Abstract
An impaired redox homeostasis is an important hallmark of biological aging. Coenzyme Q10 is an endogenous lipophilic antioxidant that decreases with age and has been linked to oxidative stress. The purpose of this study was to evaluate the effect of CoQ10 supplementation on redox homeostasis and levels of inflammatory cytokines in young and old rats. Male Wistar rats (young and old) were randomly divided into four groups (n = 6). Group I: young control, Group II: young rats treated with CoQ10, Group III: old control, Group IV: old rats treated with CoQ10. CoQ10 (20 mg/kg) was administered daily to Group II and IV via oral gavage. After 28 days of treatment, rats were sacrificed and biomarkers of oxidative stress and inflammatory cytokines were evaluated. Results demonstrated a significant (p ≤ 0.05) increase in malondialdehyde, protein carbonyl oxidation, advanced oxidation protein products, inflammatory cytokines: CRP, IL-6, TNF-α, and a decline in levels of superoxide dismutase, catalase, reduced glutathione, ferric reducing antioxidant potential in plasma and plasma membrane redox system in old rats when compared to young rats. After treatment with CoQ10 significant decrease in the level of MDA, PCO, AOPP, CRP, IL-6, and TNF-α was observed. Also, significant up-regulation of SOD, CAT, GSH, FRAP, and PMRS was observed. The results show that supplementing rats with CoQ10 aids in the maintenance of redox equilibrium with replenishment of antioxidant reserves and down-regulation of inflammatory biomarkers. Thus CoQ10 supplementation could be a potential anti-aging therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aging is an intricate process that is characterized by the gradual decline in the physical, mental, and reproductive capacity, leading to loss of function, vulnerability to diseases, and ultimately the end of life (Jia et al. 2017). Among the various theories put forward, the free radical theory of aging is one of the most accepted postulations which states that oxidative damage is the underlying cause of aging (Harman 2003). The body has a well-defined antioxidant defense mechanism that is powerful enough to fend off oxidative stress. However, with aging the efficiency of the body to manage the disequilibrium induced by an increase in ROS decreases (López-Otín et al. 2013). Furthermore, it leads to impairment in the systemic redox system that is one of the mechanistic factors for the development of age-related diseases predominantly vascular diseases linked to aging, diabetes, arthritis, and neurodegenerative disorders (Akçay et al. 2000; Cebe et al. 2014; Telci et al. 2002).
Various supplements and natural antioxidant products have shown to scavenge ROS when supplemented in different rat models. Antioxidants can counteract the damage caused by ROS in cells at relatively low concentrations thereby protecting physiological targets such as lipids, DNA, and proteins (Bast and Haenen 2015). A significant number of studies has found a relationship between total antioxidant capacity in serum or plasma and the progression of aging and age-related diseases (Conti et al. 2016). All this evidence leads to the search for natural or synthetic antioxidants, and the establishment of several treatment strategies aimed at counteracting oxidative stress by supplementing exogenous antioxidants, either solely or in combination.
One of the major contributors to aging-related pathologies is oxidative stress which leads to mitochondrial dysfunction and activation of downstream cell death pathways (Prajapati et al. 2017). During aging the number of defective mitochondria increases, disrupting the redox homeostasis and signaling mechanism (Scialo and Sanz 2021). Coenzyme Q10 (CoQ10) is an essential cofactor in the electron transport chain and the only endogenously synthesized lipophilic antioxidant. Coenzyme Q10 along with other molecules plays a significant role in the synthesis of ATP by channeling electrons in the mitochondrial electron transport chain. It is also involved in many signaling, metabolic and antioxidant activities depicting a central role of CoQ10 in multiple functions of the body (López-Lluch 2021). A gradual decline in the level of Coenzyme Q10 in plasma as well as tissues is observed during aging that is related to secondary deficiency of CoQ10 which is a result of advancing mitochondrial malfunction that occur during aging which further leads to decline in biological and behavioural activity (Navas 2020; Dohlmann et al. 2022; Hosseini et al. 2022). Triphenyl phosphine (TPP) has been frequently employed in the development of antioxidants that target mitochondria. Plastoquinone based SKQ1 and MitoQ10 are analogues of Coenzyme Q10 that function similarly to deliver quinonoid to mitochondria at cellular and functional level via TPP conjugation (Atayik and Çakatay 2022).
Moreover, the deficiency of Coenzyme Q10 and elevated level of oxidative stress disturbs the homeostatic balance of the body and leads to the progression of various age-related disorders (Rodríguez-Hernández et al. 2009). Many studies have demonstrated that exogenous supplementation of CoQ10 may help to restore the diminished levels and enhance the activity by stabilizing the level of oxidative stress, which further exerts a protective mantle (Chis et al. 2020). However, the role of CoQ10 as a potent antioxidant that could scavenge free radicals efficiently to maintain redox homeostasis in young and aged rats remains unclear (Mantle and Hargreaves 2019). In line with this assertion, the present study was focused on the assessment of the role of Coenzyme Q10 supplementation in young and old rats.
Materials and methods
Chemicals and reagents
2,4,6-Tri(2-pyridyl)-S-triazine (TPTZ), 4,7-diphenyl-1,10-phenanthroline disulfonic acid disodium salt (DPI), reduced glutathione (GSH), 2,4-dinitrophenylhydrazine(DNPH), dithiobis nitro benzoic acid (DTNB) were procured from Sigma Aldrich, St. Louis, MA, USA. Coenzyme Q10 was purchased from SRL chemicals (India) Pvt. Ltd. All other chemicals were of analytical grade available from Merck, Germany, and SRL, India.
Experimental design
The experiment was carried out on healthy young (4 months) and old (24 months) male Wistar rats. Rats were obtained from the CSIR-Indian Institute of Toxicology Research (CSIR-IITR) animal house in Lucknow and bred in the University of Allahabad animal house. Rats were kept in a controlled environment with a 12-h light/dark cycle (25 ± 2 °C). All rats were fed a standard laboratory diet of nutrient-rich pellets and had full access to water. The rats were then randomly assigned into the following four groups (n = 6):
-
Group I—Young control (YC): (4 months old, b.w. = 150 g ± 10 g) Rats were administered with olive oil (as a vehicle) once daily for 28 days.
-
Group II—Young treated (YC + CoQ10): (4 months old, b.w. = 150 g ± 10 g) Young rats were treated with a suspension of Coenzyme Q10 orally (20 mg/kg b.w.) once every afternoon for 28 days.
-
Group III—Old control (OC): (24 months old, b.w. = 350 g ± 20 g) Rats were administered with olive oil (as a vehicle) once daily for 28 days.
-
Group IV—Old treated (OC + CoQ10): (24 months old, b.w. = 350 g ± 20 g) Old rats were treated with a suspension of Coenzyme Q10 orally (20 mg/kg b.w.) once every afternoon for 28 days.
The route of administration and dose of Coenzyme Q10 was decided from previously published research (Luo et al. 2019). A separate study in which rats were supplemented with olive oil was also performed; our results showed that there were no significant changes when compared with the control rats (data not shown). The entire study was carried out following the protocols and procedures approved by the ethics committee of University of Allahabad.
Isolation of blood and separation of packed red blood cells (PRBCs)
Rats were sacrificed under mild anesthesia when the scheduled treatment was completed. Blood was taken into heparinized syringes via cardiac puncture and centrifuged at 800 g for 10 min at 4 °C. The red blood cells (RBCs) were washed twice with cold phosphate buffer saline (PBS) at physiological pH after the plasma was withdrawn. The remaining packed RBCs (PRBCs) were suspended in 0.09% glucose-containing PBS and plasma was kept at − 80 °C for further analysis. The plasma protein content was evaluated by Folin’s method (Lowry et al. 1951) using bovine serum albumin as standard.
-
A.
Lipid profile parameters
Serum was diluted using PBS (pH 7.4) in the ratio of 1:50. The diluted serum samples were then used for determining triglycerides and total cholesterol levels using kits from ERBA diagnostics (Mannheim, Germany) and the result was analyzed on an Erba Mannheim Chem-7 analyzer.
-
B.
Measurement of oxidative stress biomarkers
-
1.
Total ferric reducing ability of plasma (FRAP)
FRAP was used to determine the total antioxidant capacity of plasma (Benzie and Strain 1996). In brief, 100 ml of plasma and 900 ml of FRAP reagent (300 mM acetate buffer, pH 3.6, 20 mM ferric chloride, and 10 mM 2,4,6-tripyridyl-s-triazine in 40 mM hydrochloric acid in a 10:1:1 ratio) were taken. For 5 min, the absorbance was measured at 593 nm at 30-s intervals. The values obtained were expressed in µmol Fe (II)/L plasma.
-
2.
Reduced glutathione (GSH)
The conventional approach for determining erythrocyte GSH was used (Beutler 1984). The approach is based on the SH (sulfhydryl) group's capacity to reduce 5, 5-dithiobis, and 2-nitrobenzoic acid to generate a yellow-colored anionic product with a 412 nm absorbance. GSH concentration is derived using a standard plot and expressed in mg/ml PRBCs. The standard plot range for GSH was from 0 to 100 µM.
-
3.
Erythrocyte PMRS activity
The activity of the erythrocyte PMRS was assessed using the procedure previously described (Rizvi et al. 2006). In short, PRBCs (0.2 ml) from all experimental groups were suspended to a final volume of 2.0 ml in PBS containing 5 mM glucose and 1 mM freshly produced potassium ferricyanide. The solution was incubated for 30 min at 37 °C before being centrifuged for 10 min at 4 °C at 800 g. Using 4,7-diphenyl-1,10-disulfonic acid disodium salt and absorbance at 535 nm (Ɛ = 20,500 M−1 cm−1), ferrocyanide concentration in the supernatant was estimated. The values obtained are expressed in µmol ferrocyanide/ ml PRBC/ 30 min.
-
4.
Malondialdehyde (MDA) content
The reaction with thiobarbituric acid was slightly modified to quantify erythrocyte MDA, a lipid peroxidation indicator (Esterbauer and Cheeseman 1990). Packed erythrocytes (0.2 ml) were suspended in 3 ml PBS containing 0.5 mM glucose at pH 7.4 and packed erythrocytes (0.2 ml) were suspended in 3 ml PBS containing 0.5 mM glucose at pH 7.4. The suspended packed erythrocytes (0.2 ml) were then combined with 1 ml of 10% trichloroacetic acid (TCA) and 2 ml of 0.67% thiobarbituric acid (TBA), then heated for 20 min at 90–100 °C and chilled. The mixture was then centrifuged for 5 min at 1000 g, and the absorbance of the supernatant was measured at 532 nm. The extinction coefficient (Ɛ = 153,000) was used to calculate the concentration of MDA in erythrocytes, which is represented as nmol/ml of packed erythrocytes.
-
5.
Plasma protein carbonyls (PCO)
The reported method was used to determine the plasma protein carbonyls (PCOs) (Levine et al. 1990). Previously published lab reports outlined an updated version of this methodology (Garg et al. 2017). Using the supernatant spectra at 370 nm, the carbonyl concentration was determined. The carbonyl concentration was calculated using an absorption coefficient of 22,000 M−1 cm−1 and expressed as nmol/ mg protein.
-
6.
Advanced oxidation protein products (AOPP)
With minor modifications, the previously published protocol (Witko-Sarsat et al. 1996) was used to determine AOPPs in plasma using a spectrophotometric assay. AOPP concentrations are measured in millimoles of chloramine-T per liter of plasma (µmol/L).
-
C.
Estimation of enzymatic antioxidants
-
1.
Superoxide dismutase (SOD)
The activity of superoxide dismutase (SOD) was determined following established protocol (Misra and Fridovich 1972). In 0.1 ml of plasma, sodium carbonate (1 ml, 50 nM), nitroblue tetrazolium (0.4 ml, 25 µM), and hydroxylamine hydrochloride (0.2 ml, 0.1 mM) were mixed to make a reaction mixture. At 560 nm, the absorbance of the reaction mixture was measured. The activity of enzyme is reported in terms of I.U. / min/ mg protein.
-
2.
Catalase (CAT)
For measuring catalase activity (CAT), the following method has been worked upon (Aebi et al. 1974). The breakdown of H2O2 was used to determine catalase activity, which was confirmed by a 1-minute drop in absorbance at 240 nm. Catalase-specific activity is calculated and the results are expressed in units of IU/ mg protein.
-
D.
Inflammatory cytokines
The level of different cytokines C-reactive protein, IL-6, and TNF-α were calculated according to the manufacturer's instructions (Krishgen Bioframework, India), as discussed in detail in a previously published lab report (Kumar et al. 2020).
Statistical analysis
The sample size has been calculated by resource equation method. Values are expressed as mean ± standard deviation (SD) of three independent experiments (n = 6 rats/ group). A test of statistical analysis was performed using Graph Pad PRISM version 5.01 software. Data were assessed by one-way analysis of variance (ANOVA) followed by a posthoc Bonferroni Multiple Comparison Test. p ≤ 0.05 was considered to be statistically significant (* when compared with young control and # when compared with the old control group).
Results
-
A.
Lipid profile parameters
A significant increase in the level of triglycerides and total cholesterol was observed in old control rats (139.93 ± 1.39* mg/dl, 107.13 ± 7.67* mg/dl) when compared with young controls (113.29 ± 8.56 mg/dl, 79.44 ± 8.86 mg/dl) respectively. After treatment, a non-significant decline in the level of triglycerides and total cholesterol was observed in young-treated rats (101.65 ± 7.45 mg/dl, 60.84 ± 5.19 mg/dl) when compared with young control. CoQ10 supplementation led to a significant down-regulation of lipid profile parameters in old treated rats (116.91 ± 11.65 mg/dl, 83.36 ± 5.22# mg/dl) when compared with old control.
-
B.
Enzymatic antioxidant levels
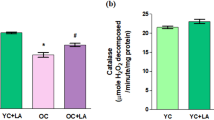
A one-way ANOVA detected a significant effect of CoQ10 treatment in levels of SOD [F (3, 8) = 20.20, p ≤ 0.0004] and CAT [F (3, 8) = 52.18, p ≤ 0.0001]. Results show a significant decrease (p ≤ 0.05) in the level of SOD and a non-significant change in CAT levels of old control rats when compared with young control. A significant up-regulation in the level of CAT was observed in young rats (50.37%) and non-significant changes were found in SOD levels of young rats after treatment with Coenzyme Q10. Whereas, in old-treated rats a significant increase was found in both SOD (48.45%) and CAT (49.48%) levels when compared with their control group shown in Fig. 1a, b.
Coenzyme Q10 supplementation up-regulates the level of enzymatic antioxidants denoted by a Superoxide Dismutase (SOD) (U/min/mg protein) b Catalase (µM of H2O2/min/mg protein) Values are given as mean ± SD of three independent experiments *p ≤ 0.05 when compared with young control and #p ≤ 0.05 when compared with the old control group
-
C.
Effect of CoQ10 supplementation on oxidative stress biomarkers
-
1.
Plasma antioxidant status
The ferric reducing ability of plasma is used to determine plasma antioxidant capacity (FRAP). A one-way ANOVA detected a significant effect of CoQ10 treatment [F (3, 8) = 26.65, p ≤ 0.0002]. A significant decrease (p ≤ 0.05) was found in the old control group when compared with the young control. Whereas after treatment there was a significant increase in the level of FRAP (Fig. 2a) in young (29.81%) and old (39.82%) treated rats when compared with their respective controls.
Effect of CoQ10 treatment on FRAP, GSH, and PMRS a Antioxidant capacity in terms of FRAP is measured and expressed in µmol Fe (II)/L plasma b Reduced glutathione (GSH) level (mg/ml PRBC) c Plasma membrane redox system (PMRS) activity (µmol ferrocyanide/mL PRBCs/30 min) Values are given as mean ± SD of three independent experiments *p ≤ 0.05 when compared with young control and #p ≤ 0.05 when compared with the old control group
-
2.
Reduced Glutathione (GSH)
A one-way ANOVA detected a significant effect of CoQ10 treatment on GSH [F (3, 8) = 33.64, p ≤ 0.0001]. Figure 2b shows a significant decline (p ≤ 0.05) in the level of reduced glutathione in old control concerning young control rats. Coenzyme Q10 supplementation led to a significant increase in the plasma GSH level in young (25%) and old (35.71%) rats when compared with the young and old control group, respectively.
-
3.
Plasma membrane redox system(PMRS) level
The erythrocyte PMRS is a good regulator of the antioxidant state. A one-way ANOVA detected a significant effect of CoQ10 treatment [F (3, 8) = 154.7, p ≤ 0.0001]. PMRS levels were found to be significantly higher (p ≤ 0.05) in young treated rats (31.32%), compared to the young control group. Similarly, in comparison to the old control, CoQ10 treatment significantly (p ≤ 0.05) increases (29.25%) PMRS levels in aged rats depicted in Fig. 2c.
-
4.
Malondialdehyde (MDA)
Figure 3a shows a significant increase in the level of MDA in old control rats when compared with young control. A one-way ANOVA detected a significant effect of CoQ10 treatment in levels of MDA [F (3, 8) = 99.31, p ≤ 0.0001]. After treatment, there was a significant down-regulation (p ≤ 0.05) in the level of malondialdehyde in young treated rats (20.65%), compared to the young control group. Similarly, in comparison with the old control, CoQ10 treatment significantly (p ≤ 0.05) decreased (22.57%) MDA levels in aged rats.
Coenzyme Q10 attenuates oxidative stress biomarkers in rats a Malondialdehyde (MDA) lipid peroxidation marker expressed in nmol/ml packed RBC b Protein oxidation marker measured as protein carbonyl (PCO) level (nmol/mg protein) c Advanced oxidation protein products (AOPPs) (μmol/L) Values are expressed as mean ± SD (n = 6). *p ≤ 0.05 when compared with young control, #p ≤ 0.05 when compared with the old control group
-
5.
Level of plasma protein oxidation
The effect of CoQ10 on plasma protein oxidation was measured using oxidative stress indicators PCO and AOPP. A significant increase in the level of both PCO and AOPP was observed in the old control after comparing with the young control. A one-way ANOVA detected a significant effect of CoQ10 treatment in levels of PCO [F (3, 8) = 38.71, p ≤ 0.0001] and AOPP [F (3, 8) = 64.38, p ≤ 0.0001]. CoQ10 supplementation significantly down-regulates (p ≤ 0.05) the level of AOPP (33.68%) in young treated rats but no significant changes were observed in PCO levels in the young treated group when compared with their control. A significant decline was found in plasma levels of PCO (30.75%) and AOPP (23.35%) in old treated rats when compared with their control groups shown in Fig. 3b, c.
-
D.
Biomarkers of inflammation
A one-way ANOVA detected a significant effect of CoQ10 treatment in the level of CRP [F (3, 4) = 40.31, p ≤ 0.0019], IL-6 [F (3, 4) = 22.64, p ≤ 0.0057] and TNF-α [F (3, 4) = 12.60, p ≤ 0.0166]. In Fig. 4a there is a significant increase in the level of CRP in old control rats when compared with young control. After treatment a significant (p ≤ 0.05) decrease (29.78%) in CRP level of young-treated rats is observed and non-significant decrease was found in old-treated rats. In Fig. 4b, c there is a significant decrease in the level of IL-6 (57.05%) and TNF-α (41.53%) in old-treated rats when compared with old control whereas a non-significant decline was found in young treated rats when compared with young control.
Effect of CoQ10 on inflammatory biomarkers The cytokine level was measured in the serum of rats as a C-Reactive Protein (CRP) in µg/ml b Interleukin-6 (IL-6) expressed in pg/ml c Tumor necrosis factor alpha (TNF-α) in pg/ml Values are expressed as mean ± SD (n = 6). *p ≤ 0.05 when compared with young control, #p ≤ 0.05 when compared with the old control group
Discussion
There are compelling authentications that support the role of oxidative stress in the progression of aging and associated disorders. The shift in the balance of antioxidants leads to an increment in free radical production resulting in high oxidative stress and deregulation of cellular function (Birben et al. 2012). To maintain sufficient ATP synthesis, several biochemical signaling systems mediate increasing oxidative stress. The mitochondrial efficiency declines with the advancing age and results in higher ROS production that causes deleterious effects on the organism (Liguori et al. 2018).Coenzyme Q10 is a ubiquitous quinine molecule, that plays a pivotal role in transfer of electrons from Complex I/II to Complex III via NADH and succinate in mitochondrial electron transport chain. Due to the involvement of CoQ10 in ATP synthesis, the role of CoQ10 for optimal biological function becomes crucial; however, a decline in its level is observed with aging (Ayunin et al. 2022).
The decline in the levels of CoQ10 leads to disturbance in numerous mitochondrial functions during aging. Imbalance in redox homeostasis, decreased mitochondrial membrane potential, changed membrane ion transport, increased proton leak, and degraded mitochondrial signalling cascades are some of them (Atayik and Çakatay 2022). The hypothesis that Coenzyme Q10 supplementation could either alleviate symptoms of aging or retard the severity of age-related disorders is backed up by several studies (Hernández-Camacho et al. 2018). CoQ10, as an antioxidant, has shown promising results as a treatment for diseases characterized by oxidative stress, and its positive benefits in the treatment of aging or age-related damages, such as the early stages of neurodegenerative disorders, cardiovascular diseases, osteoporosis, renal disorders, etc. (Gutierrez-Mariscal et al. 2020).
In the present investigation, there was a non-significant change in the body weight of treated rats. The decline observed in the levels of triglycerides was also not significant. However, the levels of total cholesterol show significant down-regulation in old treated rats when compared with old control. The results obtained from this study support the previously published reports (Al-Attar 2010). Many diverse enzymatic and non-enzymatic antioxidant mechanisms exist in blood plasma, which helps to reduce free radical levels and allow them to perform essential biological processes without inflicting severe harm (Irato and Santovito 2021). Antioxidant enzymes, represented by superoxide dismutase (SOD) and catalase (CAT), have been reported to remove excess ROS. After SOD transforms superoxide anion to hydrogen peroxide, the enzyme catalase is engaged in H2O2 detoxification in the antioxidant defense mechanism of the body (Matés 2000). An up-regulation in the activity of SOD and CAT was observed in treated rats when compared to control rats in the present study. CoQ10 supplementation appears to enhance the antioxidant defense mechanism by effectively scavenging free radicals through the stimulation of antioxidant enzymes (Song et al. 2017).
The ferric reducing ability of plasma (FRAP) is an extensively used measure of plasma antioxidant reserve. Oral supplementation of Coenzyme Q10 increases the level of FRAP in the plasma because of its ability to recycle and regenerate other antioxidants like α-tocopherol or ascorbic acid in their respective reduced and active state (Navas et al. 2007).
Reduced glutathione (GSH) has been found a protective mechanism against oxidative stress. It neutralizes free radicals and also protects against oxidative stress throughout aging. A decline in the level of GSH in erythrocytes and the brain is linked with the elevated levels of reactive oxygen species depicted in several studies (Maurya et al. 2015). A low level of GSH and its replenishment after treatment with Coenzyme Q10 was observed. The ability of Coenzyme Q10 as an antioxidant helps in replenishing the glutathione reserves by combating the adverse actions of free radicals. Supplementation with CoQ10 significantly increases the level of GSH in erythrocytes of diabetic rats (Al-Thakafy et al. 2004).
Regulation of the redox cycle of Coenzyme Q10 in which ubiquinone is reduced by reductases that transfer electrons from cytosolic NAD(P)H is necessary to maintain its antioxidant action (López-Lluch et al. 2010). The most critical enzymes that maintain ubiquinol levels in cell membranes are CytB5R3 and NQO1; these are an integral part of the plasma membrane redox system (PMRS) (Rodríguez-Aguilera et al. 2000). The activation of these reductases results in the local accumulation of NAD+, which then modulates numerous important biological functions linked to aging, such as the activity of sirtuins and the cAMP pathway (Olgun 2009). As per published data, the erythrocyte PMRS activity is elevated to provide a stabilizing mechanism to counteract increasing ROS generation and oxidative damage with aging (Kumar and Rizvi 2014). Further, PMRS activity is associated with enhanced mitochondrial activity that leads to a delay in senescence (López-Lluch 2020).
As a result of which malondialdehyde is produced, this is an important indicator of oxidative damage. CoQ10 is a major anti-oxidant that protects membrane phospholipids from peroxidation by maintaining the plasma membrane and other intracellular membranes (Gutierrez-Mariscal et al. 2019). The quinol structure of CoQ10 inhibits lipid peroxidation by preventing the generation and propagation of lipid peroxy radicals and is oxidized to quinone and non-radical lipid hydroperoxide (Conti et al. 2016). The results obtained by the present study exhibit a decrease in the levels of MDA in both young and old treated rats when compared with the control, thus explaining the effectiveness of CoQ10 in inhibiting lipid peroxidation. Studies show significant improvement in the oxidative stress parameters like MDA when rats were treated with CoQ10 (Ulla et al. 2017).
Under stress, ROS also damages plasma proteins, causing carbonyl to form on a variety of amino acids (Tripathi et al. 2020). PCO and AOPP are key indicators for plasma protein oxidation that indicate the severity of protein degradation. During stress conditions, cross-linking protein products are formed namely advanced oxidation protein products (AOPP) which is a powerful marker of protein oxidation (Pandey et al. 2010). A significant decrease in PCO and AOPP levels was observed after treatment with Coenzyme Q10. The way CoQ10 quenches the initiating peroxy radicals not only prevents lipid peroxidation but also protects proteins from oxidation thereby exhibiting protection against damage (Varela-López et al. 2016).
An array of transcription factors are activated by the elevated levels of oxidative stress that leads to differential expression of the genes that are incorporated in inflammatory pathways (Hussain et al. 2016). The imbalance caused by oxidative stress can alter immune system activity; by decreasing cellular functions due to oxidative damage implying its role in the progression of immunosenescence (Hernández-Camacho et al. 2020). Chronic inflammation is a common denominator of aging. Substantial production of superoxide, either within the cell by mitochondria or intracellular redox activities, or outside the cell by NADPH oxidases seen in inflammatory cells, can cause cell damage. (López-Lluch 2021). Our findings show a significant reduction of C-Reactive Protein (CRP) an important marker of inflammation, pro-inflammatory cytokines viz. interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α) levels in treated rats when compared with control.
Recent advances in the field of aging have brought to the fore some possible mechanisms for the early detection of onset of aging related alterations of which aging-induced proteinopathies is probably of high merit. Citrullination, protein carbamylation, abnormal S-nitrosylation, nitrosylation and protein dityrosination can be an epitope for autoantibody formation that can prove to be a novel biomarker (Yanar et al. 2020). Our study however, lacks the quantification of such novel biomarkers which could be of further help in detection and establishment of promising future therapeutics.
Conclusion for future biology
Coenzyme Q10, a putative antioxidant, was successfully able to preserve systemic redox equilibrium while maintaining redox homeostasis in rat erythrocytes during aging. The exogenous administration of Coenzyme Q10 targeted the mitochondrial dysfunction and inflammation that is a common denominator of aging. Our findings support the concept that consumption of Coenzyme Q10 rich diet or commercially available supplements like Mito Q10 and SKQ1 could be of high therapeutic value and provide a viable intervention strategy for achieving a healthy lifespan.
Data availability
Supplementary data to this article can be found online at https://doi.org/10.17632/yzxbf9tpsc.1.
References
Aebi H, Wyss SR, Scherz B, Skvaril F (1974) Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem 48:137–145
Akçay T, Dinçer Y, Kayali R, Colgar U, Oral E, Cakatay U (2000) Effects of hormone replacement therapy on lipid peroxides and oxidation system in postmenopausal women. J Toxicol Environ Health A 59:1–5
Al-Attar AM (2010) Hypolipidemic effects of Coenzyme Q10 in experimentally induced hypercholesterolemic model in female rats. Am J Pharmacol Toxicol 5:14–23
Al-Thakafy HS, Khoja SM, Al-Marzouki ZM, Zailaie MZ, Al-Marzouki KM (2004) Alterations of erythrocyte free radical defense system, heart tissue lipid peroxidation, and lipid concentration in streptozotocin-induced diabetic rats under coenzyme Q10 supplementation. Saudi Med J 25:1824–1830
Atayik MC, Çakatay U (2022) Mitochondria-targeted senotherapeutic interventions. Biogerontology 23:401–423
Ayunin Q, Miatmoko A, Soeratri W, Erawati T, Susanto J, Legowo D (2022) Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel. Sci Rep 12:906
Bast A, Haenen GRMM (2015) Nutritional antioxidants: it is time to categorise. In: Lamprecht M. (eds), Antioxidants in sport nutrition. CRC Press/Taylor & Francis, Boca Raton (FL)
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Beutler (1984) Red cell metabolism. A manual of biochemical methods. Ann Intern Med 83:919
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Cebe T, Atukeren P, Yanar K, Kuruç AI, Ozan T, Kunbaz A, Sitar ME, Mirmaroufizibandeh R, Aydın S, Çakatay U (2014) Oxidation scrutiny in persuaded aging and chronological aging at systemic redox homeostasis level. Exp Gerontol 57:132–140
Chis BA, Chis AF, Muresan A, Fodor D (2020) Q10 Coenzyme supplementation can improve oxidative stress response to exercise in metabolic syndrome in rats. Int J Vitam Nutr Res 90:33–41
Conti V, Izzo V, Corbi G, Russomanno G, Manzo V, De Lise F, Di Donato A, Filippelli A (2016) Antioxidant supplementation in the treatment of aging-associated diseases. Front Pharmacol 7:24
Dohlmann TL, Kuhlman AB, Morville T, Dahl M, Asping M, Orlando P, Silvestri S, Tiano L, Helge JW, Dela F, Larsen S (2022) Coenzyme Q10 supplementation in statin treated patients: a double-blinded randomized placebo-controlled trial. Antioxidants (basel) 11:1698
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Garg G, Singh S, Singh AK, Rizvi SI (2017) Metformin alleviates altered erythrocyte redox status during aging in rats. Rejuvenation Res 20:15–24
Gutierrez-Mariscal FM, Yubero-Serrano EM, Villalba JM, Lopez-Miranda J (2019) Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit Rev Food Sci Nutr 59:2240–2257
Gutierrez-Mariscal FM, Arenas-de Larriva AP, Limia-Perez L, Romero-Cabrera JL, Yubero-Serrano EM, López-Miranda J (2020) Coenzyme Q10 Supplementation for the reduction of oxidative stress: clinical implications in the treatment of chronic diseases. Int J Mol Sci 21
Harman D (2003) The free radical theory of aging. Antioxid Redox Signal 5:557–561
Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P (2018) Coenzyme Q10 supplementation in aging and disease. Front Physiol 9:44
Hernández-Camacho JD, Meza-Torres C, López-Lluch G (2020) Immunosenescence and CoQ10. In: López LG (ed) Coenzyme Q in aging. Springer International Publishing, Cham, pp 269–282
Hosseini L, Majdi A, Sadigh-Eteghad S, Farajdokht F, Ziaee M, Rahigh AS, Farzipour M, Mahmoudi J (2022) Coenzyme Q10 ameliorates aging-induced memory deficits via modulation of apoptosis, oxidative stress, and mitophagy in aged rats. Exp Gerontol 168:111950
Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N (2016) Oxidative stress and inflammation: What polyphenols can do for us? Oxid Med Cell Longev 2016:7432797
Irato P, Santovito G (2021) Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants (Basel) 10
Jia L, Zhang W, Chen X (2017) Common methods of biological age estimation. Clin Interv Aging 12:759–772. https://doi.org/10.2147/CIA.S134921
Kumar D, Rizvi SI (2014) A critical period in lifespan of male rats coincides with increased oxidative stress. Arch Gerontol Geriatr 58:427–433
Kumar R, Akhtar F, Rizvi SI (2020) Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin Exp Pharmacol Physiol 47:571–582
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772
López-Lluch G (2020) Extramitochondrial Coenzyme Q10 in aging. In: López LG (ed) Coenzyme Q in aging. Springer International Publishing, Cham, pp 91–111
López-Lluch G (2021) Coenzyme Q homeostasis in aging: response to non-genetic interventions. Free Radic Biol Med 164:285–302
López-Lluch G, Rodríguez-Aguilera JC, Santos-Ocaña C, Navas P (2010) Is coenzyme Q a key factor in aging? Mech Ageing Dev 131:225–235
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Lowry OH, NiraJ R, Farr AL, RoseJ R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Luo K, Yu JH, Quan Y, Shin YJ, Lee KE, Kim HL, Ko EJ, Chung BH, Lim SW, Yang CW (2019) Therapeutic potential of coenzyme Q10 in mitochondrial dysfunction during tacrolimus-induced beta cell injury. Sci Rep 9:7995
Mantle D, Hargreaves I (2019) Coenzyme Q10 and degenerative disorders affecting longevity: an overview. Antioxidants (Basel) 8
Matés JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Maurya PK, Kumar P, Chandra P (2015) Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol 5:216–222
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Navas P (2020) The Current Coenzyme Q Science and Knowledge. In: López LG (ed) Coenzyme Q in Aging. Springer International Publishing, Cham, pp 3–9
Navas P, Villalba JM, de Cabo R (2007) The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 7(Suppl):S34-40
Olgun A (2009) Converting NADH to NAD+ by nicotinamide nucleotide transhydrogenase as a novel strategy against mitochondrial pathologies during aging. Biogerontology 10:531–534
Pandey KB, Mehdi MM, Maurya PK, Rizvi SI (2010) Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis Mark 29:31–36
Prajapati SK, Garabadu D, Krishnamurthy S (2017) Coenzyme Q10 prevents mitochondrial dysfunction and facilitates pharmacological activity of atorvastatin in 6-OHDA induced dopaminergic toxicity in rats. Neurotoxicol Res 31:478–492
Rizvi SI, Jha R, Maurya PK (2006) Erythrocyte plasma membrane redox system in human aging. Rejuvenat Res 9:470–474
Rodríguez-Aguilera JC, López-Lluch G, Santos-Ocaña C, Villalba JM, Gómez-Díaz C, Navas P (2000) Plasma membrane redox system protects cells against oxidative stress. Redox Rep 5:148–150
Rodríguez-Hernández A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, Gómez IL, Cotán D, Navas P, Sánchez-Alcázar JA (2009) Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 5:19–32
Scialo F, Sanz A (2021) Coenzyme Q redox signalling and longevity. Free Radic Biol Med 164:187–205
Song M-H, Kim H-N, Lim Y, Jang I-S (2017) Effects of coenzyme Q10 on the antioxidant system in SD rats exposed to lipopolysaccharide-induced toxicity. Lab Anim Res 33:24–31
Telci A, Cakatay U, Akhan SE, Bilgin ME, Turfanda A, Sivas A (2002) Postmenopausal hormone replacement therapy use decreases oxidative protein damage. Gynecol Obstet Investig 54:88–93
Tripathi SS, Kumar R, Bissoyi A, Rizvi SI (2020) Baicalein maintains redox balance in experimental hyperlipidemic rats. Arch Physiol Biochem 1–9
Ulla A, Mohamed MK, Sikder B, Rahman AT, Sumi FA, Hossain M, Reza HM, Rahman GMS, Alam MA (2017) Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol Toxicol 18
Varela-López A, Giampieri F, Battino M, Quiles JL (2016) Coenzyme Q and its role in the dietary therapy against aging. Molecules 21:373
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313
Yanar K, Atayik MC, Simsek B, Çakatay U (2020) Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 21:531–548
Acknowledgements
Authors acknowledge the Department of Biotechnology, Government of India for providing partial financial support under the “Research Resources, Service Facilities, and Platforms” program.
Author information
Authors and Affiliations
Contributions
PS and SIR conceived and designed the experiments. PS and JKA performed the experiments. AKV and SIR analyzed the data. PS and SIR wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors have no competing interests to be declared.
Ethical approval
All animal care and exploratory methods conformed with the guidelines of the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India and also follow guidelines for the Care and Use of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, P., Verma, A.K., Arya, J.K. et al. Modulatory effect of exogenous Coenzyme Q10 on redox and inflammatory biomarkers during aging in rats. BIOLOGIA FUTURA 73, 473–481 (2022). https://doi.org/10.1007/s42977-022-00140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-022-00140-5