Abstract
Up to 25% of ovulating women suffer from primary dysmenorrhea, a condition associated with pain and transient-reduced quality of life, along with greater irritability and impaired sleep. In the present study, we asked whether and if so to what extent melatonin and meloxicam can improve subjective and objective sleep and reduce pain among women with primary dysmenorrhea (PD). To this end, we conducted a double-blind cross-over clinical trial lasting for three menstrual cycles. A total of 14 women (mean age M = 27.5 years) with primary dysmenorrhea took part in the study. At baseline, that is, during the first menstruation, they completed a visual analogue scale to rate pain; sleep continuity was assessed via actigraphs, and overall sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI). Next, participants were randomly assigned to one of two conditions, either melatonin during the second, and meloxicam during the third menstruation, or meloxicam during the second, and melatonin during the third menstruation. Neither participants nor investigators were aware of participants’ study assignment. During the second and third menstruations, the assessments described above were repeated. At baseline, sleep assessed both objectively and subjectively was impaired, and pain was high. Subjective sleep improved and pain decreased during the second and third menstruations irrespective of whether melatonin or meloxicam was administered first or second. Likewise, objective sleep efficiency increased and objective sleep latency shortened. The efficacy of melatonin was superior to that of meloxicam. The present pattern of results suggests that both melatonin and meloxicam are suitable to treat pain and PD-related sleep complaints among women with primary dysmenorrhea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abdominal cramping pain at the onset of and during menstruation and in the absence of identifiable pelvic pathology is defined as primary dysmenorrhea (PD; Iacovides et al. 2015). Other symptoms include headache, lack of energy, insomnia, low mood, decreased quality of life, stress, and socio-behavioral issues (Iacovides et al. 2015). Prevalence rates range from 25 to 45% of menstruating women (Dawood 2006; Iacovides et al. 2015). Furthermore, in about 20% of women with PD, pain severity may impact negatively on everyday activities and lead to absence from school or work (Latthe and Champaneria 2014; Dawood 2006).
Abd-El-Maeboud et al. (2014) listed the following interventions to treat PD-related pain: pain relievers, herbal medications, dietary therapies, acupuncture, transcutaneous electrical nerve stimulation, and laparoscopic presacral neurectomy. Caruso et al. (2015, 2016) also found positive effects of contraceptives in the treatment of PD-related pain. In the present study, we investigated the efficacies of a pain reliever (meloxicam) and a sleep remedy (melatonin).
As regards sleep, Baker et al. (1999) and Iacovides et al. (2015, 2009) showed that, compared to women without PD, women with PD had both subjectively and objectively reduced sleep. Reduced sleep includes subjective lower sleep quality, shorter sleep duration, more awakenings after sleep onset, and lower sleep efficiency. Additionally, poor sleep and increased pain processing are associated. Lautenbacher (2017) and Lautenbacher et al. (2006) showed that sleep deprivation increased pain in an almost linear function (cf. Onen et al. 2001; Roehrs and Roth 2005). Correspondingly, pain impairs sleep (Lautenbacher 2017; Marshansky et al. 2017; Roehrs and Roth 2005). We took all these observations into account and asked whether and if so to what extent a pain reliever (meloxicam) could both relieve PD-related pain and improve PD-related sleep during menstruation. We also asked whether a sleep remedy (melatonin) might have similar benefits.
Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and fever-reducing effects. In double-blind studies of treatments for PD-related pain, meloxicam proved to be superior to placebo (Chantler et al. 2008) and equal to mefenamic acid (de Mello et al. 2004), but no study has yet investigated the influence of meloxicam on sleep either in general or among women with PD.
Melatonin is a hormone that is produced by the pineal gland and regulates sleep and wakefulness. As a medication, melatonin appeared to have the potential to improve sleep among adults with insomnia (Auld et al. 2017). Additionally, research on assisted reproductive technologies indicates that melatonin seems to increase fertilization rate (Vitale et al. 2016). Exogenous melatonin also has the potential to perform as an analgesic (Zhu et al. 2017), probably via a down-regulation of inflammatory pathways (Posa et al. 2017). Accordingly, it is conceivable that melatonin impacts positively both on sleep and on pain in women with PD.
The following hypotheses were formulated. First, following others (Chantler et al. 2008; de Mello et al. 2004), we expected that meloxicam would reduce pain. Second, following the rationale of the pain-sleep-link (Lautenbacher 2017; Lautenbacher et al. 2006; Roehrs and Roth 2005), we expected improved sleep when pain was reduced under meloxicam. Third, following others (Auld et al. 2017), we expected that sleep would improve under melatonin. Fourth, following Posa et al. (2017), we expected that melatonin would have positive effects with respect to pain as well as sleep. To test the hypotheses, we performed a double-blind study with a cross-over design in which women with PD were randomly assigned either to a melatonin-first–meloxicam-second, or a meloxicam-first–melatonin-second condition. We believe that the results might help both women with PD and professionals to cope with both PD and PD-related poor sleep.
Method
Procedure
Eligible women with primary dysmenorrhea were informed about the aims of the study, and the confidential and anonymous handling of the data. Afterwards, participants gave their written informed consent. There were three time points for assessment, corresponding to three menstruations. Sleep was assessed both subjectively and objectively; pain was assessed via a visual analogue scale (VAS) during the days of each menstruation. After baseline and at the second menstrual cycle, participants were randomly assigned to one of the following two study conditions: Participants given melatonin during the second menstrual cycle received meloxicam for the third menstrual cycle; participants given meloxicam during the second menstrual cycle received melatonin for the third menstrual cycle.
This randomized crossover trial was conducted between June 2015 and August 2016 at the Kermanshah University of Medical Sciences (KUMS), Kermanshah, Iran. All procedures were approved by the institutional ethics committee of the KUMS, and performed in accordance with the rules laid down in the Declaration of Helsinki (Iranian Registry of Clinical Trials: IRCT2015031521475N1).
Sample
Eligible participants were thoroughly screened for medical and psychiatric issues. Inclusion criteria were (1) aged between 18 and 35 years, (2) self-reported abdominal pain (six of more points on the visual analogue scale) immediately prior to and during the first 3 days of menstruation, (3) regular menstruation with a cycle duration of 28 days ± 3 days, and (4) impaired sleep (Pittsburgh Sleep Quality Index score of five and higher) immediately prior to and during the first 3 days of menstruation. Exclusion criteria were (1) diagnosis of secondary dysmenorrhea; (2) sleep-related issues such as RLS, snoring, sleep apnea, shift-work, and sleep-wake disorders; (3) psychiatric disorders such as major depressive disorders, eating disorders, bipolar disorders, and substance use disorder; (4) 2 weeks prior to the first screening and throughout the entire study excessive intake of sleep-altering substances; (5) hormonal contraceptives; and (6) breastfeeding (exclusion criteria 1–6 based on a thorough medical, psychiatric, and sleep-related interview performed by medical doctors, psychiatrists, and clinical psychologists).
Power analysis and randomization
Power analysis was performed with G*Power®3.1.9.3. A minimum sample size of 11 participants per cycle was required to detect a mean difference of 2.0, standard deviation of 2.0, 90% power, and 5% type I error, between baseline period and medication period. Julious (2005) has suggested that samples of 12 participants should be sufficient to run pharmaceutical pilot studies. Randomization occurred via computerized software (www.randomizer.org®).
Tools
Subjective sleep: Pittsburgh Sleep Quality Index (PSQI; Buysse et al. 1989)
The PSQI is a self-report scale that is completed in 5 min; it consists of 19 items and contains seven subscales (subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleeping medication, and daytime dysfunction), each weighted equally on a scale from 0 to 3, with higher scores indicating poorer sleep quality. The seven components are then summed to obtain an overall PSQI score, ranging from 0 (good sleep quality) to 21 (poor sleep quality). Total scores of ≥ 5 reflect poor sleep, associated with considerable sleep complaints. Farrahi et al. (2012) validated the psychometric properties of the Farsi version (Cronbach’s alpha = .83).
Objective sleep assessment
Actigraphy (Ambulatory Monitoring, Inc., USA) was used to record objective sleep continuity. The actigraph is a portable device which records patients’ movements to assess sleep parameters. Participants wore a digital movement-measuring instrument on the wrist of the non-dominant hand. The tool registers every movement above 0.012 g in a bi-axial direction. The data, recorded in 30-s intervals, were digitally integrated and afterwards translated into sleep measures using the software program (based on sleep/wake algorithm as defined by Gorny et al. 1997). The following parameters are scored: total sleep time (TST), sleep onset latency (SOL), number of awakenings after sleep onset (NWAK), and sleep efficiency (SE). Participants wore the actigraph during their menstrual periods.
Subjective pain
To assess participants’ dysmenorrhea-related pain, a 100-mm visual analogue scale (VAS) was employed, with the anchor points 0 (= “no pain at all”) to 10 (= “the worst pain I have ever felt”). Previous studies have shown that the VAS provides both reliable and valid assessments of pain (Coll and Ameen 2006; Collins et al. 1997).
Medication
Capsules were administrated to be taken shortly before going to bed during each night of their menstrual period. Each capsule consisted either of melatonin (3 mg) or meloxicam (7.5 mg) in gelatine capsules. The melatonin and meloxicam capsules were identical in shape, color, weight, scent, consistency, and packaging.
Further analgesic use
If participants required further pain relief (over the counter: OTCs without prescriptions) after taking the study medication, they were allowed to take their own medication, reporting the total number of items consumed during the entire menstrual phase.
Statistical analysis
ANOVAs for repeated measures were performed with Time (baseline, second, and third menstruation), study condition (melatonin-first–meloxicam-second vs. meloxicam first–melatonin-second), and the Time by Study condition interactions as factors, and subjective and objective sleep parameters and subjective pain and analgesic use as dependent variables. Post hoc tests after Bonferroni-Holm corrections for p values were performed to examine differences between the three specific time points both between and within the two study conditions. The nominal level of significance was set at alpha < .05. In case of deviation from sphericity, Greenhouse-Geisser corrected degrees of freedom and epsilon ε values were used. All statistics were performed with SPSS® 22.0 (IBM Corporation, Armonk NY, USA) for Apple® Mac®.
Results
Sample
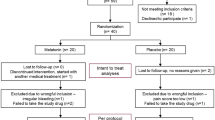
Forty-eight eligible participants were screened, and 16 fulfilled the inclusion criteria. Of these, 14 agreed to participate in the study; thus, data from 14 completers were analyzed (Fig. 1). Mean age was 27.5 ± 4.2 years (range 22–34 years). The mean body mass index (BMI) was 28.2 ± 4.3 kg/m2. Menses duration was 6.2 ± 0.6 days.
Tables 1 and 2 report all descriptive and inferential statistical indices.
Pain
Pain decreased over time (Fig. 2). A significant Time by Group interaction was observed; compared to the meloxicam condition, pain was lower in the melatonin condition. Post hoc analyses showed that, compared to the baseline, pain perception declined during both the second (either melatonin or meloxicam) and the third menstruation (either meloxicam or melatonin). Post hoc analyses within the study conditions showed that pain decreased continuously in the melatonin, but not in the meloxicam condition.
Subjective sleep (PSQI)
Subjective sleep (PSQI total score) improved over time (Fig. 3). Total sleep time and sleep efficiency increased, sleep onset latency shortened, and the number of awakenings after sleep onset and the PSQI total score decreased. No significant group differences and no significant Time by Group interactions were observed. Descriptively (large effect size), sleep duration increased more in the melatonin than in the meloxicam condition. Post hoc analyses showed that compared to the baseline, subjective sleep increased during both the second (either melatonin or meloxicam) and third menstruation (either meloxicam or melatonin). Specifically, subjective sleep duration increased, the subjective number of awakenings after sleep onset decreased, and sleep efficacy increased both under melatonin and meloxicam, and always compared to the baseline.
Objective sleep (actigraphy)
Total sleep time (TST), sleep onset latency (SOL), the number of awakenings after sleep onset, and sleep efficacy (SE) did not change significantly over time, and no significant Group and Time by Group interactions were observed (see Table 2). However, there were large effect sizes over time for shortened sleep onset latency, while a medium effect size was observed for increased sleep efficiency. Furthermore, compared to the meloxicam condition, the melatonin condition was superior (large effect sizes) for shortened sleep onset time (Fig. 4) and for sleep efficiency.
Use of analgesic medications
The use of other analgesic medications decreased over time, and irrespective of melatonin or meloxicam.
Discussion
The key findings of the present study were that, in a sample of women with primary dysmenorrhea, compared to the baseline, administration of both melatonin and meloxicam improved subjective sleep and reduced pain. As regards objective sleep, large effect sizes were observed over time for shortened sleep onset time and increased sleep efficiency. The present results add to the current literature in that we showed that both a pain reliever (meloxicam) and a sleeping drug (melatonin) produced improvements in subjective sleep, pain, use of additional analgesic medication, and dimensions of objective sleep.
Four hypotheses were formulated and each of these is now considered.
Our first hypothesis was that meloxicam would reduce pain, and this was confirmed. The results expanded upon previous research (Chantler et al. 2008; de Mello et al. 2004) in that these results emerged in a double-blind RCT to treat PD-related pain.
Our second hypothesis was that the administration of meloxicam would also have a positive impact on participants’ sleep patterns, and again, this was confirmed. Accordingly, we were able to expand upon previous research in showing that a pain reliever (meloxicam) could improve sleep among women with PD. Importantly, sleep improved both subjectively and objectively (medium effect sizes for sleep efficiency and awakenings after sleep onset). While the data available could not shed light on the underlying mechanisms, we follow others (Lautenbacher 2017; Lautenbacher et al. 2006; Roehrs and Roth 2005), who have argued that pain and pain elaboration interrupts sleep and up-regulates psychophysiological arousal. Consistent with this view is the literature to showing that, among people suffering from insomnia, sleep disturbances are maintained by dysfunctional beliefs and negative expectancies (Riemann et al. 2010). We suggest that, in parallel fashion, negative expectancies as regards pain and poor sleep among women with PD may trigger and maintain poor sleep (and pain) during menstruation. On this basis, we argue that reduced pain during menstruation also resulted in improved sleep.
Our third hypothesis was that sleep would improve with melatonin, and this hypothesis was supported. Thus, again, we expanded upon previous results (Auld et al. 2017) in that melatonin was administered for the first time for sleep complaints in women with PD.
Our fourth hypothesis was that administration of melatonin would also reduce pain, and this too was confirmed. The present results are thus in accord with previous research (Posa et al. 2017), but again, the current research adds to this in demonstrating the pain-relieving effect of melatonin for women with PD.
The pattern of results indicated that both melatonin and meloxicam improved subjective and objective sleep and reduced pain; these treatments also resulted in a reduced intake of OTC pain relievers. Can melatonin and meloxicam therefore be regarded as interchangeable? A closer inspection of the data reveals a more fine-grained pattern. As regards pain, melatonin did significantly and continuously reduce pain, while this was not the case for meloxicam. By the third time-point, pain was significantly lower in the melatonin than in the meloxicam condition. Likewise, compared to the meloxicam condition, in the melatonin condition, pain intensity was lower. It therefore appears that of these two options, melatonin is clearly the better.
A similar observation holds true for subjective sleep onset latency—compared to the baseline condition, the decrease in SOL was more pronounced in the melatonin than the meloxicam condition—and for subjective sleep efficiency—compared to the baseline condition, the increase in sleep efficiency (SE) was more pronounced in the melatonin than the meloxicam condition. This pattern of results was further confirmed by actigraph-based objective SOL and SE values: There were large effect sizes for the melatonin—but not the meloxicam condition.
Changes in the use of additional OTC pain relievers showed that their intake had increased by the third time-point in the meloxicam, but not in the melatonin condition. It is unclear why additional intake of pain reliever increased in the former condition. We believe that there are three possible reasons for this. First, it is conceivable that meloxicam was associated with poorer subjective and objective sleep, and that poor sleep increased pain perception (Brand et al. 2010; Lautenbacher 2017; Lautenbacher et al. 2006; Roehrs and Roth 2005, 2017). Consistent with this is the finding by Roehrs et al. (2006) that experimentally curtailed sleep duration and REM sleep had a hyperalgesic effect among young healthy pain-free and normal sleepers. Thus, a similar process might have occurred in the meloxicam but not the melatonin condition. A second reason might be that melatonin had a more pronounced impact, as melatonin is known positively to impact both sleep and pain (Posa et al. 2017). Third, it is possible that unassessed and latent psychophysiological factors might have biased the present pattern of results.
The data available from this study were unable to shed light on the neurophysiological mechanisms underlying the associations between sleep and pain among women with PD when they were treated with either melatonin or meloxicam. In the absence of more direct evidence, therefore, we offer the following speculations. Price (2000) proposed a dual process of pain signaling and cognitive-emotional pain elaboration: In addition to the sensory-discriminative afferent pathway that transmits pain signals from the periphery to the central nervous system (“Where does it hurt?”), an affective-motivational afferent pathway (“How much does it hurt?”) involves brain regions responsible for emotional-cognitive processes. Coghill et al. (2003) noted that pain signals appear to be elaborated via the affective-motivational afferent pathway in the anterior circular cortex (ACC), the insula cortex, prefrontal cortex (PFC), and the amygdala, thus underlining that pain and pain elaboration are not linear “if-then” mechanisms, but are instead highly cognitive-emotional processes. Thus, the possible directions of influence are as follows. We assume that at baseline, poor objective and subjective sleep and high self-rated pain were reciprocally related. Under melatonin and meloxicam, pain decreased and both sleep quality and sleep continuity improved.
Several limitations warrant against overgeneralization of these findings. First, we employed actigraphs to estimate dimensions of sleep continuity, while sleep-EEG recordings could have provided more insight into the association between pain and dimensions of sleep architecture (REM sleep and non-REM sleep; duration and percentage relative to the total sleep time). Specifically, it would have been interesting to know if REM sleep changed both under melatonin and meloxicam and as a function of reduced pain perception, as it is believed that REM sleep, pain perception, and pain consolidation may be functionally related (Roehrs et al. 2006; Roehrs and Roth 2005). Second, it is conceivable that additional latent and unassessed psychophysiological dimensions might have biased two or more variables in the same or opposite direction. This holds particularly true given that we relied upon self-assessments, while experts’ ratings might have allowed assessment of symptoms of depression and anxiety. Third, a placebo condition would have allowed estimation of both the physiological and psychological impact of melatonin and meloxicam on patients’ sleep and pain.
Conclusions
While the underlying neurophysiological mechanisms remain unclear, both melatonin and meloxicam administered during menstruation improved both subjective and objective sleep and reduced pain among ovulating women suffering from primary dysmenorrhea.
References
Abd-El-Maeboud KH, Kortam MA, Ali MS, Ibrahim MI, Mohamed RM (2014) A preliminary pilot randomized crossover study of uzara (Xysmalobium undulatum) versus ibuprofen in the treatment of primary dysmenorrhea. PLoS One 9(8):e104473. https://doi.org/10.1371/journal.pone.0104473
Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL (2017) Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev 34:10–22. https://doi.org/10.1016/j.smrv.2016.06.005
Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D (1999) High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Phys 277(6 Pt 1):E1013–E1021
Brand S, Gerber M, Puhse U, Holsboer-Trachsler E (2010) The relation between sleep and pain among a non-clinical sample of young adults. Eur Arch Psychiatry Clin Neurosci 260(7):543–551. https://doi.org/10.1007/s00406-010-0113-2
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Caruso S, Iraci M, Cianci S, Casella E, Fava V, Cianci A (2015) Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Investig 38(11):1211–1218. https://doi.org/10.1007/s40618-016-0460-6.
Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A (2016) Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 microg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Investig 39(8):923–931. https://doi.org/10.1007/s40618-015-0383-7
Chantler I, Mitchell D, Fuller A (2008) The effect of three cyclo-oxygenase inhibitors on intensity of primary dysmenorrheic pain. Clin J Pain 24(1):39–44. https://doi.org/10.1097/AJP.0b013e318156dafc
Coghill RC, McHaffie JG, Yen YF (2003) Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A 100(14):8538–8542. https://doi.org/10.1073/pnas.1430684100
Coll AM, Ameen J (2006) Profiles of pain after day surgery: patients’ experiences of three different operation types. J Adv Nurs 53(2):178–187. https://doi.org/10.1111/j.1365-2648.2006.03713.x
Collins SL, Moore RA, McQuay HJ (1997) The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 72(1–2):95–97
Dawood MY (2006) Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 108(2):428–441. https://doi.org/10.1097/01.AOG.0000230214.26638.0c
de Mello NR, Baracat EC, Tomaz G, Bedone AJ, Camargos A, Barbosa IC, de Souza RN, Rumi DO, Martinez Alcala FO, Velasco JAA, Cortes RJR (2004) Double-blind study to evaluate efficacy and safety of meloxicam 7.5 mg and 15 mg versus mefenamic acid 1500 mg in the treatment of primary dysmenorrhea. Acta Obstet Gynecol Scand 83(7):667–673. https://doi.org/10.1111/j.0001-6349.2004.00433.x
Farrahi MJ, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A (2012) Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath 16:79–82
Gorny SW, Allen RP, Krausmann DT, Cammarata J, Earley CJ (1997) A parametric and sleep hysteresis approach to assessing sleep and wake from wrist activity meter with enhanced frequency range. Paper presented at the 11th annual meeting of the associated professional sleep societies, June 10–15. San Francisco, CA
Iacovides S, Avidon I, Baker FC (2015) What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 21(6):762–778. https://doi.org/10.1093/humupd/dmv039
Iacovides S, Avidon I, Bentley A, Baker FC (2009) Diclofenac potassium restores objective and subjective measures of sleep quality in women with primary dysmenorrhea. Sleep 32(8):1019–1026
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4(4):287–291. https://doi.org/10.1002/pst.185
Latthe PM, Champaneria R (2014) Dysmenorrhoea. BMJ Clin Evid
Lautenbacher S (2017) Sleep and pain are definitely coupled-but how tight is this coupling? Pain 159:3–4. https://doi.org/10.1097/j.pain.0000000000001082
Lautenbacher S, Kundermann B, Krieg JC (2006) Sleep deprivation and pain perception. Sleep Med Rev 10(5):357–369. https://doi.org/10.1016/j.smrv.2005.08.001
Marshansky S, Mayer P, Rizzo D, Baltzan M, Denis R, Lavigne GJ (2017) Sleep, chronic pain, and opioid risk for apnea. Prog Neuro-Psychopharmacol Biol Psychiatry. https://doi.org/10.1016/j.pnpbp.2017.07.014
Onen SH, Alloui A, Gross A, Eschallier A, Dubray C (2001) The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res 10(1):35–42
Posa L, De Gregorio D, Gobbi G, Comai S (2017) Targeting melatonin MT2 receptors: a novel pharmacological avenue for inflammatory and neuropathic pain. Curr Med Chem 24:1. https://doi.org/10.2174/0929867324666170209104926
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288(5472):1769–1772
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C (2010) The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 14(1):19–31. https://doi.org/10.1016/j.smrv.2009.04.002
Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T (2006) Sleep loss and REM sleep loss are hyperalgesic. Sleep 29(2):145–151
Roehrs T, Roth T (2005) Sleep and pain: interaction of two vital functions. Semin Neurol 25(1):106–116. https://doi.org/10.1055/s-2005-867079
Roehrs TA, Roth T (2017) Increasing presurgery sleep reduces postsurgery pain and analgesic use following joint replacement: a feasibility study. Sleep Med 33:109–113. https://doi.org/10.1016/j.sleep.2017.01.012
Vitale SG, Rossetti P, Corrado F, Rapisarda AM, La Vignera S, Condorelli RA, Valenti G, Sapia F, Lagana AS, Buscema M (2016) How to achieve high-quality oocytes? The key role of Myo-inositol and melatonin. Int J Endocrinol 2016:4987436. https://doi.org/10.1155/2016/4987436
Zhu C, Xu Y, Duan Y, Li W, Zhang L, Huang Y, Zhao W, Wang Y, Li J, Feng T, Li X, Hu X, Yin W (2017) Exogenous melatonin in the treatment of pain: a systematic review and meta-analysis. Oncotarget 8(59):100582–100592. https://doi.org/10.18632/oncotarget.21504
Acknowledgements
We thank Nick Emler (University of Surrey, Surrey, UK) for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
FK, FM, SB, DSB, FA, HK, and MRG designed the study.
FK, FM, DSB, FA, HK, and MRG wrote the proposal for the ethical committee.
FK, FM, FA, HK, and MRG were highly involved in the recruitment of patients, the assessments, and interventions.
SB, DSB, and MRG performed the statistical analysis.
DSB, SB, and MRG wrote the draft of the manuscript.
DSB and SB integrated the co-authors’ comments.
DSB, SB, HK, and MRG completed the final version of the manuscript and submitted it.
Corresponding author
Ethics declarations
All procedures were approved by the institutional ethics committee of the KUMS, and performed in accordance with the rules laid down in the Declaration of Helsinki (Iranian Registry of Clinical Trials: IRCT2015031521475N1).
Conflict of interest
The authors declare that they have no conflicts of interests.
Financial disclosure
The entire study was performed without external funding.
Rights and permissions
About this article
Cite this article
Keshavarzi, F., Mahmoudzadeh, F., Brand, S. et al. Both melatonin and meloxicam improved sleep and pain in females with primary dysmenorrhea—results from a double-blind cross-over intervention pilot study. Arch Womens Ment Health 21, 601–609 (2018). https://doi.org/10.1007/s00737-018-0838-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-018-0838-x