of serum oxytocin differences between healthy controls (n = 15) and women with primary (n = 19) or secondary (n = 12) dysmenorrhea. The primary outcome (serum oxytocin concentration) was assessed for normality using the Shapiro-Wilk test. Student’s t-tests were used to determine group differences in clinical profiles for the following variables: VAS for pelvic/bowel/urinary pain, demographic characteristics, Genitourinary Pain Index [19], menstrual pain (NRS and VAS), Pain Catastrophizing Scale [20], PROMIS [21] measures (anxiety, depression, pain behavior, pain interference, social satisfaction) and oxytocin. A one-way ANOVA was used to determine group differences in serum oxytocin concentrations based on race. Pearson correlation coefficients were calculated to compare oxytocin to age, BMI, menstrual pain (NRS and VAS), Pain Catastrophizing Scale, and PROMIS measures. Partial correlation coefficients were calculated to compare oxytocin to menstrual pain at the visit or menstrual pain over the past 3 months while accounting for depression as a potential confounder.
Results
Age, BMI, race, and education did not differ between healthy controls, participants with a history of primary dysmenorrhea (Dys), or participants with confirmed secondary dysmenorrhea (Dys-S) (Table 1). As expected, dysmenorrheic participants collectively reported greater menstrual pain and corresponding absenteeism (p’s < 0.001) when compared to healthy controls. A detailed comparison within the clinical pain characteristics (Tables 2 and 3) showed that Dys-S participants reported greater Genitourinary Pain Index scores (p < 0.01) and greater pain interference (p < 0.01) than Dys participants. This is consistent with what would be expected in a secondary dysmenorrheic cohort.
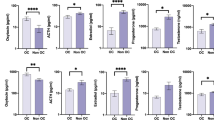
At the time of the study visit, we confirmed that Dys (5.6 ± 0.5, NRS) and Dys-S (6.6 ± 0.5) participants continued to experience greater menstrual pain while healthy controls were pain free (0 ± 0, p’s < 0.001). Circulating blood samples collected during menses were used to compare serum oxytocin concentrations between the three groups (Fig. 1). When compared to healthy controls (966 ± 55 pg/mL), Dys (704 ± 33 pg/mL) and Dys-S participants (711 ± 66 pg/mL) had significantly lower oxytocin concentrations (p’s < 0.01). We also conducted post hoc sensitivity analyses to determine if age, BMI, or race were potential cofounders. Pearson correlation analyses demonstrated that age (r = 0.22, p = 0.143) and BMI (r = 0.05, p = 0.729) were not significantly associated with serum oxytocin concentrations. Additionally, there were no significant differences in serum oxytocin concentrations based on participant’s race (p = 0.192).
Building on the above findings, we next explored whether lower serum oxytocin concentrations were associated with pain-related or potentially confounding psychological variables (Tables 3 and 4). Lower oxytocin concentrations were primarily associated with greater menstrual pain reported at the study visit (r = −0.45; p = 0.002) and over the past 3 months (r = −0.58; p < 0.001). Low oxytocin concentrations were also associated with increased pain catastrophizing (r = −0.39; p = 0.006), increased pain-related behavior (r = −0.32; p = 0.025), and increased pain interference (r = −0.31; p = 0.033). Interestingly, serum oxytocin concentration was only marginally correlated with depression (r = −0.28; p = 0.052) and not significantly correlated with anxiety (r = −0.21; p = 0.157) or social satisfaction (r = 0.22; p = 0.142). When considering depression as a potential confounder, menstrual pain reported at the study visit (r = −0.38; p = 0.009) and over the past 3 months (r = −0.54; p < 0.001) remained significantly correlated with serum oxytocin concentrations. Therefore, serum oxytocin’s association with dysmenorrhea status is not likely confounded by depression (Table 4).
Comment
Our results show that, unexpectedly, women with a history of primary or secondary dysmenorrhea have lower serum oxytocin concentrations when compared to eumenorrheic women. Our results also show that oxytocin is more closely associated with pain intensity. Despite group differences between the dysmenorrheic cohorts and healthy controls, depression, anxiety, and social satisfaction were not associated with serum oxytocin concentrations.
Our results differ from those of Liedman and colleagues [5], who reported higher plasma oxytocin concentrations in dysmenorrheic women than healthy controls. They used extracted plasma samples and radioimmunoassays. With this extraction technique, the vast majority of oxytocin, which is protein-bound, will be excluded [22]. Although a gold standard for oxytocin measurement is currently debated, it remains to be determined whether protein-bound oxytocin is biologically active. In any case, the bound fraction of oxytocin can rapidly translate to being functional since the dissociation rate of oxytocin is 2 s−1 [23, 24]. For this study, we used a sensitive and discriminative assay validated for the use of unextracted plasma or serum with liquid chromatography and mass spectrometry [16] and diluted our samples per the manufacturer’s protocol to avoid matrix interference. Using a similar assay and methods, Erickson et al. reported that serum oxytocin concentration increases from 1651 ± 121 to 1717 ± 127 pg/mL in breastfeeding mothers following lactation [25]. Given that this subtle oxytocin increase is associated with the lactation letdown reflex, we anticipate that our reported group difference of approximately 250 pg/mL is biologically plausible and relevant.
With regard to psychosocial factors, our study did not find strong associations between serum oxytocin and anxiety, depression, or social satisfaction. We expected to detect an association given prior studies have reported correlations between oxytocin and anxiety [18], depression [26], and social function [27]. However, those studies drew from populations with formally diagnosed psychosocial disorders. In contrast, our use of PROMIS scales quantifies the relative level of anxiety, depression, and social satisfaction in a cohort primarily characterized by their menstrual pain condition rather than overt psychosocial dysfunction. A meta-analysis has suggested that inconsistent study results on circulating oxytocin and depression could be due to differences in illness phase and comorbidities [28]. Pain, a factor correlated with depression [29], could represent an important confounder often not addressed in prior studies. Our use of a partial correlation analysis confirmed an independent association between menstrual pain and oxytocin despite dysmenorrheic participants having worse depression than healthy controls.
Our findings provide additional mechanistic insight as to why prior studies investigating oxytocin antagonists for the treatment of primary dysmenorrhea had mixed results. These studies were based on work demonstrating that high oxytocin concentrations elicit uterine hypercontractility in humans [3]. One explanation for these mixed results from atosiban [10, 11] on dysmenorrhea is the use of intrauterine pressure catheters for measurement; the foreign object’s sensation is not physiological and may have exacerbated any normal relationship between oxytocin, uterine hypercontractility, and reported pain. Rather, oxytocin may have protective effects such as increasing uterine perfusion via splanchnic vasodilation [30]. Even during pregnancy, oxytocin elicits hypotension via arterial vasodilation [31]. Considering its protective effects and that circulating oxytocin concentrations are typically lowest during menses [32], it is unlikely that elevated oxytocin is a trigger for dysmenorrhea.
Stepping back from oxytocin’s role in uterine contractility, its overall association with pain symptoms could reflect a greater influence of this neuropeptide in pain regulation. Oxytocin modulates analgesia via a spinal circuit (for review, see [33]). Preclinical studies have demonstrated that endogenous oxytocin is released from the hypothalamus and brainstem upon peripheral noxious stimulation and that exogenous oxytocin increases pain tolerance [34, 35]. Low circulating oxytocin concentrations have been observed in other chronic pain conditions. For example, patients with recurrent abdominal pain [36] have 18% lower plasma oxytocin concentrations when compared to healthy controls. Notably, Yang found that patients with both acute and chronic back pain had 29% and 69% lower plasma oxytocin concentrations, respectively, than healthy controls [37]. These results are consistent with the hypothesis that oxytocin is analgesic. When considering these data in the context of the aforementioned work, low oxytocin concentrations observed in our patients might reflect unsuspected, shared pain modulatory deficits seen in these full-blown chronic pain states. Based on this mechanistic hypothesis, dysmenorrheic women might have pain relief with acute oxytocin administration. Given its favorable safety profile [38], it would be interesting to see whether oxytocin receptor agonists are effective for menstrual pain in a research trial.
Other clinical studies have demonstrated that both myometrial oxytocin receptor expression and binding affinity are increased in adenomyosis [39, 40] and endometriosis [41]. When compared to our findings, increased myometrial receptor concentrations may serve as a compensatory mechanism induced by acute or chronic reductions in oxytocin. Conversely, increased myometrial oxytocin receptor activity could be mediated by uterine-derived oxytocin concentrations that may not be reflected in circulation [42]. To tease apart this mechanism, circulating oxytocin, uterine oxytocin, and uterine oxytocin receptor expression would need to be quantified and compared during menses.
In this study, blood samples were collected exclusively during menses. Subsequent studies continuing to examine circulating oxytocin in dysmenorrhea should consider collecting plasma or serum sampled during non-menses or across the menstrual cycle. This comparison would further inform how oxytocin mechanistically contributes to menstrual pain. For example, if circulating oxytocin concentrations in dysmenorrheic women were consistently low throughout the menstrual cycle when compared to healthy controls, it would further suggest that dysmenorrhea is associated with dysfunctional endogenous pain modulation.
The strengths of this study are the careful characterization of pain in both primary and secondary dysmenorrhea and the use of correlation analysis with validated questionnaires to account for confounding by psychosocial factors. The study is limited by its sample size, the collection of serum samples without regard to fasting or time of day, and the inclusion of serum samples collected only in the menses phase. Although it was not possible to rule out occult endometriosis among participants with primary dysmenorrhea, the simultaneous acquisition of uterine imaging suggested that there were not any obvious anatomical factors within this subgroup. Future studies should also explore whether women with endometriosis, leiomyoma, or adenomyosis who do not experience dysmenorrhea have altered oxytocin concentrations.
Unexpectedly, our study found that women with a history of primary or secondary dysmenorrhea had lower serum oxytocin concentrations during menses when compared to eumenorrheic women. When considered alongside the existing literature, our findings suggest that low circulating oxytocin could be a sign of dysfunctional endogenous pain regulation.
References
Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83.
Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol. 2003;24:27–61.
Bossmar T, Akerlund M, Szamatowicz J, Laudanski T, Fantoni G, Maggi M. Receptor-mediated uterine effects of vasopressin and oxytocin in nonpregnant women. Br J Obstet Gynaecol. 1995;102:907–12.
Hellman KM, et al. Cine MRI during spontaneous cramps in women with menstrual pain. Am. J. Obstet. Gynecol. 2018;218:506.e1–8.
Liedman R, et al. Reproductive hormones in plasma over the menstrual cycle in primary dysmenorrhea compared with healthy subjects. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2008;24:508–13.
Liedman R, et al. Endometrial expression of vasopressin, oxytocin and their receptors in patients with primary dysmenorrhoea and healthy volunteers at ovulation. Eur J Obstet Gynecol Reprod Biol. 2008;137:189–92.
Anderberg UM, Uvnäs-Moberg K. Plasma oxytocin levels in female fibromyalgia syndrome patients. Z Rheumatol. 2000;59:373–9.
Alfvén G. Plasma oxytocin in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2004;38:513–7.
Grewen KM, Light KC, Mechlin B, Girdler SS. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethn Health. 2008;13:219–41.
Akerlund M. Can primary dysmenorrhea be alleviated by a vasopressin antagonist? Results of a pilot study. Acta Obstet Gynecol Scand. 1987;66:459–61.
Valentin L, Sladkevicius P, Kindahl H, Broeders A, Marsal K, Melin P. Effects of a vasopressin antagonist in women with dysmenorrhea. Gynecol Obstet Investig. 2000;50:170–7.
Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–57.
Oladosu FA, et al. Abdominal skeletal muscle activity precedes spontaneous menstrual cramping pain in primary dysmenorrhea. Am. J. Obstet. Gynecol. 2018;219:91.e1–7.
Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manag. 2011;41:1073–93.
Casper RF, Powell AM. Premenstrual syndrome: documentation by a linear analog scale compared with two descriptive scales. Am J Obstet Gynecol. 1986;155:862–7.
Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–22.
Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124:13–21.
Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701.
Clemens JQ, Calhoun EA, Litwin MS, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74(5):983–7 e1-3.
Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the pain Catastrophizing scale. J Behav Med. 1997;20:589–605.
Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11.
Brandtzaeg OK, et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep. 2016;6:31693.
Blumenstein M, Hruby VJ, Yamamoto DM. Evidence from hydrogen-1 and carbon-13 nuclear magnetic resonance studies that the dissociation rate of oxytocin from bovine neurophysin at neutral pH is slow. Biochemistry (Mosc). 1978;17(4971–4977).
Pardridge WM. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981;2:103–23.
Erickson EN, Carter CS, Emeis CL. Oxytocin, Vasopressin and Prolactin in New Breastfeeding Mothers: Relationship to Clinical Characteristics and Infant Weight Loss. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2019:890334419838225. https://doi.org/10.1177/0890334419838225.
Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–10.
Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–7.
Engel S, Laufer S, Knaevelsrud C, Schumacher S. The endogenous oxytocin system in depressive disorders: a systematic review and meta-analysis. Psychoneuroendocrinology. 2018;101:138–49.
Westling AM, Tu FF, Griffith JW, Hellman KM. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am. J. Obstet. Gynecol. 2013;209:422e.1–422.e10.
Altura BM, Altura BT. Actions of vasopressin, oxytocin, and synthetic analogs on vascular smooth muscle. Fed Proc. 1984;43:80–6.
Rabow S, Olofsson P. Pulse wave analysis by digital photoplethysmography to record maternal hemodynamic effects of spinal anesthesia, delivery of the baby, and intravenous oxytocin during cesarean section. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2017;30:759–66.
Engel S, Klusmann H, Ditzen B, Knaevelsrud C, Schumacher S. Menstrual cycle-related fluctuations in oxytocin concentrations: a systematic review and meta-analysis. Front Neuroendocrinol. 2018. https://doi.org/10.1016/j.yfrne.2018.11.002.
Xin Q, Bai B, Liu W. The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochem Int. 2017;103:57–64.
Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28:1113–9.
Eliava M, et al. A new population of Parvocellular oxytocin neurons controlling Magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–304.
Alfvén G, de la Torre B, Uvnäs-Moberg K. Depressed concentrations of oxytocin and cortisol in children with recurrent abdominal pain of non-organic origin. Acta Paediatr Oslo Nor. 1994;1992(83):1076–80.
Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine. 1994;19:867–71.
MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–26.
Nie J, Liu X, Guo S-W. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am. J. Obstet. Gynecol. 2010;202:346.e1–8.
Guo S-W, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil Steril. 2013;99:231–40.
Mechsner S, et al. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005;83(Suppl 1):1220–31.
Mitchell BF, Fang X, Wong S. Oxytocin: a paracrine hormone in the regulation of parturition? Rev Reprod. 1998;3:113–22.
Acknowledgments
We would like to acknowledge Dr. Gerald Gebhart for his editorial advice. This work was funded by NICHD HD081709, HD091502, and NorthShore University HealthSystem.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
F.F.T. is a consultant and speaker for AbbVie Pharmaceuticals. The remaining authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was done at NorthShore University HealthSystem
Rights and permissions
About this article
Cite this article
Oladosu, F.A., Tu, F.F., Garfield, L.B. et al. Low Serum Oxytocin Concentrations Are Associated with Painful Menstruation. Reprod. Sci. 27, 668–674 (2020). https://doi.org/10.1007/s43032-019-00071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00071-y