Abstract
The dynamics and distribution of endogenous cytokinins (CKs), gibberellic (GA3) and salicylic (SA) acids in wheat (Triticum aestivum L., ‘Podolyanka’) and spelt wheat (Triticum spelta L., ‘Frankenkorn’) plants was analyzed using HPLC–MS. Fourteen-day-old plants that had been exposed to short-term heat stress (+ 40 °C, 2 h) and 21-day-old plants after recovery were studied. Heat stress induced rapid changes, both specific and nonspecific, in hormone levels and distribution. The level of GA3 decreased in the shoots and roots of both winter and spelt wheat. A reduction in SA content was observed in wheat, while an increase was observed in spelt. The pool of CKs significantly increased in wheat, while in spelt—it decreased more than twofold. After recovery, an increase in GA3 content occurred in both species, but not to the levels measured in control plants. More active accumulation of GA3 was observed in the roots. The content of SA in the shoots of wheat continued to decrease, while in the roots it increased. In spelt, hormone concentration decreased, but it remained higher than in 21-day-old control plants. In shoots of both plants the pool of CKs decreased, while in wheat roots it did not change, and in spelt roots it decreased. The total CKs content in stressed wheat plants was twice as high as in spelt. In general, we established significant hormonal fluctuations, which indicate a direct involvement of endogenous cytokinins, gibberellic and salicylic acids in wheat and spelt response to heat stress. Screening of stress-resistant genotypes of cereals may benefit from quantitation of CKs, GA3, and SA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 40% of worldwide areas where wheat is grown are exposed to high temperature stresses and an increase by 1 °C from the average of 23 °C reduces yield by approximately 10% (Narayanan 2018). Heat stress causes negative changes in the water regime (Hasanuzzaman et al. 2013), inhibits photosynthetic activity (Ashraf and Harris 2013) and metabolic processes (Farooq et al. 2011), induces the accumulation of reactive oxygen species (ROS) (Wang et al. 2011), and changes in hormonal status (Abhinandan et al. 2018). The initial stages of wheat ontogenesis are especially sensitive to high temperatures (Abid et al. 2018).
Phytohormones are signaling biomolecules with different chemical structures and physicochemical properties, which act in nanomolar concentrations to regulate physiological and metabolic processes in plants. More that 130 forms of gibberellins exist, but physiological activity is characteristic only of certain gibberellic acids (GAs) (GA1, GA3, GA4, GA5, GA6 and GA7), while others constitute their precursors and inactive forms (Sponsel and Hedden 2010). The main functions of GAs are the regulation of seed germination, coordination of cell division and elongation, sex determination, pollen and flower development, flowering induction, seed and fruit formation (Gantait et al. 2015). Following a reduction in the endogenous GAs content, plant growth is stunted, whereas enhanced hormone biosynthesis prevents stress damage (Colebrook et al. 2014). Cytokinins (CKs)—another important component of the phytohormone complex—are present as free bases: isopentenyladenine (iP), dihydrozeatin, cis-zeatin, and trans-zeatin (t-Z) and their conjugates (ribosides and nucleotides). t-Z and its derivatives are the most active dominant forms (Kieber and Schaller 2018). CKs are involved in regulation of cell division, formation of meristems, photosynthesis, aging, absorption of macro- and microelements and responses to the negative effects of the environment (Ha et al. 2012; Veselov et al. 2017; Cortleven et al. 2019). In the early phases of heat stress, CKs stimulate the opening of the stomata and transpiration and activate the antioxidant system (Prerostova et al. 2020). Salicylic acid (SA) is a phenolic compound involved in the regulation of such important physiological processes as photosynthesis, respiration, transpiration and thermogenesis (Janda and Ruelland 2015), and enhances plant resistance to a wide range of abiotic and biotic stressors (Kang et al. 2014; Kumar 2014; Jayakannan et al. 2015). SA induces stress resistance by promoting the accumulation of osmolites and antioxidant defense, as well as through cross-talk with other hormones (Khan et al. 2015; Janda 2019).
The winter wheat Triticum aestivum L., which is a main grain crop in Ukraine, is cultivated on almost 240 million hectares. An important element of its cultivation technology, which affects productivity, involves the use of high-yielding genotypes tolerant to biotic and abiotic stressors (Morgun et al. 2016). Spelt wheat (Triticum spelta L.) is a husked-wheat species, is considered a wild precursor of winter wheat, has the same genomic composition, is easily crossed and used as a donor of valuable agricultural traits (Babenko et al. 2018). High nutritional qualities and adaptability to organic farming make spelt an attractive option in many European countries (Lacko-Bartošová et al. 2010; Escarnot et al. 2012).
Here, we quantify and compare the dynamics and distribution of endogenous gibberellic acid GA3, CKs and SA in shoots and roots of wheat and spelt plants and to identify specific and nonspecific traits of phytohormonal status associated with response to heat stress and recovery. The study of adaptation mechanisms provides useful information for the selection of stress-resistant varieties of cereals in light of expected future climate change.
Materials and methods
Plant material
Experiments were performed at the M. G. Kholodny Institute of Botany of the NAS of Ukraine in 2018–2019. Plants of wheat (T. aestivum L., cultivar ‘Podolyanka’) and spelt wheat (T. spelta L., cultivar ‘Frankenkorn’) were studied. Spelt seeds were obtained from the collection of the National Centre for Plant Genetic Resources of Ukraine in Kharkiv, and wheat seeds—from the collection of the Institute of Plant Physiology and Genetics of the NAS of Ukraine (Kyiv). The seeds were calibrated, sterilized in 80% ethanol, washed with distilled water, and placed for 3 h in a cuvette with water. Then, the seeds were planted in 2-L vessels. River sand sterilized by calcination was used as the substrate. Plants were grown under controlled conditions at a temperature of + 20/17 °C (day/night), light intensity 190 μmol·m−2·s–1, photoperiod 16/8 h (day/night), relative air humidity—70 ± 5%, substrate humidity of 60% from full moisture content. The plants were watered with 50 ml of Knop solution per vessel daily.
Abiotic stress treatments and sample collection
To simulate temperature stress, 14-day-old plants were placed in a thermostat at a temperature of + 40 °C for 2 h, under the illumination of 190 μmol·m−2·s–1. For recovery, the plants were grown to 21-day-old in standard conditions (see above). Shoots and roots of 14- and 21-day-old plants were collected for further study.
Extraction of GA3, SA, and CKs
Samples of shoots and roots (2 g) were frozen and ground in liquid nitrogen using 10 ml of extraction solution—methanol, distilled water, and formic acid (15:4:1 ratio). The homogenate was incubated at + 4 °C for 24 h in the dark. Extracts containing plant hormones were obtained by 30 min centrifugation at 15,000 RPM and + 4 °C and separation of the supernatant. The precipitate was resuspended in 5 ml of extraction solution. The suspension was incubated for 30 min and centrifuged again. The combined supernatants were evaporated to an aqueous residue under reduced pressure in a vacuum evaporator at + 40 °C. Further purification was performed on two solid-phase SPE cartridges: C18 Sep-Pak Plus, Waters and Oasis MCX, 6 cc/150 mg, Waters (Kosakivska et al. 2020). The C18 Sep-Pak Plus cartridge was used to remove lipophilic substances, proteins and pigments. Sorption and separation of phytohormones of different classes were performed on the Oasis MCX cartridge. Elution of GA3 and SA was performed with 100% methanol (first fraction). The second fraction with cytokinins was obtained using an alkaline solution of methanol (60 ml of methanol, 2.5 ml of 25% ammonia and deionized water to 100 ml). The obtained fractions were evaporated to dryness in concentrator flasks using a vacuum rotary evaporator at a temperature not exceeding + 40 °C. Each dry residue was dissolved to 200 μl with 45% methanol prior to analysis.
Quantification of GA3, SA, and CKs
Analytical determination of phytohormones was performed using high-performance liquid chromatography on Agilent 1200 LC/MS series instrument (USA) with diode-array detector G1315B and single quadrupole mass detector Agilent G6120A. Phytohormone content was analyzed and calculated using Agilent OpenLAB CDS ChemStation Edition chromatograph software (rev. C.01.09). Chromatographic separation was carried out using an Agilent ZORBAX Eclipse Plus C18 column 4.6 × 250 mm with a lipophilic-modified sorbent, particle size 5 μm (reverse phase chromatography).
To determine the content of SA, 10 μl aliquots of the first fraction was separated using a solvent system (acetonitrile, deionized water, acetic acid in a volume ratio of 45:54.9:0.1) and SA was detected at an analytical wavelength of 302 nm. The flow rate of the mobile phase was 0.8 ml/min. After separating an aliquot of 20 μl of the first fraction with a solvent system (acetonitrile, deionized water, acetic acid—30:69.9:0.1), GA3 was quantitatively detected by the mass spectrometer signal. The flow rate of the mobile phase during the GA3 detection was 0.5 ml/min.
The 20 μl aliquots of the fraction with cytokinins were separated using a system of solvents (methanol, deionized water, acetic acid), the detection was performed at a wavelength of 269 nm. A step gradient system was used to elute the cytokinins, namely: 0 min: CH3OH/0.5% solution of CH3COOH in deionized water (37/63)—25 min: CH3OH/0.5% solution of CH3COOH (70/30)—35 min: CH3OH/0.5% CH3COOH solution (100/0) at a constant flow rate of 0.5 ml per minute. The duration of the column equilibration after analysis (post-run) was 15 min.
Unlabeled GA3, SA, trans-zeatin-O-glucoside (t-ZOG), (t-Z), trans-zeatinribozid (t-ZR), iP, and isopentenyladenosine (iPa) manufactured by Sigma-Aldrich (USA) were used as chemical standards for the creation of calibration tables in the chromatographic methods of the instrument software. The content of analytes in the samples was monitored using a mass spectrometer in the combined mode (electrospray and chemical ionization at atmospheric pressure) with ionization of molecules of analytes in negative polarity and positive—for cytokinins during analysis. For quantitation of GA3, the molecular weight of which is 346, we used the signal of the mass detector in SIM (setting 50% of the scan time for single ion monitoring of the 345 m/z value [346-H]−).
Statistical analyses
All measurements were performed with three biological and three analytical replicates. Phytohormonal content was analyzed and calculated using Agilent OpenLAB CDS ChemStation Edition (rev. C.01.09), a program for controlling the HPLC/MS (high-performance liquid chromatography–mass spectrometry) instrument and processing the data). Statistical analysis was carried out in Statistica, version 6.0 (StatSoft Inc.). One-way analysis of variance (ANOVA) was conducted, with tests at P ≤ 0.05 considered statistically significant (Van Emden 2008).
Results
Morphometric parameters of T. aestivum and T. spelta after short-term hyperthermia and in recovery period
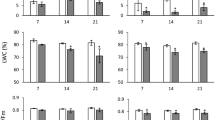
A short-term high temperature stress caused minor changes in shoot height and primary root length of wheat and spelt plants. Some changes were observed in the fresh (FW) and dry weight (DW) of organs. FW and DW of wheat shoots did not change appreciably. On the other hand, in roots an increase in FW was accompanied by a decrease in DW, as a result of the elevated hydration of root cells and the intensification of water transport to shoots. In spelt wheat, FW of shoots and roots declined, while DW of roots increased. The negative effects of stress manifested on the 21st day. Neither species was able to completely recover its morphometric parameters. The height of the shoots remained less than in control. The growth of spelt roots was slower than control. A decrease in DW and FW of stressed plants organs was observed (Table 1).
Thus, the negative effects of short-term hyperthermia were manifested as a decline in morphometric parameters in wheat and spelt plants on the 21st day after recovery. Wheat plants were more resistant.
Dynamics and distribution of endogenous gibberellic acid in T. aestivum and T. spelta after short-term hyperthermia and in recovery period
We observed that GA3 concentration in the roots of fourteen and twenty one-day-old control plants of wheat exceeded the corresponding indicators in shoots by 2.7- and 2.3-fold, respectively. Temperature stress caused a decrease in GA3 levels. Roots were more sensitive to high temperature: the amount of GA3 decreased by 37.3%, while in shoots it decreased by 23.2%. On the 21st day after recovery, an increase in hormone content was seen, but the recorded values were below control. GA3 accumulation after recovery was more intensive in wheat roots (Fig. 1).
Dynamics and distribution of endogenous gibberellic acid in the organs of 14-day-old plants of Triticum aestivum L. cv. Podolyanka and Triticum spelta L. cv. Frankenkorn after short-term hyperthermia (+ 40 °C, 2 h), and on the 21st day after recovery (ng/g FW); differences between the mean values were evaluated using Bonferroni-corrected ANOVA, considered to be significant at P < 0.05;*P < 0.05; **P < 0.01;***P < 0.001 compared to control at this stages of vegetation
At the same time, endogenous GA3 dominated in shoots of 14-day-old control spelt plants and in roots of 21-day-old plants. During growth, the content of endogenous GA3 in shoots and roots of control 21-day-old spelt plants decreased 3.7- and 1.6-fold, respectively. In shoots and roots of post-stress spelt plants the level of endogenous GA3 decreased by 17% and 48%, respectively. In the roots of 21-day recovery plants, GA3 content increased in relation to post-stress plants 17% and exceeded the control 21-day-old plants by 27%. In shoots of 21-day-old post-stress plants, the GA3 content was 20.7% higher than in control, but 2.5-fold lower than in 14-day-old post-stress plants (Fig. 1).
Thus, endogenous GA3 dominated in the roots of control and post-stress wheat plants, while in spelt the site of hormone accumulation was in the shoots. Heat stress inhibited the accumulation of hormones. After recovery, GA3 accumulated in the roots of both species.
Dynamics and distribution of endogenous salicylic acid in T. aestivum and T. spelta after short-term hyperthermia and in recovery period
The ratio of SA content between shoots and roots of 14- and 21-day-old wheat plants was 3:1 and 6:1, and spelt 2.5:1 and 1.9:1, respectively. On the 21st day, the amount of hormone in the wheat shoots increased by 24.1% and in the roots—decreased by 35%. Maximum accumulation of SA was recorded in the roots of 14-day-old and shoots of 21-day-old wheat plants. During growth, the amount of SA in spelt plants decreased. On the 21st day, its level decreased almost fourfold in shoots and threefold in roots.
After heat stress, in shoots and roots of 14-day-old wheat plants SA content decreased by 30.0% and 46.7%, respectively. During the recovery period on the 21st day, the level of SA in shoots continued to decrease, while in roots increased 2.1-fold compared to control and 2.6-fold compared to post-stress plants (Fig. 2).
Dynamics and distribution of endogenous salicylic acid in the organs of 14-day-old plants of Triticum aestivum L. cv. Podolyanka and Triticum spelta L. cv. Frankenkorn after short-term hyperthermia (+ 40 °C, 2 h) and on the 21st day after recovery (ng/g FW); differences between the mean values were calculated using Bonferroni-corrected ANOVA, considered to be significant at P < 0.05;*P < 0.05; **P < 0.01;***P < 0.001 compared to control at this stages of vegetation
Dynamics and distribution of SA in post-stress spelt plants differed from those in wheat. In shoots, the SA content increased by 16%, and in roots—by 13%. On the 21st day after recovery, the amount of hormone decreased, but was higher than in 21-day control plants (Fig. 2).
Therefore, the accumulation of endogenous SA in control wheat plants occurred much more intensely than in spelt. In both species, SA dominated in shoots. Heat stress inhibited the accumulation of the hormone in wheat. After recovery, the site of SA accumulation was in the shoots of both species.
Dynamics and distribution of endogenous cytokinins in T. aestivum and T. spelta after short-term hyperthermia and after recovery
Among the studied forms of CKs in wheat we identified t-Z, t-ZOG and iP. t-ZR and iPa were present in trace amounts. After heat stress the content of t-Z increased 1.3-fold, t-ZOG—4.7-fold and iP—2.4-fold in wheat roots, whereas in shoots the content of t-Z decreased 1.4-fold, and iP and t-ZOG rose 2.3-fold and 2.6-fold, respectively. Total CKs content in shoots after heat stress rose 1.7-fold, and in roots—2.5-fold. On the 21st day after recovery in shoots of wheat, the pool of endogenous cytokinins decreased 1.4-fold, while in roots it did not change. The content of iP in shoots declined almost 13-fold, while, conversely, in roots, it increased 3.9-fold. In roots t-Z content rose 1.7-fold (Table 2).
We found five forms of CKs in spelt. After heat stress in the roots, the content of t-Z and iP increased 2.3-and twofold, and the content of t-ZOG significantly (7.4-fold) decreased. A decrease in the content of t-Z (1.3-fold), t-ZR (2.2-fold), iPa (up to trace amounts) and t-ZOG (2.8-fold) was detected in shoots. The total content of CKs in shoots after heat stress decreased 2.4-fold, and in roots 2.2-fold. On the 21st day after recovery, the pool of endogenous cytokinins decreased 1.5-fold in shoots, and 1.8-fold—in roots. In shoots, the levels of t-Z (2.3-fold), ZR (twofold) and ZOG (1.6-fold) also decreased, while the amount of iPa (2.3-fold) and iP (1.2-fold) increased. In roots, the content of all CK forms declined significantly: t-Z and ZR 4.4-fold, iPa 13-fold, iP to trace amounts, while the content of t-ZOG almost did not change (Table 3).
Discussion
Since the beginning of the century the ambient temperature has been steadily rising and is expected to rise further. At higher temperatures, the duration of all stages of ontogenesis, photosynthetic activity, stability of cell membranes, relative water content and leaf area index, total biomass and wheat yield decrease (Narayanan 2018). One of the critical periods of wheat ontogenesis is the three-leaf stage, when a transition from nutrition drawn from grain reserves to the absorption of nutrients from the outside through the root system occurs. High-yielding modern wheat genotypes, local species and wild precursors are characterized by specific temperature optima and some anatomical-morphological and biochemical differences. As compared to wild species, modern wheat varieties have higher stability of the photosynthetic apparatus, and wider ranges of optimal temperatures, which contribute to increased productivity throughout the growing season (Brestic et al. 2018). Genes involved in the regulation of phytohormone biosynthesis under stress conditions affect the ontogenesis and resistance of agricultural crops. In particular, changes in the metabolism and signaling of gibberellins and brassinosteroids determine stress tolerance, while yields are regulated mainly by cytokinins (Nadolska-Orczyk et al. 2017). The expression of genes encoding enzymes involved in GAs synthesis is regulated by external signals. Under the influence of negative factors, GA synthesis is regulated by GA2ox genes, which encode GA2-inactivating enzymes, as well as the DELLA RGL3 gene, which encodes a growth suppressor (Colebrook et al. 2014; Minguet et al. 2014). We have found that in the initial stages of ontogenesis, the nature of the accumulation and distribution of gibberellic acid in the organs of wheat and spelt follow specific patterns. The content of GA3 in spelt shoots was more than threefold higher than its content in wheat shoots. On the other hand, the amount of GA3 in roots of both species was approximately the same, with a slight predominance in wheat. The response of wheat and spelt to hyperthermia was not species-specific. In 14-day post-stress wheat and spelt plants, GA3 accumulation was inhibited. However, the preservation of the distribution of the hormone between the organs turned out to be specific. In wheat, GA3 dominated in roots, and in spelt—in shoots. After short-term hyperthermia, the biomass of wheat roots increased by 33%, while the biomass of spelt roots decreased by 5% (Table 1). The architecture of the root system is the main interface between the plant and abiotic factors, as it identifies and responds to negative influences, and helps overcome them. Length and density of the main and lateral roots play a crucial role in tolerance acquiring. Thus, an increase in density and diameter of the main root and development of lateral roots improved access to moisture at greater soil depths, increased hydration, photosynthetic activity and stomatal conductance, growth and drought resistance of rice and corn plants (Lynch et al. 2014; Zhan et al. 2015). Gibberellins have a positive effect on root growth. A recent study showed that HDT1/2 (histone deacetylases) mediates the early transition from root tip cell division to their elongation by inhibition the transcription of the GA2ox2 gene, a gibberellin inactivator (Li et al. 2017). On the 21st day after recovery, the amount of GA3 in wheat did not reach control levels, while in spelt it exceeded control levels. GA3 was predominant in the roots of both species. On the 21st day, the weight of the roots of post-stressed wheat plants was 17%, and spelt—12% less than that of control plants (Table 1).
Heat stress in general had a negative effect on the accumulation of GA3 in the organs of 14-day-old plants, but a sufficiently high level of the hormone in wheat roots induced an increase in their biomass and an acquisition of tolerance. In spelt roots, active accumulation of GA3 and growth of biomass wad observed after recovery on the 21st day, indicating a successful completion of post-stress adaptation.
The mechanism of stress tolerance under the action of SA is quite complex and not clearly understood. It includes the production of osmolites, induction of antioxidant activity and interaction with other hormones (Khan et al. 2015). We found that in the initial stages of ontogenesis after the transition from heterotrophic to autotrophic nutrition, SA accumulated in shoots of 14-day-old plants. The content of SA in wheat shoots was threefold higher than in roots. A similar distribution was observed in spelt, where the level of the hormone in shoots was 2.5-fold higher than in roots. The amount of SA in wheat shoots was 7.6-fold higher than in spelt, similar results were obtained for roots where hormone content was 6.3-fold higher. In 14-day-old post-stress wheat plants, the amount of SA decreased significantly, while in spelt organs, on the contrary, it increased slightly. On the 21st day after recovery, the SA content in spelt shoots and roots was threefold higher than in control, while in wheat it decreased in shoots 2.2-fold and doubled in roots. An accumulation of endogenous SA was observed in response to water stress in soybean plants (Hamayun et al. 2010), in rice plants under salt stress (Sawada et al. 2006), and in wheat under heavy metal (zinc) pollution (Kosakivska et al. 2019). It should be noted that SA content in plants under abiotic stresses, as a rule, grows more slowly than does the generation of ROS (reactive oxygen species). Because of this, SA is considered to be a signaling molecule involved in the perception, amplification and transduction of primary ROS signals (Larkindale and Huang 2004).
Our study has shown that during the formation of wheat and spelt reaction to short-term heat stress, a complex modifications of the cytokinins hormones occur, the nature of which depends on the species and organ of the plant. Indeed, after heat stress, the pool of CKs in wheat shoots and roots increased significantly. In roots, t-Z, t-ZOG and iP accumulated, while in shoots—t-Z content decreased, and iP and t-ZOG content increased. On the other hand, the total content of CKs in spelt shoots and roots more than halved. The content of t-Z and iP in spelt roots rose and the level of t-ZOG significantly declined. In shoots, a decrease in the content of t-Z, t-ZR, iPa and t-ZOG was recorded.
It should be noted that spelt plants significantly surpassed wheat plants by the total quantitative content of cytokinins in the control conditions. At the same time, the pool of cytokinins in wheat shoots was higher than in spelt in post-stress plants. On the 21st day after recovery in shoots of wheat and spelt, the pool of endogenous cytokinins decreased, while in wheat roots it did not change, and in spelt roots it decreased. The total CKs content in stressed wheat plants was twice as high as in spelt. Earlier we showed that after short-term heat stress in the roots of 14-day-old wheat plants of frost-resistant Volodarka variety, the CKs pool increased due to a significant accumulation of endogenous c-Z and iPa. Conversely, in the shoots, the general level of CKs was halved (Kosakivska et al. 2016).
The effect of high temperature on the balance of cytokinins has been studied mainly in the reproductive period of cereal development (Cheikh and Jones 1994; Wang et al. 2020). In rice inflorescences and roots under the action of high temperature the content of active cytokinins forms decreased (Wu et al. 2017). Under short-term heat stress, changes in the content of certain forms of cytokinins have been recorded (Farkhutdinov et al. 1997; Todorova et al. 2005). At a temperature of + 40 °C for the first 30 min, the level of active forms of cytokinins increased in the leaves and decreased in the roots, and after 2 h of stress reduction of cytokinins content was detected both in the leaves and in the roots of arabidopsis plants (Dobrá et al. 2015). Zeatin and zeatinribozid play a key role in the regulation of growth processes of higher plants, while in shoots they act as positive growth regulators, and in roots—as growth suppressors. Therefore, it is plausible that the changes in the dynamics and distribution of cytokinins after short-term hyperthermia revealed in this work, are aimed at reducing the growth activity of wheat and spelt. The simultaneous surge in the accumulation of iP and t-ZOG in wheat is due to continued cytokinins biosynthesis (as its primary products), and a shift in the synthesis of the hormone under the action of stress on conjugation to O-glucoside. At the same time, in spelt plants, accumulation of iP was recorded in shoots, while the content of t-ZOG decreased significantly in both shoots and in roots.
Plants integrate ecological and endogenous signals through a complex network of phytohormonal interactions, forming reaction and regulating growth and development. The involvement of cytokinins, gibberellins and SA in plant responses to stresses is broadly appreciated, and evident from the results of exogenous applications of hormones. Thus, priming with solutions of gibberellic and salicylic acids induced the germination of rye grains Secale montanum, increased the germination index and coefficient of germination rate in drought conditions. After treatment with phytohormones, the content of antioxidant enzymes catalase and ascorbate peroxidase increased (Ansari et al. 2013). Treatment with a SA solution contributed to the formation of stress resistance of wheat to hyperthermia (Asif et al. 2019), maintained photosynthetic activity, reducing oxidative damage to photosynthetic membranes due to the production of antioxidants glutathione and ascorbate (Chen et al. 2016), induced proline accumulation, which promoted the normalization of hydration (Khan et al. 2013). A positive effect of cytokinin treatment was revealed for wheat (Gupta et al. 2003) and barley (Hosseini et al. 2008). The mechanism of this action is related to the ability of these hormones not only to stimulate cell division, but also to increase the attracting ability of seeds and prolong the period of active photosynthesis (Hönig et al. 2018).
Our study showed that heat stress induced rapid changes, both specific and nonspecific, in the hormonal balance in 14-day-old wheat and spelt plants, the nature of which depended on the species and organ of plant. Our data also revealed a prolonged effect of heat stress on phytohormones accumulation and distribution in the organs of 21-day-old wheat and spelt post-stress plants. It is worth noting that in control conditions the content of endogenous SA was significantly higher in wheat, while the content CKs was higher in spelt. These fluctuations indicate a direct involvement of endogenous cytokinins, gibberellic and salicylic acids in the formation of a response to heat stress, and may be useful in screening of stress-resistant genotypes of wheat and spelt taking into account future extreme climatic changes.
Abbreviations
- GA3 :
-
Gibberellic acid
- SA:
-
Salicylic acid
- CKs:
-
Cytokinins
- iP:
-
Isopentenyladenine
- iPa:
-
Isopentenyladenosine
- t-Z:
-
trans-Zeatin
- t-ZR:
-
trans-Zeatin riboside
- t-ZOG:
-
trans-Zeatin-O-glucoside
- HPLC–MS:
-
High-performance liquid chromatography-mass spectrometry
- ROS:
-
Reactive oxygen species
- FW:
-
Fresh weight
- DW:
-
Dry weight
References
Abhinandan K, Skori L, Stanic M, Hickerson NMN, Jamshed M, Samuel MA (2018) Abiotic stress signaling in wheat—an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front Plant Sci 9:734. https://doi.org/10.3389/fpls.2018.00734
Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T (2018) Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci Rep 8:1–15. https://doi.org/10.1038/s41598-018-21441-7
Ansari O, Azadi MS, Sharif-Zadeh F, Younesi L (2013) Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum) seeds under drought stress conditions. J Stress Physiol Biochem 9(3):61–71
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190. https://doi.org/10.1007/s11099-013-0021-6
Asif M, Jamil HMA, Hayat MT, Mahmood Q, Ali S (2019) Use of phytohormones to improve abiotic stress tolerance in wheat. In: Hasanuzzaman M, Nahar K, Hossain M (eds) Wheat Production in changing environments. Springer, Singapore, pp 465–479. https://doi.org/10.1007/978-981-13-6883-7_18
Babenko LM, Hospodarenko HM, Rozhkov RV, Pariy YF, Pariy MF, Babenko AV, Kosakivska IV (2018) Triticum spelta: origin, biological characteristics and perspectives for use in breeding and agriculture. Regul Mech Biosyst 9(2):250–257. https://doi.org/10.15421/021837
Brestic M, Zivcak M, Hauptvoge P, Misheva S, Kocheva K, Yang X, Li X, Allakhverdiev S (2018) Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth Res 136(2):245–255. https://doi.org/10.1007/s11120-018-0486-z
Cheikh N, Jones RJ (1994) Disruption of maize kernel growth and development by heat stress (role of cytokinin/abscisic acid balance). Plant Physiol 106(1):45–51. https://doi.org/10.1104/pp.106.1.45
Chen YE, Cui JM, Li GX, Yuan M, Zhang ZW, Yuan S, Zhang HY (2016) Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol Plant 60:139–147. https://doi.org/10.1007/s10535-015-0564-4
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217(1):67–75. https://doi.org/10.1242/jeb.089938
Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2019) Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ 42:998–1018. https://doi.org/10.1111/pce.13494
Dobrá J, Černý M, Štorchová H, Dobrev P, Skalák J, Jedelský PL, Lukšanová H, Gaudinová A, Pešek B, Malbeck J, Vanek T, Brzobohatý B, Vanková R (2015) The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant Sci 231:52–61. https://doi.org/10.1016/j.plantsci.2014.11.005f
Escarnot E, Jacquemin J-M, Agneessens R, Paquot M (2012) Comparative study of the content and profiles of macronutrients in spelt and wheat, a review. Biotechnol Agron Soc Environ 16(2):243–256
Farkhutdinov RG, Kudoyarova GR, Veselov SY, Valke R (1997) Influence of temperature increase on evapotranspiration rate and cytokinin content in wheat seedlings. Biol Plant 39:289–291. https://doi.org/10.1023/A:1000627916005
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:491–507. https://doi.org/10.1080/07352689.2011.615687
Gantait S, Sinniah UR, Ali MN, Sahu NC (2015) Gibberellins—a multifaceted hormone in plant growth regulatory network. Curr Protein Pept Sci 16:406–412. https://doi.org/10.2174/1389203716666150330125439
Gupta NK, Gupta S, Shukla DS, Deshmukh PS (2003) Differential responses of BA injection on yield and specific grain growth in contrasting genotypes of wheat (Triticum aestivum L.). Plant Growth Regul 40:201–205. https://doi.org/10.1023/A:1025023822806
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179. https://doi.org/10.1016/j.tplants.2011.12.005
Hamayun M, Khan SA, Shinwari ZK, Khan AL (2010) Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pak J Bot 42(2):977–986
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K (2018) Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int J Mol Sci 19:4045. https://doi.org/10.3390/ijms19124045
Hosseini SM, Poustini K, Ahmadi A (2008) Effects of foliar application of BAP on source and sink strength in four six-rowed barley (Hordeum vulgare L.) cultivars. Plant Growth Regul 54:231–239. https://doi.org/10.1007/s10725-007-9245-4
Janda M, Ruelland E (2015) Magical mystery tour: salicylic acid signaling. Environ Exp Bot 114:117–128. https://doi.org/10.1016/j.envexpbot.2014.07.003
Janda T, Khalil R, Tajti J, Pal M, Darko E (2019) Responses of young wheat plants to moderate heat stress. Acta Physiol Plant 41:137. https://doi.org/10.1007/s11738-019-2930-x
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2015) Salicylic acid in plant salinity stress signalling and tolerance. J Plant Growth Regul 75:25–40. https://doi.org/10.1007/s10725-015-0028-z
Kang GZ, Li G, Guo T (2014) Molecular mechanism of salicylic acid induced abiotic stress tolerance in higher plants. Acta Physiol Plant 36:2287–2297. https://doi.org/10.1007/s11738-014-1603-z
Khan MI, Iqbal N, Masood A, Per TS, Khan AN (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav 8(11):e26374. https://doi.org/10.4161/psb.26374
Khan MI, Fatma M, Per TS et al (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462. https://doi.org/10.3389/fpls.2015.00462
Kieber JJ, Schaller GE (2018) Cytokinin signaling in plant development. Development 145:dev149344. https://doi.org/10.1242/dev.149344
Kosakivska IV, Voytenko LV, Likhnyovskiy RV (2016) Peculiarities of cytokinin accumulation and distribution in Triticum aestivum L. seedlings under temperature stresses. J Stress Physiol Biochem 12(2):32–38
Kosakivska IV, Voytenko LV, Vasyuk VA, Shcherbatiuk MM (2019) Effect of zinc on growth and phytohormones accumulation in Triticum aestivum L. priming with abscisic acid. Dopov Nac Akad Nauk Ukr 11:93–99. https://doi.org/10.15407/dopovidi2019.11.093
Kosakivska IV, Shcherbatiuk MM, Voytenko LV (2020) Profiling of hormones in plant tissues: history, modern approaches, use in biotechnology. Biotechnol Acta 13(4):14–25. https://doi.org/10.15407/biotech13.04.014
Kumar D (2014) Salicylic acid signaling in disease resistance. Plant Sci 228:127–134. https://doi.org/10.1016/j.plantsci.2014.04.014
Lacko-Bartošová M, Korczyk-Szabó J, Ražný R (2010) Triticum spelta—a specialty grain for ecological farming systems. Res J Agric Sci 42(1):143–147
Larkindale J, Huang B (2004) Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J Plant Physiol 161(4):405–413. https://doi.org/10.1078/0176-1617-01239
Li H, Torres-Garcia J, Latrasse D, Benhamed M, Schilderink S, Zhou W, Kulikova O, Hirt Y, Bisseling T (2017) Plant-specific histone deacetylases HDT1/2 regulate GIBBERELLIN 2-OXIDASE2 expression to control Arabidopsis root meristem cell number. Plant Cell 29(9):2183–2196. https://doi.org/10.1105/tpc.17.00366
Lynch JP, Chimungu JG, Brown KM (2014) Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J Exp Bot 65:6155–6166. https://doi.org/10.1093/jxb/eru162
Minguet GE, Alabadi D, Blázquez MA (2014) Gibberellin implication in plant growth and stress responses. In: Tran L-SP, Pal S (eds) Phytohormones: a window to metabolism, signaling and biotechnological application. Springer, New York, pp 119–161. https://doi.org/10.1007/978-1-4939-0491-4_5
Morgun VV, Dubrovna OV, Morgun BV (2016) Modern biotechnologies of obtaining stress-resistant wheat plants. Fiziol Rast Genet 48(3):196–214. https://doi.org/10.15407/frg2016.03.196
Nadolska-Orczyk A, Rajchel IK, Orczyk W, Gasparis S (2017) Major genes determining yield-related traits in wheat and barley. Theor Appl Genet 130:1081–1098. https://doi.org/10.1007/s00122-017-2880-x
Narayanan S (2018) Effects of high temperature stress and traits associated with tolerance in wheat. J Sci 2(3):177–186. https://doi.org/10.15406/oajs.2018.02.00067
Prerostova S, Dobrev PI, Kramna B, Gaudinova A, Knirsch V, Spichal L, Zatloukal M, Vankova R (2020) Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of Arabidopsis. Front Plant Sci 11:87. https://doi.org/10.3389/fpls.2020.00087
Sawada H, Shim I, Usui K (2006) Induction of benzoicacid-2-hydroxylase and salicylic acid biosynthesis: Modulation by salt stress in rice seedlings. Plant Sci 171:263–270. https://doi.org/10.1016/j.plantsci.2006.03.020
Sponsel VM, Hedden P (2010) Gibberellin biosynthesis and inactivation. In: Davies PJ (ed) Plant hormones. Springer, Dordrecht, pp 63–94. https://doi.org/10.1007/978-1-4020-2686-7_4
Todorova D, Genkov T, Vaseva-Gemisheva I, Alexieva V, Karanov E, Smith A, Hall M (2005) Effect of temperature stress on the endogenous cytokinin content in Arabidopsis thaliana (L.) Heynh plants. Acta Physiol Plant 27:13–18. https://doi.org/10.1007/s11738-005-0031-5
Van Emden H (2008) Statistics for terrified biologists. Wiley-Blackwell, Oxford
Veselov DS, Kudoyarova GR, Kudryakova NV, Kusnetsov VV (2017) Role of cytokinins in stress resistance of plants. Russ J Plant Physiol 64:15–27. https://doi.org/10.1134/S1021443717010162
Wang X, Cai J, Jiang D, Liu F, Dai T, Cao W (2011) Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J Plant Physiol 168:585–593. https://doi.org/10.1016/j.jplph.2010.09.016
Wang HQ, Liu P, Zhang JW, Zhao B, Ren BZ (2020) Endogenous hormones inhibit differentiation of young ears in maize (Zea mays L.) under heat stress. Front Plant Sci 11:533046. https://doi.org/10.3389/fpls.2020.533046
Wu C, Cui K, Wang W, Li Q, Fahad S, Hu Q, Huang J, Nie L, Mohapatra PK, Peng S (2017) Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front Plant Sci 8:371. https://doi.org/10.3389/fpls.2017.00371
Zhan A, Schneider H, Lynch J (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168:1603–1615. https://doi.org/10.1104/pp.15.00187
Acknowledgements
The publication contains the results of research conducted within the project funded by the National Academy of Sciences of Ukraine № III-82-17.454 “Phytohormonal system of new genotypes of T. aestivum L. and its wild precursors under the action of extreme climatic factors” (2017–2021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kosakivska, I.V., Vasyuk, V.A., Voytenko, L.V. et al. Changes in hormonal status of winter wheat (Triticum aestivum L.) and spelt wheat (Triticum spelta L.) after heat stress and in recovery period. CEREAL RESEARCH COMMUNICATIONS 50, 821–830 (2022). https://doi.org/10.1007/s42976-021-00206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-021-00206-5