Abstract
Coenzyme Q10 (CoQ10) is an essential component of the electron transport system and the only lipid-soluble compound synthesized endogenously present in all cell membranes with bioenergetics and antioxidant properties.
Aging, neurodegenerative disorders, cardiovascular disease and other aged-related diseases, as well as genetic mutations, have been associated with CoQ10 deficiency. Since both limited uptake and low bioavailability of dietary CoQ10 might influence in this deficiency, supplementation with CoQ10 must be considered in those cases as therapeutic solution. However, more research is needed in order to identify the appropriate dose, the effectiveness and the bioavailability of orally-administered CoQ10. Furthermore research must be developed in order to design therapeutic agents to induce the endogenous synthesis CoQ10 specially in elderly people.
This review will focus in the most relevant biochemical characteristics of this important antioxidant, including its main functions, levels and distribution in human organism and the therapeutic potential of CoQ10, especially, during aging and the associated diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Coenzyme Q10
Coenzyme Q10, also known as CoQ10, vitamin Q10, ubiquinone, and ubidecarenone, is a benzoquinone compound, identified as a component of the mitochondrial respiratory chain (Crane et al. 1989; Schultz and Clarke 1999). It has been isolated and characterized as an ubiquitous quinone substance that received the name of ubiquinone (Festenstein et al. 1955). After its isolation, CoQ10 was identified as an essential electron carrier in the inner membrane of mitochondria as member of the mitochondrial electron transport chain (Festenstein et al. 1955; Crane et al. 1957).
CoQ10 is the short name of 2,3-dimethoxy-5-methyl-6-decaprenyl-1,4-benzoquinone. It is a lipid-soluble quinone with a very high biological activity. CoQ10 has two different parts, a polar benzoquinone ring and a lipidic isoprenoid side chain whose length depends on the organism. Its structure is similar to vitamin E. The name CoQ10 indicates that the quinone ring is bound to ten isoprenyl subunits that are part of this compound’s structure. This is the predominant human form of this molecule. Moreover, the term “coenzyme” denotes it as an organic (contains carbon atoms), non-protein molecule necessary for the proper functioning of its protein partner (an enzyme or an enzyme complex) (Jeya et al. 2010). The principal characteristic of CoQ10 is its presence in three redox states: the fully oxidized ubiquinone form, a semiquinone form (that acts as free radical) and the fully reduced ubiquinol (Fig. 17.1) (Alcazar-Fabra et al. 2016). CoQ10 is found in all cell membranes but the highest presence is in the inner membrane of mitochondria in every cell in the human organism. In mitochondria, CoQ10 is essential as a cofactor in the mitochondrial electron transport chain, and them, indispensable for aerobic cellular respiration and for the production of ATP and cell bioenergetics in aerobic organisms (Alcazar-Fabra et al. 2016; Acosta et al. 2016).
1.1 Coenzyme Q10 Functions
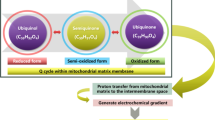
As member of the mitochondrial electron transport chain, CoQ10 accepts electrons from different donors named reductases, mainly NADH-coenzyme Q oxidoreductase (Complex I) and succinate-dehydrogenase (Complex II). The reduced form is later oxidized by transferring electrons to ubiquinol-cytochrome c reductase complex (Complex III) (Fig. 17.2). Its electron transport activity is accompanied by a pumping capacity that transfers protons from mitochondrial matrix to the intermembrane space contributing to create a proton gradient between mitochondrial matrix and cytosol (Crane 2001). This redox activity permits to mitochondria to participate in cell growth and maintenance (Overvad et al. 1999). Through this process, CoQ10 maintains a permanent redox equilibrium between the reduced form (ubiquinol) and the oxidized form (ubiquinone ). This equilibrium is maintained in mitochondria mainly by the activity of complexes I and II as electron donors and Complex III as acceptor. Other researches have revealed that CoQ10 is also a co-factor for the function of uncoupling proteins. For these reasons, CoQ10 is essential in the control of bioenergetics homeostasis in cells (Littarru and Tiano 2007; Potgieter et al. 2013).
In other cell membranes, at least three enzymes are known as CoQ10-reductases: NADH/NADPH oxidoreductase (DT diaphorase), NADH cytochrome b5 reductase and NADPH coenzyme Q reductase (Villalba and Navas 2000). In these membranes, ubiquinol acts as a potent antioxidant protecting cells from oxidative damage and contributing to the stability of the cell membranes, proteins, glycoproteins and DNA. Further reduced CoQ10 form has also been reported to protect LDL from oxidation (Lopez-Lluch et al. 2010). LDLs tend suffer more oxidation during aging probably by the reduction of the levels of CoQ10. For this reason, CoQ10 supplementation could be a good therapy to decrease LDL oxidation reducing the high risk of cardiovascular disease during aging (Yubero-Serrano et al. 2011).
In addition to direct antioxidant radical scavenging, CoQ10, and particularly the semiquinone intermediate (Fig. 17.1), recycles and regenerates other membrane antioxidants, such as α-tocopherol and also cytosolic and extracellular antioxidants such as ascorbic acid. CoQ10 is essential to maintain them in their reduced and activity state (Navas et al. 2007). All these activities make CoQ10 as the main lipidic antioxidant , more powerful than vitamin E, present in relative high concentrations and able to regenerate intracellular reducing mechanisms (Forsmark-Andree et al. 1995).
1.2 Levels and Distribution of Coenzyme Q10 in the Humans
All human cells studied so far can synthesize CoQ10. Its amount in these cells depends on the organs and tissues. In humans, CoQ10 ranges from 8 μg/g in lung to 114 μg/g in heart. In some determinations, a shorter form, CoQ9 has been found but only in small quantities (2–7%) (Jeya et al. 2010). In general, ubiquinol levels are higher than the levels of the oxidized form, ubiquinone , in most of the human tissues except in the case of lung and brain (Bhagavan and Chopra 2006). In the case of brain, the increase in the ratio ubiquinol/ubiquinone can be associated with neurological diseases due to mitochondrial dysfunction (Spinazzi et al. 2019).
Generally, tissues with high metabolic activity, such as the heart, kidney, liver and muscle, contain relatively high concentrations of CoQ10 (Ernster and Dallner 1995). At the cellular levels, most of the CoQ10 (40–50%) is localized at the mitochondrial inner membrane. It is present in the rest of cell membranes although at smaller amounts in the other organelles and in the cytosol.
Cell and tissue CoQ10 is coming from endogenous synthesis although it can be also obtained from food intake or oral supplementation. Interestingly, the range of CoQ10 concentration in humans show a high range and also depend on age, sex and race, and on the health of the individual (Sohal and Forster 2007). In healthy young individuals, total body content of CoQ10 is around 0.99 ± 0.3 mg/L (from 0.55 mg/L and 1.87 mg/L). However, these levels decrease during aging, and in age-related diseases such as in patients with cardiomyopathies, congestive heart failure and degenerative diseases (Fotino et al. 2012; Shetty et al. 2012).
1.3 Biosynthesis and Transport of CoQ10
The main although not unique source of CoQ10 in humans is the endogenous synthesis. This synthesis depends on the mevalonate pathway (Fig. 17.3). CoQ10 synthesis Shares the mevalonate pathway with cholesterol, dolichol, dolychil-phoshate and isoprene chains that bind to aminoacid residues in proteins (Villalba et al. 2010). De novo CoQ10 synthesis in humans is initiated by the union of the benzoquinone ring precursor, 4-hydroxybenzoate, and the isoprenoid side chain produced from farnesyl pyrophosphate. These two molecules are condensed by the polyprenil-4-hydroxybenzoate transferase, COQ2. After that, a complex with many other components, at least eight enzymes (encoded by COQ3–10) modify the benzene ring with subsequent methylation, decarboxylation and hydroxylation reactions to (Quinzii et al. 2007; Turunen et al. 2004).
Cholesterol and Coenzyme Q10 pathways share Mevalonate intermediate and Statin interruption
3-Hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) is changed into Mevalonate by the action of the enzyme HMG-CoA reductase. From here, Mevalonate can be used to synthesize both cholesterol and coenzyme Q10. Statins work by inhibiting the action of HMG-CoA reductase, thereby decreasing the amount of mevalonate available to make either cholesterol or coenzyme Q10
It seems clear that CoQ10 synthesis is located in mitochondria and from this, it is distributed in all the subcellular compartments. Then, a transport system from the mitochondria to the rest of cellular membranes must exist. Using in vivo labeling and cell fractionation in spinach leaves, it was demonstrated that CoQ10 is transported from the endoplasmic reticulum to other compartments through a vesicle-mediated process involving the Golgi system (Wanke et al. 2000). This cellular transport system was also found in human cells in culture (Fernández-Ayala et al. 2005a). Interestingly, exogenous CoQ10 can enter the cell through plasma membrane and incorporate to cell organelles including mitochondrial inner membrane (Fernández-Ayala et al. 2005b).
1.4 Uptake and Distribution of CoQ10
CoQ10 is found in many dietary sources including animals and vegetables and can be also obtained from many dietary supplements. Large amounts are present in food from animal sources such as chicken legs, heart, liver and herrings. In comparison with meat and fish, lower levels are found in vegetables probably by the lower amount of mitochondria in comparison with animal cells (Table 17.1). In general, dietary intake of CoQ10 has been estimated as 3–5 mg/day. However, this intake is not necessary in situations without endogenous CoQ10 synthesis dysfunction in which the quinone reaches a saturation level in cells and tissues (Bhagavan and Chopra 2006).
To understand the distribution of CoQ10 in tissues after oral ingestion it is necessary to take into consideration its lipophilic nature. The absorption of CoQ10 is enhanced in the presence of lipids, then supplementation with CoQ10 must be performed with fat-rich meals. Due its biochemical characteristics, CoQ10 is absorbed slowly from the small intestine, possibly because it has a high molecular weight and is not very water soluble, passes into the lympha, and finally to the blood bound to chylomicrons and further to tissues, mainly liver. This mechanism is the same than vitamin E used to be incorporated from dietary sources (Zhang et al. 1995). In blood plasma, the reduced form, ubiquinol, is bound to lipoproteins, mostly to LDL (Bhagavan et al. 2007; Zhang et al. 1995). It has been considered that circulating concentrations of CoQ10 may be a putative biomarker to indicate their general status in the body and for monitoring the bioavailability of CoQ10 supplementation.
Accordingly, the number of different formulations developed to improve the incorporation of CoQ10 into human body, the importance of the vehicle and the solubility of CoQ10 in the preparation is clear in order to increase its bioavailability (López-Lluch et al. 2019). During the last decade, CoQ10 supplements have been developed as oil-based, softgel or powder-filled capsules and hard tablets. Comparisons between studies have indicated that CoQ10 bioavailability is influenced by the type of formulation, and that it is better to take CoQ10 with fatty foods (Villalba et al. 2010).
2 CoQ10 and Aging
We can define aging as the normal decline in survival suffering by all organisms along time. Understanding the molecular and cellular mechanisms underlying aging process would permit to develop strategies to resolve the problems associated to the increase of aged population that affects the whole world. Aging events have been studied from different points of view. One of the most accepted theories suggest that aging, is associated with the increase of oxidative damage in cells and tissues that drives aging and the age-related degenerative diseases (Su et al. 2010). In this theory, reactive oxygen species (ROS) are the factors that trigger the deleterious, irreversible changes and macromolecular damage associated with aging (Fig. 17.4) (Miquel 1998; Sohal et al. 2002).
Theory of aging
One of the most accepted theory of aging suggest that aging is produced by the deleterious, irreversible changes and macromolecular damage produced by ROS . Some modifications (mainly those related to DNA) are not completely repaired and thus accumulate, leading to cell death, organism malfunction, and the “aging phenotype”
Normal aerobic cell metabolism releases ow amounts of ROS as results of the partial reduction of molecular oxygen. These low ROS levels have beneficial effects maintaining antioxidant machinery. However, when there is a ROS overproduction, the accumulation of these reactive species produces oxidative modifications affecting many cell components including all the organic molecules present in cells (Valko et al. 2006). To avoid oxidative damage, organisms contains antioxidant mechanisms (Fig. 17.4) such as enzymes (superoxide dismutase, catalase, glutathione peroxidase) and small hydrophilic and hydrophobic molecules that act directly as non-enzymatic antioxidants (ascorbic acid, tocopherol, glutathione, CoQ10, and others). Thus, we can define oxidative stress as the damage produced by the imbalance between ROS production rate and the capacity to eliminate ROS by and antioxidant defences, in favour of ROS . This imbalance seems to be a hallmark of aging since oxidative stress has been associated with many age-associated diseases such as chronic-degenerative disease, such as cancer, metabolic and disease cardiovascular diseases.
Oxidative stress causes some of the modifications that cannot be completely repaired by antioxidant and cell turnover mechanisms and thus accumulate. The accumulation of oxidized structures in cells leads to cell senescence, cell death, organism malfunction, and the “aging phenotype.” Nowadays, a version of the free-radical theory to explain aging, related with mitochondria as the main source and, at the same time target for ROS-dependent damage, is one of the most popular theories of aging (Barja 2007; Miquel et al. 1980). This theory postulates that mitochondrial DNA (mtDNA) suffer higher oxidative damage as the organism ages and this leads to the accumulation of mtDNA. This accumulation produces a vicious cycle in which an initial ROS-induced mtDNA damage increases oxidants production that, in turn, leads to more mitochondrial damage that produces more mtDNA damage (Gilmer et al. 2010).
According to this concept, mitochondria and mitochondrial ROS would play an important role in the development of strategies to delay and improve the aging process. These strategies must be focused on extending lifespan and/or retarding age-associated biological changes, including age-related diseases (Lee et al. 2004). Among these strategies, nutritional and pharmacological interventions studied in several model organisms, including yeast, flies, mice and rats, as well as monkeys. Accordingly, with this strategy, some antioxidants have proved to be useful as dietary antiaging therapies (Duntas 2011; Lopez-Dominguez et al. 2012).
2.1 Coenzyme Q10 Deficiency in Aging
As it has been indicated before, all cells in the organism synthesize CoQ10 (Schultz and Clarke 1999). Along human life, CoQ10 increases until 20 years; however, it seems that the organism begin to lose its ability to synthesise CoQ10 during maturity and aging when and the coenzyme becomes deficient (Blatt and Littarru 2011; Gutierrez-Mariscal et al. 2011; Ochoa et al. 2007). Besides a decrease in biosynthetic capacity, other factors or situations may affect the levels of CoQ10, including an increase in its degradation (Nakamura et al. 1999) or changes in membrane composition as occurs in different age-related diseases (Kagan and Quinn 1996). However, it is difficult to determine the importance of the changes in CoQ10 levels during aging since they are tissue- and organ-dependent. It has been shown that levels of CoQ10 in mitochondria of old rat brain increase (Battino et al. 1997) whereas they decrease in skeletal muscle (Lass et al. 1999). These differences make very complex the study of the importance of CoQ10 in aging process and further research is needed in order to clarify the importance of CoQ10 in aging progression.
Furthermore, dietary supplementation with CoQ10 does not affects all the organs. In young and healthy rodents, dietary CoQ10 is easily incorporated into liver and spleen; however, in older animals supplemental CoQ10 seems to restore normal levels (Beal 1999; Rosenfeldt et al. 1999).
This decrease in CoQ10 during aging has been related to a higher oxidative stress associated with aging and its related diseases. Thus, oral CoQ10 supplementation could be an effective antioxidant strategy to many age-associated diseases such as neurodegenerative disorders, diabetes, and cancer, muscular and cardiovascular diseases in which oxidative stress is an important factor.
3 Therapeutic Uses of CoQ10 in Age-Related Diseases
The fundamental role of CoQ10 in mitochondria, bioenergetics and antioxidant protection is the base of the therapeutic importance of CoQ10 supplementation. The studies performed in animals demonstrate that large doses of CoQ10 can reach all tissues and subcellular components including heart and brain mitochondria. This capacity has implications in therapies for many human diseases in which oxidative stress is a main factor. Several evidence have been recorded about the beneficial effects in cardiovascular, neurodegenerative and many other aged-related diseases (Bhagavan and Chopra 2006; Villalba et al. 2010; Gonzalez-Guardia et al. 2015; Gutierrez-Mariscal et al. 2012; Gutierrez-Mariscal et al. 2014; Yubero-Serrano et al. 2013).
3.1 CoQ10 and Cardiovascular Disease
Cardiovascular disease (CVD) is one of the major causes of death and disability worldwide. We can suspect that the importance of disease will increase with the increase of elderly and the higher levels of obesity and sedentary lifestyles. Today, around 17 million deaths per year are associated to CVD (Flowers et al. 2014).
One of the main priorities in public health systems is to design a strategy to prevent CVD by modifying lifestyle. In this strategy, diet plays an important role. Several dietary factors have been related with a rise in the risk to suffer CVD, such as a low consumption of fruit and vegetables, a high intake of saturated fat and salt (Eilat-Adar et al. 2013). In the pathogenesis of CVD, oxidative stress plays a central role.
Oxidative stress has been also associated with congestive heart failure, hypertension and ischemic heart disease. The high-energy requirements and high mitochondria amount in heart muscle cells is the main cause of the high levels of CoQ10 found in this cell type. In samples from human heart it has been detected a significant decrease of the CoQ10 content in cardiomyopathies. This deficiency showed a direct correlation with the severity of disease (Folkers et al. 1985; Nobuyoshi et al. 1984). A recent meta-analysis demonstrated that CoQ10 supplementation in the clinical treatment of CVD shoes improvement of congestive heart failure, indicated by best left ventricular ejection fraction (LVEF). Further, the New York Heart Association classification (NYHA), showed that subjects treated with CoQ10 supplements improved in the ejection fraction in comparison with controls (placebo) (Fotino et al. 2013). Moreover, a prospective, randomized, double-blind, placebo-controlled, multicentre trial in which CoQ10 (Q-SYMBIO) is used as an adjunctive treatment of chronic heart failure has demonstrated that the treatment with this quinone is safe, well tolerated, and associated with a reduction in general symptoms (Mortensen et al. 2014). These clinical results could be based on the important molecular functions of CoQ10, as integral component of mitochondrial respiratory chain (Littarru and Tiano 2010), and the only lipid-soluble antioxidant that slows lipid peroxidation in the circulation (Littarru and Tiano 2007).
CVD in elderly is accompanied with other complications such as diabetes and hypercholesterolemia. Certain drugs used in hypercholesterolemic disease can cause depletion of CoQ10 in particular statins that affect the first enzyme (hydroxyl-methylglutaryl-coenzyme A reductase; HMG-CoA reductase) in cholesterol and CoQ10 synthesis pathway (Schaars and Stalenhoef 2008) (Fig. 17.3). Statins are widely prescribed to reduce cholesterol levels by inhibiting HMG-CoA reductase (Folkers et al. 1990). Chronic statin treatment can reduce endogenous-synthesized cholesterol levels but, at the same time, they also lower CoQ10 levels. This can be the reason why chronic statin treatment is associated with muscle-related symptoms, pain or myopathies that can be improved with CoQ10 supplementation (Caso et al. 2007).
In relationship with the treatment of heart diseases several studies have concluded that supplementation with CoQ10 (50–300 mg/day) can be the safe and optimal dose, although higher doses such as 1200 mg/day have been also safely used (Gao et al. 2011). The majority of these clinical studies indicate that the treatment with CoQ10 significantly improve the heart muscle function, increasing ATP synthesis and enhancing myocardial contractility (Folkers et al. 1985). Importantly, these treatments have demonstrated no adverse effects or drug interactions (Kaikkonen et al. 2002).
3.2 CoQ10 and Hypertension
Hypertension is also associated with aging. Hypertension is also a key risk factor for stroke, myocardial infarction, congestive heart failure, kidney failure, and peripheral vascular disease. Although many pharmacological treatment have shown efficacy lowering blood pressure and modestly decrease stroke, myocardial infarction, and mortality, hypertension remains with high prevalence especially in old population and additional treatments are needed (Musini et al. 2009). The health effects of CoQ10 as additional treatment have been investigated in several controlled intervention studies in human subjects in a range of CoQ10 doses from 100 mg to 200 mg/day (Young et al. 2011; Yubero-Serrano et al. 2011, 2013). CoQ10 affects vasodilatation by improving endothelium and vascular smooth muscle activity, counteracting vasoconstriction and lowering blood pressure. Among treated patients, it has been reported a decrease in systolic blood pressure ranged from 11–17 mmHg and 8 mmHg decrease in diastolic blood pressure after the treatment with CoQ10. These results indicate a putative role of CoQ10 as a hypotensive agent and probably a safe adjuvant in the treatment with conventional anti-hypertensive pharmacological products.
3.3 CoQ10 and Endothelial Function
The progression and clinical manifestations of atherosclerosis and cardiovascular diseases depends on the dysfunction of endothelium. Several studies have determined the effect of oral CoQ10 supplementation on the physiology of endothelium in patients suffering coronary artery disease or diabetes mellitus or in elderly people (Gao et al. 2011; Tiano et al. 2007; Watts et al. 2002). In most of the individuals treated with CoQ10, endothelial function, determined by flow-mediated dilation (FMD) or by nitro-glycerine-mediated dilation (NMD) and the activity of extracellular superoxide dismutase, improved. This effect has been associated with the antioxidant and anti-inflammatory activity of CoQ10 (Yubero-Serrano et al. 2012). CoQ10 treatment decreases the rate of production of peroxynitrite from nitric oxide (NO) that reacts with superoxide radicals. Likely, under conditions of oxidative stress, CoQ10 can reduce the levels of superoxide radicals (Yubero-Serrano et al. 2011, 2013; Tiano et al. 2007). Furthermore, in vitro studies have demonstrated that CoQ10 can efficiently prevent apoptosis of endothelial cells produced by high glucose and the adhesion to monocytes. This effect is very important in the prevention of the development of atherosclerosis (Tsuneki et al. 2007). Further, the inhibition of the LDL-oxidation by ubiquinol adds an important factor in the prevention of atherogenesis (Thomas et al. 1997).
3.4 CoQ10 and Renal Failure
Chronic kidney (CKD) and end-stage renal (ESRD) diseases are also associated with oxidative stress (Himmelfarb and Hakim 2003). Five hundred thousand patients in the United States receive maintenance haemodialysis for ESRD, with life expectancies less than 17–34% than those of the general population (US Renal Data System 2013). In these patients, the balance between ROS and antioxidants is disturbed and oxidative stress is produced. The high ratio of mortality has been attributed to an increased risk of cardiovascular disease produced by this high oxidative stress (Kuchta et al. 2011). The putative role of CoQ10 in CKD patients has been studied only in a few studies. Lippa et al. (1994) determined the levels of CoQ10 in 48 patients under chronic haemodialysis, in comparison with 15 uremic patients and a control group of healthy subjects (Lippa et al. 2000). In this study, CoQ10 levels were significantly lower in CKD patients. In a recent study, the levels of CoQ10 and oxidative stress biomarkers were determined in CKD, haemodialysis and peritoneal dialysis (PeD) patients. Contrary to the study of Lippa et al. (1994), in this study, researchers did not find differences in CoQ10 levels between those of CKD and haemodialysis groups. However, they did observe higher levels of members of the antioxidant system in patients undergoing PeD in comparison with CKD patients (Gokbel et al. 2011).
In other study, supplementation with CoQ10 (120 mg/day) to patients with CKD, reduced the number of patients on dialysis in comparison with placebo after 28 days of treatment (Singh et al. 2000). As in other cases, the tolerability and safety of oral CoQ10 administration was determined until doses as higher as 1800 mg/day in CKD patients (Yeung et al. 2015). Authors conclude that in these patients, CoQ10 supplementation is important since it reduces systemic oxidative stress and improves mitochondrial function in patients receiving haemodialysis (Yeung et al. 2015).
3.5 CoQ10 and Neurodegenerative Diseases
Mitochondrial dysfunction is a common characteristic of the neurodegenerative diseases. This dysfunction is accompanied by abnormal energy metabolism and higher oxidative stress. As we have previously described, CoQ10 levels in the brain and other tissues in humans and animals have been shown to decline with age. As an antioxidant molecule, CoQ10 has been involved in the development of neurodegenerative diseases such as Parkinson’s disease (PD), Huntington’s disease (HD) and other neurodegenerative disorders (Koroshetz et al. 1997; Shults et al. 1997). There is, therefore, a robust scientific rationale for testing this agent in neuroprotective therapies.
CoQ10 has shown protective effects in the nigrostriatal dopaminergic system in many preclinical studies of PD (Liu et al. 2011). Mitochondrial dysfunction has been strongly associated with PD and, probably for this reason, CoQ10 levels are significantly lower in mitochondria from PD patients. A randomised, placebo controlled and double-blind study performed in these patients demonstrated that a CoQ10 dose as high as 1200 mg/day, was safe and reduced the worsening of PD (Shults et al. 2002). Treatment with CoQ10 was accompanied by significant increases in plasma levels of CoQ10 and a higher NADH-cytochrome C reductase activity in white blood cells (Shults et al. 2002). Everyday activities of the patients, such as dressing, bathing and feeding, were significantly improved with the treatment with CoQ10. Accordingly with this study, another placebo-controlled, double-blind trial showed that CoQ10 supplementation provided a mild but significant benefit on PD patients in comparison with (Muller et al. 2003).
In the case of HD, strong evidence indicate the existence of early oxidative stress. As in the other cases, this oxidative stress is coupled with mitochondrial dysfunction and the impairment of energy metabolism in which the deficiency in CoQ10 can be an important factor (Stack et al. 2008). In HD patients, CoQ10 doses, ranging from 600 to 1200 mg/day were tested during six-months in an open-label trial. Although no significant effect on clinical scores were found, the treatment with CoQ10 significantly decreased the levels of cortical lactate concentrations. This decrease was reversed following withdrawal of therapy indicating a protective effect of CoQ10 supplementation (Delanty and Dichter 1998). Again, the effect of CoQ10 in the behaviour of metabolic markers indicate the bioenergetics effect of oral CoQ10 in the mitochondrial metabolism of brain (Koroshetz et al. 1997).
4 Conclusions
The importance of CoQ10 is because this quinone is not just an agent in the transit of electrons in energy transduction in mitochondria. CoQ10 is a strong antioxidant able to regenerate the redox capacity in many tissues and organs. In normal conditions, its biosynthesis in mitochondria and endoplasmic reticulum provides sufficient CoQ10, but in some conditions such as genetic failure or aging and age-related diseases, CoQ10 deficiency can be an important factor in the progression of incapacity or disease. A number of studies have demonstrated that CoQ10 can be easily and safely used as nutritional supplement to delay and mitigate the effects caused by its depletion.
The clear beneficial effects of CoQ10 are reinforced because its excellent safety record. CoQ10 is very well tolerated even at high doses and for prolonged periods with null or very limited side effects. However, it is necessary to increase the research to determine the appropriate dose, effectiveness, and to increase the bioavailability of orally administered CoQ10, specially, in the elderly. Further, a promising strategy can be centred in the design of therapeutic agents that increment the endogenous synthesis of CoQ10.
References
Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, Salviati L (2016) Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta 1857(8):1079–1085. https://doi.org/10.1016/j.bbabio.2016.03.036
Alcazar-Fabra M, Navas P, Brea-Calvo G (2016) Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim Biophys Acta 1857(8):1073–1078. https://doi.org/10.1016/j.bbabio.2016.03.010
Barja G (2007) Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 10(2):215–224
Battino M, Svegliati Baroni S, Littarru GP, Bompadre S, Leone L, Gorini A, Villa RF (1997) Coenzyme Q homologs and vitamin E in synaptic and non-synaptic occipital cerebral cortex mitochondria in the ageing rat. Mol Asp Med 18(Suppl):S279–S282
Beal MF (1999) Coenzyme Q10 administration and its potential for treatment of neurodegenerative diseases. BioFactors (Oxford, England) 9(2–4):261–266
Bhagavan HN, Chopra RK (2006) Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res 40(5):445–453
Bhagavan HN, Chopra RK, Craft NE, Chitchumroonchokchai C, Failla ML (2007) Assessment of coenzyme Q10 absorption using an in vitro digestion-Caco-2 cell model. Int J Pharm 333(1–2):112–117
Blatt T, Littarru GP (2011) Biochemical rationale and experimental data on the antiaging properties of CoQ(10) at skin level. BioFactors (Oxford, England) 37(5):381–385
Caso G, Kelly P, McNurlan MA, Lawson WE (2007) Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 99(10):1409–1412. https://doi.org/10.1016/j.amjcard.2006.12.063
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20(6):591–598
Crane FL, Hatefi Y, Lester RL, Widmer C (1957) Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta 25(1):220–221
Crane FL, Hatefi Y, Lester RL, Widmer C (1989) Isolation of a quinone from beef heart mitochondria. 1957. Biochim Biophys Acta 1000:362–363
Delanty N, Dichter MA (1998) Oxidative injury in the nervous system. Acta Neurol Scand 98(3):145–153
Duntas LH (2011) Resveratrol and its impact on aging and thyroid function. J Endocrinol Investig 34(10):788–792
Eilat-Adar S, Sinai T, Yosefy C, Henkin Y (2013) Nutritional recommendations for cardiovascular disease prevention. Nutrients 5(9):3646–3683. https://doi.org/10.3390/nu5093646
Ernster L, Dallner G (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271(1):195–204
Fernández-Ayala DJ, López-Lluch G, García-Valdés M, Arroyo A, Navas P (2005a) Specificity of coenzyme Q10 for a balanced function of respiratory chain and endogenous ubiquinone biosynthesis in human cells. Biochim Biophys Acta 1706(1-2):174–83. https://doi.org/10.1016/j.bbabio.2004.10.009
Fernández-Ayala DJ, Brea-Calvo G, López-Lluch G, Navas P (2005b) Coenzyme Q distribution in HL-60 human cells depends on the endomembrane system. Biochim Biophys Acta 1713(2):129–37. https://doi.org/10.1016/j.bbamem.2005.05.010
Festenstein GN, Heaton FW, Lowe JS, Morton RA (1955) A constituent of the unsaponifiable portion of animal tissue lipids (lambda max. 272 m mu). Biochem J 59(4):558–566
Flowers N, Hartley L, Todkill D, Stranges S, Rees K (2014) Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 12:CD010405. https://doi.org/10.1002/14651858.CD010405.pub2
Folkers K, Vadhanavikit S, Mortensen SA (1985) Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci U S A 82(3):901–904
Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H (1990) Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A 87(22):8931–8934
Forsmark-Andree P, Dallner G, Ernster L (1995) Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Radic Biol Med 19(6):749–757
Fotino AD, Thompson-Paul AM, Bazzano LA (2012) Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis. Am J Clin Nutr
Fotino AD, Thompson-Paul AM, Bazzano LA (2013) Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am J Clin Nutr 97(2):268–275. https://doi.org/10.3945/ajcn.112.040741
Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L (2011) Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis 221(2):311–316
Gilmer LK, Ansari MA, Roberts KN, Scheff SW (2010) Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev 131(2):133–143
Gokbel H, Atalay H, Okudan N, Solak Y, Belviranli M, Turk S (2011) Coenzyme Q10 and its relation with oxidant and antioxidant system markers in patients with end-stage renal disease. Ren Fail 33(7):677–681
Gonzalez-Guardia L, Yubero-Serrano EM, Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Marin C, Camargo A, Delgado-Casado N, Roche HM, Perez-Jimenez F, Brennan L, Lopez-Miranda J (2015) Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. J Gerontol 70(1):78–84. https://doi.org/10.1093/gerona/glu098
Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Lista J, Yubero-Serrano EM, Camargo A, Delgado-Casado N, Cruz-Teno C, Santos-Gonzalez M, Rodriguez-Cantalejo F, Castano JP, Villalba-Montoro JM, Fuentes F, Perez-Jimenez F, Lopez-Miranda J (2011) Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age (Dordr) 34(2):389–403
Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Lista J, Yubero-Serrano EM, Camargo A, Delgado-Casado N, Cruz-Teno C, Santos-Gonzalez M, Rodriguez-Cantalejo F, Castano JP, Villalba-Montoro JM, Fuentes F, Perez-Jimenez F, Lopez-Miranda J (2012) Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age (Dordr) 34(2):389–403. https://doi.org/10.1007/s11357-011-9229-1
Gutierrez-Mariscal FM, Yubero-Serrano EM, Rangel-Zuniga OA, Marin C, Garcia-Rios A, Perez-Martinez P, Delgado-Lista J, Malagon MM, Tinahones FJ, Perez-Jimenez F, Lopez-Miranda J (2014) Postprandial activation of p53-dependent DNA repair is modified by Mediterranean diet supplemented with coenzyme Q10 in elderly subjects. J Gerontol 69(7):886–893. https://doi.org/10.1093/gerona/glt174
Himmelfarb J, Hakim RM (2003) Oxidative stress in uremia. Curr Opin Nephrol Hypertens 12(6):593–598
Jeya M, Moon HJ, Lee JL, Kim IW, Lee JK (2010) Current state of coenzyme Q(10) production and its applications. Appl Microbiol Biotechnol 85(6):1653–1663. https://doi.org/10.1007/s00253-009-2380-2
Kagan VENH, Quinn JP (1996) Coenzyme Q: its role in scavenging and generation of radicals in membranes. In: Cardenas E, Packer LM (eds) Handbook of antioxidants. M Dekker, New York, pp 157–201
Kaikkonen J, Tuomainen TP, Nyyssonen K, Salonen JT (2002) Coenzyme Q10: absorption, antioxidative properties, determinants, and plasma levels. Free Radic Res 36(4):389–397
Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF (1997) Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann Neurol 41(2):160–165
Kuchta A, Pacanis A, Kortas-Stempak B, Cwiklinska A, Zietkiewicz M, Renke M, Rutkowski B (2011) Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res 34(1):12–19. https://doi.org/10.1159/000321508
Lass A, Kwong L, Sohal RS (1999) Mitochondrial coenzyme Q content and aging. BioFactors (Oxford, England) 9(2–4):199–205
Lee CK, Pugh TD, Klopp RG, Edwards J, Allison DB, Weindruch R, Prolla TA (2004) The impact of α-lipoic acid, coenzyme Q10, and caloric restriction on life span and gene expression patterns in mice. Free Radic Biol Med 36:1043–1057
Lippa S, Colacicco L, Bondanini F, Calla C, Gozzo ML, Ciccariello M, Angelitti AG (2000) Plasma levels of coenzyme Q(10), vitamin E and lipids in uremic patients on conservative therapy and hemodialysis treatment: some possible biochemical and clinical implications. Clin Chim Acta Int J Clin Chem 292(1–2):81–91
Lippa S, Colacicco L, Callà C, Sagliaschi G, Angelitti AG (1994) Coenzyme Q10 levels, plasma lipids and peroxidation extent in renal failure and in hemodialytic patients. Mol Aspects Med 15(Suppl):s213–9. https://doi.org/10.1016/0098-2997(94)90031-0
Littarru GP, Tiano L (2007) Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol 37(1):31–37
Littarru GP, Tiano L (2010) Clinical aspects of coenzyme Q10: an update. Nutrition 26(3):250–254. https://doi.org/10.1016/j.nut.2009.08.008
Liu J, Wang L, Zhan SY, Xia Y (2011) Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst Rev 12:CD008150. https://doi.org/10.1002/14651858.CD008150.pub2
Lopez-Dominguez JA, Khraiwesh H, Gonzalez-Reyes JA, Lopez-Lluch G, Navas P, Ramsey JJ, de Cabo R, Buron MI, Villalba JM (2012) Dietary fat modifies mitochondrial and plasma membrane apoptotic signaling in skeletal muscle of calorie-restricted mice. Age (Dordr)
Lopez-Lluch G, Rodriguez-Aguilera JC, Santos-Ocana C, Navas P (2010) Is coenzyme Q a key factor in aging? Mech Ageing Dev 131(4):225–235. https://doi.org/10.1016/j.mad.2010.02.003
López-Lluch G, Del Pozo-Cruz J, Sánchez-Cuesta A, Cortés-Rodríguez AB, Navas P (2019) Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. 57:133–140. https://doi.org/10.1016/j.nut.2018.05.020. Epub 2018 Jun 27
Miquel J (1998) An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol 33(1–2):113–126
Miquel J, Economos AC, Fleming J, Johnson JE Jr (1980) Mitochondrial role in cell aging. Exp Gerontol 15(6):575–591
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP, Investigators QSS (2014) The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail 2(6):641–649. https://doi.org/10.1016/j.jchf.2014.06.008
Muller T, Buttner T, Gholipour AF, Kuhn W (2003) Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci Lett 341(3):201–204
Musini VM, Tejani AM, Bassett K, Wright JM (2009) Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 4:CD000028. https://doi.org/10.1002/14651858.CD000028.pub2
Nakamura T, Ohno T, Hamamura K, Sato T (1999) Metabolism of coenzyme Q10: biliary and urinary excretion study in Guinea pigs. BioFactors (Oxford, England) 9(2–4):111–119
Navas P, Villalba JM, de Cabo R (2007) The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 7(Suppl):S34–S40. https://doi.org/10.1016/j.mito.2007.02.010
Nobuyoshi M, Saito T, Takahira H, Yamano Y, Kanazawa T (1984) Levels of coenzyme Q10 in biopsies of left ventricular muscle and influence of administration of coenzyme Q10. In: Folkers K, Yamamura Y (eds) Biomedical and clinical aspects of coenzyme Q, vol 4. Elsevier, Amsterdam, pp 221–229
Ochoa JJ, Quiles JL, Lopez-Frias M, Huertas JR, Mataix J (2007) Effect of lifelong coenzyme Q10 supplementation on age-related oxidative stress and mitochondrial function in liver and skeletal muscle of rats fed on a polyunsaturated fatty acid (PUFA)-rich diet. J Gerontol 62(11):1211–1218
Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S (1999) Coenzyme Q10 in health and disease. Eur J Clin Nutr 53(10):764–770
Potgieter M, Pretorius E, Pepper MS (2013) Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr Rev 71(3):180–188. https://doi.org/10.1111/nure.12011
Quinzii CM, DiMauro S, Hirano M (2007) Human coenzyme Q10 deficiency. Neurochem Res 32(4–5):723–727
Rosenfeldt FL, Pepe S, Ou R, Mariani JA, Rowland MA, Nagley P, Linnane AW (1999) Coenzyme Q10 improves the tolerance of the senescent myocardium to aerobic and ischemic stress: studies in rats and in human atrial tissue. BioFactors (Oxford, England) 9(2–4):291–299
Schaars CF, Stalenhoef AF (2008) Effects of ubiquinone (coenzyme Q10) on myopathy in statin users. Curr Opin Lipidol 19(6):553–557. https://doi.org/10.1097/MOL.0b013e3283168ecd
Schultz JR, Clarke CF (1999) In: Cardenas E, Packer L (eds) Mitochondria, oxidants and ageing. M Dekker, New York, pp 95–118
Shetty RA, Forster MJ, Sumien N (2012) Coenzyme Q(10) supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age (Dordr)
Shults CW, Haas RH, Passov D, Beal MF (1997) Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol 42(2):261–264
Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M (2002) Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59(10):1541–1550
Singh RB, Khanna HK, Niaz MA (2000) Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in chronic renal failure: discovery of a new role. J Nutr Environ Med 10:281–288
Sohal RS, Forster MJ (2007) Coenzyme Q, oxidative stress and aging. Mitochondrion 7(Suppl):S103–S111. https://doi.org/10.1016/j.mito.2007.03.006
Sohal RS, Mockett RJ, Orr WC (2002) Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med 33(5):575–586
Spinazzi M, Radaelli E, Horré K, Arranz AM, Gounko NV, Agostinis P, Maia TM, Impens F, Morais VA, Lopez-Lluch G, Serneels L, Navas P, De Strooper B (2019) PARL deficiency in mouse causes Complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc Natl Acad Sci U S A 116(1):277–286. https://doi.org/10.1073/pnas.1811938116
Stack EC, Matson WR, Ferrante RJ (2008) Evidence of oxidant damage in Huntington’s disease: translational strategies using antioxidants. Ann N Y Acad Sci 1147:79–92. https://doi.org/10.1196/annals.1427.008
Su Y, Sun H, Fang J, Hu G, Xiao M (2010) Brain mitochondrial dysfunction in ovariectomized mice injected with D-galactose. Neurochem Res 35(3):399–404
Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP (2007) Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J 28(18):2249–2255
Thomas SR, Neuzil J, Stocker R (1997) Inhibition of LDL oxidation by ubiquinol-10. A protective mechanism for coenzyme Q in atherogenesis? Mol Aspects Med 18(Suppl):S85–103. https://doi.org/10.1016/S0098-2997(97)00031-9
Tsuneki H, Sekizaki N, Suzuki T, Kobayashi S, Wada T, Okamoto T, Kimura I, Sasaoka T (2007) Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol 566(1–3):1–10. https://doi.org/10.1016/j.ejphar.2007.03.006
Turunen M, Olsson J, Dallner G (2004) Metabolism and function of coenzyme Q. Biochim Biophys Acta 1660(1–2):171–199
US Renal Data System UADRAoE-sRDitUSB, MD (2013) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; www.usrds.org
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40
Villalba JM, Navas P (2000) Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid Redox Signal 2(2):213–230
Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ (2010) Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs 19(4):535–554
Wanke M, Dallner G, Swiezewska E (2000) Subcellular localization of plastoquinone and ubiquinone synthesis in spinach cells. Biochim Biophys Acta 1463(1):188–194
Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V (2002) Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia 45(3):420–426
Yeung CK, Billings FT, Claessens AJ, Roshanravan B, Linke L, Sundell MB, Ahmad S, Shao B, Shen DD, Ikizler TA, Himmelfarb J (2015) Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrol 16:183. https://doi.org/10.1186/s12882-015-0178-2
Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Nicholls MG, Scott RS, George PM (2011) A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am J Hyper 25(2):261–270
Yubero-Serrano EM, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Tasset-Cuevas I, Santos-Gonzalez M, Caballero J, Garcia-Rios A, Marin C, Gutierrez-Mariscal FM, Fuentes F, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J (2011) Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age (Dordr) 33(4):579–590. https://doi.org/10.1007/s11357-010-9199-8
Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P, Delgado-Casado N, Cruz-Teno C, Tinahones FJ, Villalba JM, Perez-Jimenez F, Lopez-Miranda J (2012) Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol 67(1):3–10. https://doi.org/10.1093/gerona/glr167
Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuniga O, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Caballero J, Marin C, Gutierrez-Mariscal FM, Tinahones FJ, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J (2013) Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q(10) in elderly men and women. Age (Dordr) 35(1):159–170. https://doi.org/10.1007/s11357-011-9331-4
Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L (1995) Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr 125(3):446–453
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yubero-Serrano, E.M., Gutierrez-Mariscal, F.M., Lopez-Miranda, J. (2020). Effects of Coenzyme Q10 Supplementation on Elderly People. In: López Lluch, G. (eds) Coenzyme Q in Aging. Springer, Cham. https://doi.org/10.1007/978-3-030-45642-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-45642-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45641-2
Online ISBN: 978-3-030-45642-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)