Abstract
Land use has transformed landscapes, altered water and soil physical–chemical parameters, reduced habitat availability, and limited species occurrence. Here, we investigated the contribution of sites (local contribution to beta diversity—LCBD) and species (species contribution to beta diversity—SCBD) to macrophyte total β-diversity in streams inserted in a gradient of land use. We also investigated which life forms are important to SCBD and which environmental parameters are related to the change in the species composition. Sampling took place in 17 streams located in Paragominas, Pará, Brazil in September 2017. We recorded 36 species and four life forms. We identified five sites with high LCBD. The species with the four highest SCBD scores belong to the amphibious life form. CDI (Catchment Disturbance Index) and canopy cover, variables that show land use degrees, drove the distribution of macrophyte species in the land use gradient. CDI presented a positive relationship with LCBD, whereas canopy cover presented a negative relationship, i.e., a greater composition of unique species and greater diversity of macrophytes life forms were found in more altered streams than in preserved ones, due to canopy openness. Nonetheless, we emphasize that although the environmental characteristics of altered streams favored the establishment of more macrophytes species, the species found could be generalists and the pattern for other types of environments is usually the opposite. Therefore, studies focusing on temporal patterns will be important for this area to understand how the macrophyte community will stabilize. This study brings important contributions to elucidate the effects of land use on macrophytes distribution and the role played by different life forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities have caused profound shifts in natural landscapes around the world for many decades (Guida-Johnson & Zuleta, 2013; Morrison et al. 2020; Vitousek et al. 1997). However, land use intensification is occurring more dramatically in some unexplored regions, such as the Amazon Forest (FAO, 2011; Gardner et al. 2013), which leads to high deforestation rates (Hansen et al. 2010; Nobre et al. 2016). The high rates of deforestation and environmental degradation in Amazon territories are linked to economic activities such as mining, logging, pasture, and agriculture (Asner et al. 2013; Gardner et al. 2013) that greatly impact the ecosystem functioning (Felipe-Lucia et al. 2020; Wang et al. 2022). Land use is expected to reduce the availability of natural habitats, modify the communities’ structure, and alter natural processes such as primary productivity, pollination, and decomposition (Felipe-Lucia et al. 2020; Guida-Johnson & Zuleta, 2013; Johnson & Angeler, 2014; Sonter et al. 2018).
Land use affects not only terrestrial environments but also lakes, rivers, and streams (Allan, 2004; Castello & Macedo, 2016). Streams are an important part of the landscape when considering the drainage basin, connecting environments, and providing species to the regional pool (Besemer, 2015; Finn et al. 2011). Changes on the ground around streams are first observed to alter the water’s physical–chemical parameters, such as the input of nutrients, turbidity, light incidence, pH, and oxygen availability. As a consequence of that, it is observed changes in species composition and ecosystem services offered by those environments (Allan, 2004; Casotti et al. 2015; de Paiva et al. 2021; Heartsill-Scalley & Aide, 2003; Leão et al. 2020).
Different levels of land use create a heterogeneous landscape that alters stream functioning and species establishment (de Paiva et al. 2021; Fares et al. 2020). An important characteristic, when analyzing this issue, is the riparian vegetation around the channel, as it controls erosion, the input of sediments, and light incidence (due to canopy cover), among others (Castello & Macedo, 2016; Johnson & Angeler, 2014; Naiman et al. 2005; Riis et al. 2020). Changes in all mentioned parameters act as environmental filters and select the species that settle in those environments, depending if they are sensitive or resistant to that environmental degradation (Akasaka et al. 2010; Casotti et al. 2015; Johnson & Angeler, 2014). These environmental filters created by land use can reduce species richness, leading to changes in species composition among sites. This pattern was already reported for ETP (Ephemeroptera, Plecoptera, Trichoptera) (Ligeiro et al. 2013), semiaquatic bugs (Heteroptera) (Cunha et al. 2022), zooplankton (Gomes et al. 2020), and fish (Leão et al. 2020) in streams affected by land use. In this context of land use affecting communities’ structure, beta diversity can be used to identify streams and species that need more attention when elaborating conservation strategies (Heino et al. 2015), especially in the Amazon region that is suffering from these intense activities.
Beta diversity is defined as the variability in species composition among sampling units for a given area (Anderson et al. 2006). An approach proposed by Legendre and de Cáceres (2013) decomposes this total variation in the community (total β-diversity) into the Local Contribution to Beta Diversity (LCBD) and the Species Contribution to Beta Diversity (SCBD). Values of LCBD represent the degree of ecological uniqueness of a specific site sampled in comparison with all others and shows strong differences in species compositions (i.e., high uniqueness of species composition). According to Legendre and de Cáceres (2013), large LCBD values may indicate sites that have unusual species combinations and high conservation value or degraded and species-poor sites in need of ecological restoration, whereas SCBD represents the relative contribution of each species to the observed patterns of β-diversity and can be related to the intrinsic characteristics of each species (Leão et al. 2020; Pozzobom et al. 2020). These metrics reflect the response of species to environmental filters (Heino, 2009) and their spatial distribution (Rocha et al. 2018), which can help to prioritize areas and species for conservation, such as in areas with intensive land use.
Aquatic macrophytes, for example, can respond to a land use gradient with an increase in β-diversity. This occurs when the reduction in riparian vegetation in altered sites leads to high light incidence and nutrients availability, which favor the occurrence of tolerant and opportunistic macrophyte species such as amphibious and emergent life forms (Akasaka et al. 2010; Kolada, 2010; Quinn et al. 2011), including exotic species, whereas submerged and free-floating life forms are usually found in more preserved environments (Fares et al. 2020). Thus, the response of macrophytes to a land use gradient could be positive as light and nutrients induce macrophytes growth and increase species richness (Elo et al. 2018), or negative if some species dominate the community (Akasaka et al. 2010). Negative responses to community structure could also come from a high abundance of macrophyte exotic species as they are favored by anthropogenic disturbances (Mackay et al. 2010; Quinn et al. 2011). Heino et al. (2009) showed that macrophyte β-diversity was higher in preserved streams compared to the ones impacted by forestry. Johnson and Angeler (2014) found high macrophytes β-diversity along agricultural landscapes (impacted sites), implying that the diversity of resistant taxa is high, while there is a loss of sensitive taxa due to disturbance, whereas a global variation in macrophytes β-diversity in lakes was driven by environmental heterogeneity (Alahuhta et al. 2017).
Thus, macrophytes can be used as a tool to analyze the integrity of different water bodies, including those degraded by land use (Alderton et al. 2017; Jiang et al. 2018). Moreover, macrophytes play an important role in ecosystems functioning by filtering nutrients and elements from the water (Jiang et al. 2018; Thomaz, 2021) and supporting the development of other communities such as macroinvertebrates (Brito et al. 2021; Nicolet et al. 2004), zooplankton (Deosti et al. 2021), periphytic algae, and birds (Bilton et al. 2006; Scheffer, 2004). Despite their great contribution, there is still a lack of information about macrophytes in streams, especially considering land use gradients.
In this way, here, (i) we identify which sites (LCBD) and macrophyte species (SCBD) that contribute to total β-diversity in streams inserted in a gradient of land use (primary vegetation, secondary vegetation, pasture, and bare soil); (ii) evaluate the environmental parameters related to macrophytes distribution in those environments; (iii) related the different degrees of land use with LCBD to identify priority areas for stream conservation, and (iv) identify which life forms contribute the most to SCBD. We expect that variables related to degraded streams such as high canopy openness, low oxygen levels, high temperature, and high catchment disturbance, among others will be positively related to high uniqueness of species composition (LCBD) because these variables favor the establishment of different macrophytes life forms, which will increase the dissimilarity in species composition between sites. Also, amphibious species will be important for SCBD values because this life form includes both tolerant and shading species that are present in altered and preserved sites, respectively, along the land use gradient leading to a high contribution to β-diversity.
Methods
Study area
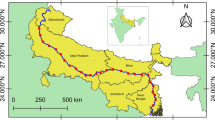
Sampling took place in 17 streams located in Paragominas, Pará, Brazil, which belong to Capim River Basin (Fig. 1, for more information about each site, please see online resource Table S1). The climate in this region is characterized as humid and hot, with a mean annual temperature of 26 °C, mean air humidity of 81%, and mean annual precipitation of 1800 mm (Pinto et al. 2009). Paragominas is inside the world’s largest remaining tropical forest, the Amazon, its natural vegetation is typical of tropical rainforest. However, several human activities take place in its territories, such as agriculture, livestock, logging, and mining (Gardner et al. 2013). More than 45% of Paragominas territory are deforested and highly degraded areas (878,000 hectares) due to economic activities (Pinto et al. 2009). Livestock, for example, occupies 80% of the open areas, familiar agriculture 14.5%, grain cultivation (rice, corn, and soybeans) 4.5%, and 1% not identified. Mining activities are mainly about bauxite and in minor proportion aluminum, kaolin, and silver (Pinto et al. 2009).
Map showing the 17 sampling sites in the municipality of Paragominas, State of Pará, Brazil. The circle sizes represent the LCBD values, as big is the circle as high is LCBD value. The colors represent the CDI values, green are low numbers (i.e., more preserved streams, bolder green represent the most preserved streams), orange are high values (i.e., more impacted streams, bolder orange represent the most impacted streams)
Sampling design
The macrophyte community was sampled in July 2017. Abundance-based composition data were obtained with a 1 m2 (1 m × 1 m) quadrat. The quadrat was randomly placed in two macrophyte stands found within a transect of 150 m in each sampling site (i.e., stream). The percentage of cover, i.e., occupancy (%) was assigned to each species inside the quadrat and used as a surrogate for macrophyte abundance-based composition, for example, if one species covered 45% of the quadrat area and another covered 12% these were their respective abundances. The quadrat considers macrophytes that occupy the above and under water area. The mean cover of stream was calculated by the sum of the cover of each species divided by the number of quadrats (in this case, two) (Mackay et al. 2010). Species richness was obtained by taking notes of all macrophyte species occurring in a 150 m transect of each aquatic ecosystem. Macrophytes were first identified in the field, the non-identified material was collected and later identified using specialized literature (Amaral et al. 2008; Lorenzi, 2008; Pott & Pott, 2000). Life forms were categorized into four groups: submerged, emergent, amphibious, and floating-leaved, following Esteves (2011) and Pott and Pott (2000). For more details about sampling and identification, please see Fares et al. (2020).
Water physical–chemical parameters were measured using a multiparameter probe (Horiba U-50) and consisted of pH, temperature (°C), turbidity (NTU), conductivity (µS/cm), and dissolved oxygen (mg/L). We also measured canopy cover above the quadrats using a densitometer, which we later converted to a percentage, according to the index proposed by Peck et al. (2006).
Land use and land cover characterization
To better summarize the effects of different land use degrees on macrophytes β-diversity, we calculated the disturbance inside a 300 m radius of land surrounding each stream (an adaptation of the Catchment Disturbance Index—CDI) for each sampling site (de Paiva et al. 2021; Ligeiro et al. 2013). For that, we used data from Fares et al. (2020) about remote sensing and land use cover that was obtained through different geoprocessing software (ArcGIS, PCI, Geomatica, and Ecognition) with atmospheric correction, the images used were from 2015. Please check all details about the methodology applied to obtain the data in Fares et al. (2020). With the mentioned data, four land use and land cover classifications were established: (a) primary vegetation that are areas occupied by tropical rainforest; (b) secondary vegetation, vegetation originated by natural succession process after total or partial primary vegetation suppression after natural or anthropogenic processes; (c) pastures, areas that are occupied by intensive and/or extensive livestock breeding; and (d) bare soil, areas of unprotected soil, especially those containing road systems such as dirt roads, highways, and mining. Thus, we weighted the different types of land use depending on the degree of anthropic change in the natural environmental conditions (de Paiva et al. 2021), and areas of mining and bare soil were weighted more than pasture, which was, in turn, weighted more than degraded forest. The index was divided by 300 to standardize 75% of its maximum value (Ligeiro et al. 2013) (CDI = 4 x % bauxite mining and bare soil + 2 x % pasture + 1 x % degraded forest /300). In this index, lower CDI values reflect more preserved sites. In the worst scenario, an extremely degraded stream would present a land use of 100% of bauxite mining and bare soil, which would lead to a CDI of 1.33 (the maximum value).
Data analysis
The local contribution to β-diversity (LCBD) and the species contribution to β-diversity (SCBD) were calculated following Legendre and de Cáceres (2013), using macrophytes abundance-composition data. For that, we applied Hellinger-transformation and ‘beta.div’ function from “adespatial” package in R program (Dray et al. 2022). We performed 999 permutations to obtain the total sum of squares (SStotal) from which is calculated total β-diversity (BDtotal), LCBD, and SCBD. We also applied adjusted-p using ‘Holm’ method to correct multiple comparisons on LCBD. High values of LCBD and SCBD indicate the local and species (respectively) that most contribute to total beta diversity.
To analyze which environmental parameters are related to the occurrence of macrophytes species, we performed a Nonmetric Multidimensional Scaling (NMDS; ‘metaMDS’ function) applying ‘envfit’ function. In the NMDS, the abundance of each macrophyte species is transformed into distances through “Bray–curtis” method and plotted in an ordination. The function ‘envfit’ correlates the environmental parameters (canopy cover, pH, conductivity, turbidity, dissolved oxygen, temperature, and CDI) with species occurrence. Before running NMDS and correlations, we standardized the environmental parameters through ‘decostand’ function and tested if they were correlated using variance inflation factors (VIF) with ‘vif.cca’ function. The variables presented no correlation (VIF < 10), and values are shown in the online resource Table S2. All functions are from “vegan” package (Oksanen et al. 2019).
To analyze how LCBD is influenced by land use variables, we performed linear regressions using ‘lm’ function from “stats” package. We ran regression models testing the variables that were significant and marginally significant in the correlations from envfit (canopy cover, CDI, temperature, and conductivity). Canopy cover and CDI composed the best model tested by Akaike’s Information Criterion (AIC). AIC was tested using ‘AIC’ function from “stats” package, the criterion used was the smaller the AIC, the better the fit (usually used when comparing models fitted by maximum likelihood to the same data). AIC and models tested are shown in the online resource Table S3. We also controlled a possible spatial effect in the regression models. The spatial factor was built through Moran’s eigenvector maps based on distance. Eigenvectors are calculated from a distance matrix based on latitude and longitude (Borcard & Legendre, 2002; Dray et al. 2006), only positive eigenvectors were selected as spatial proxies (Borcard & Legendre, 2002). For that, we used ‘dbmem’ function from “adespatial” package followed by ‘moran.randtest’ function with 999 permutations to find significant eigenvectors. Three eigenvectors were significant from the permutations and used as predictors in the regression models, and they represent broad spatial structures (regional filters). Also, to further understand LCBD values in the gradient of land use, we performed a linear regression using species richness as predictor in the ‘lm’ function.
Finally, we created a scatterplot using the SCBD values and the number of sites occupied by each species, and their life forms, in order to identify which life form contributes the most to SCBD. All graphics were performed using “ggplot2” package and ‘ggplot’ function. All analyses were performed in R version 4.0.2 an R Studio version 1.3.1093 (R Core Team, 2020).
Results
Description of the community structure
We recorded 36 species, divided into 23 families and four life forms (Table 1). The BDtotal was 0.75 and SStotal was 11.99. Values of Local Contribution to Beta Diversity (LCBD) ranged from 0.093 (P12) to 0.037 (P16) and five points were significant: P1 (LCBD: 0.076, adj-p: 0.026), P2 (LCBD: 0.083, adj-p: 0.017), P3 (LCBD: 0.081, adj-p: 0.017), P8 (LCBD: 0.087, adj-p: 0.017), and P12 (LCBD: 0.093, adj-p: 0.017) (all LCBD values are shown in the online resource Table S4).
Values of SCBD ranged from 0.001 to 0.114, with average of 0.028. The five species that most contributed were Calyptrocarya glomerulata (0.114), Triplophyllum dicksonioides (0.076), Scleria microcarpa (0.073), Adiantum humile (0.065), and Utricularia sp. (0.064) (Table 1). Most species found in the study belonged to the amphibious life form (23 species), followed by emergent (eight species). The four species with the highest SCBD scores all belong to the amphibious life form (Table 1), but they varied in sites occupied: C. glomerulata, for example, presented the highest SCBD and the highest occurrence (13 sites), T. dicksonioides and A. humile presented an occurrence of seven and six sites, respectively, while S. microcarpa occurred in only three sites (Fig. 2). The submerged macrophyte Utricularia sp occurred in four sites (Fig. 2).
Relationship between environmental and land use variables, species occurrence, and LCBD
Of all environmental parameters (canopy cover, pH, conductivity, turbidity, dissolved oxygen, temperature, and CDI), only canopy cover and CDI were significantly related to the occurrence of macrophytes species in the streams (Table 2; Fig. 3). CDI had great variation among sites, the most impacted site had a CDI of 0.75, while canopy cover had a low range (from 64.9 to 97.06%; Table 2).
NMDS ordination showing the distribution of the sampling sites and species and their correlation with environmental variables. CDI = Catchment Disturbance Index. Bold variables with red arrows are significant correlations (p < 0.05). Sites with blue labels indicate the ones with the highest LCBD values. Species named in the figure have the highest SCBD values
Macrophyte LCBD was positively related to CDI index (p = 0.045) and negatively related to canopy cover (p = 0.006) (Table 3; Fig. 4a, b). Spatial component also had an effect on macrophyte LCBD (p = 0.013). The model presented an adjusted-R2 of 0.73, F: 8.18, and p value: 0.002. LCBD was also positively related to macrophyte species richness (adjusted-R2: 0.18, F: 4.56, and p value: 0.039; Fig. 4c).
Discussion
Investigating local and species contribution to beta diversity of macrophytes (LCBD and SCBD) helps to better understand the effects of land use on aquatic ecosystems. Here, we found that canopy cover and CDI (variables that represent land use degrees) drove the occurrence of macrophyte species in the land use gradient, as expected. CDI presented a positive relationship with LCBD, whereas canopy cover presented a negative relationship. This means that higher ecological uniqueness of species composition was observed in more degraded sites with greater canopy openness and greater species richness. And as expected, amphibious species (A. humile, C. glomerulata, T. dicksonioides, and S. microcarpa) contributed the most to SCBD.
For LCBD, we observed the highest values in more impacted streams, which also presented greater canopy openness, this means that some species considered unique were only favored in those environments. Streams under the effect of land use usually present high light incidence (Allan, 2004; Casotti et al. 2015) a key factor supporting aquatic plants growth (Bleich et al. 2015; Elo et al. 2018). Hoyer et al. (2004) reported that little or no aquatic vegetation can develop where the substrate receives less than 10% of light incidence. In this way, although it is expected altered environments to have lower species diversity than preserved ones (de Paiva et al. 2021; Montag et al. 2019), the pattern for macrophytes in streams might be different (Fares et al. 2020; Hoyer et al. 2004; Kuhar et al. 2007) because of their great light dependency and the characteristics of preserved streams (a well-preserved riparian forest and consequently low light incidence on the channel). The same pattern was observed by Schneck et al. (2022) for diatoms that are also high light-dependent and for insects. High LCBD values can be related to sites in both extremes, with great species richness and unique species composition, and to sites with low species richness and poor species composition (Legendre & de Cáceres, 2013). In our study, high values of LCBD were related to sites with greater species richness, showing that a unique composition of macrophytes was favored by environmental factors present in altered environments, which contributed to high values of total beta diversity. These streams are important to maintain macrophytes diversity on a regional scale and should be taken into consideration when elaborating conservation strategies for this region. An important point to be mentioned is that it was not observed extreme values of degradation (CDI reaching 1.33) and very degraded streams could lead to a more homogeneous community with only tolerant species.
Regarding the species contribution to beta diversity, amphibious species were the most representative. Amphibious life forms are usually present in altered environments, where the ecological succession is starting and there are high light incidence and nutrients availability (Akasaka et al. 2010; Kolada, 2010; Kuhar et al. 2007; Quinn et al. 2011). However, in our study, amphibious macrophytes represented also more preserved environments that have greater canopy cover (such as P11—following the CDI index). This life form is also resistant to more shaded and humid environments (Drucker et al. 2008; Paixão et al. 2013), which can explain their selection on SCBD (with the highest values). They may have an advantage in these streams as other macrophyte life forms might not resist under great canopy cover (Kuhar et al. 2007). T. dicksonioides, for example, was recorded on water bodies with great riparian vegetation and canopy cover (Fares et al. 2020) that provides a microclimate suitable for the development of ferns such as this species (Mackay et al. 2010). Thus, shade-tolerant species (such as some amphibious species) can represent environments with great canopy cover, i.e., more preserved streams (Fares et al. 2020) instead of altered as first thought (Akasaka et al. 2010; Kolada, 2010; Kuhar et al. 2007; Quinn et al. 2011). However, this could also mean that environments with high light incidence and greater macrophytes richness are not suitable for this amphibious species, because it cannot compete with those species established there.
Amphibious was also the richest life form in the study and presented the highest number of occurrences. The pattern found here was different from Pozzobom et al. (2020), which, despite not using the term amphibious in their study, found a relationship between higher values of SCBD and intermediate occupancy of floating macrophytes. However, Pozzobom et al. (2020) studied lakes from Pantanal wetlands that present a very different dynamic compared to Amazon streams, these lakes are naturally less shaded still-waters and suffer with hydrological stress (flooding/drought phases) throughout the year, which lead to a constant turnover of species and life forms in the community (Catian et al. 2018). The studied streams, on the other hand, are shaded by the riparian vegetation and have two hydrological phases: dry and rainy season. Furthermore, the lack of clearer relationships between SCBD and occurrence of life forms, especially floating and submerged, is possible due to the few numbers of species representing these life forms and the high number of ‘rare’ species we observed.
Alterations promoted by land use create a heterogeneous landscape that can increase species dissimilarity among sites, i.e., beta diversity (Heino et al. 2015). Most studies report that although species dissimilarity is increasing in the gradient from preserved to altered environments, the macrophyte species that are in altered sites are indicators of land use change and belong to emergent life form (Alahuhta et al. 2014), which may lead to a more homogeneous community (Akasaka et al. 2010; Fares et al. 2020). This is because emergent macrophytes are considered resistant and opportunistic species (Akasaka et al. 2010). However, we observed greater species richness in more altered streams, a different pattern than reported in other studies for lakes and ponds that observed greater species richness in preserved sites (Akasaka et al. 2010; Alahuhta et al. 2014), but in concordance with what is expected for stream systems (Hoyer et al. 2004; Kuhar et al. 2007). Variables related to the channel morphology and the stream bottoms (not analyzed here) could be playing a role in the establishment of macrophytes species in streams (Bunn & Arthington, 2002; Kuhar et al. 2007), where more elevated grounds (preserved streams) favor only the presence of emergent and amphibious life forms. The width of the channel is another factor that we did not analyze but is positively related to canopy openness and doing so could play a role in macrophyte establishment as it allows greater light incidence in the channel.
Macrophytes support the development of several communities. Amphibious and emergent life forms that live in the water-land interface create micro-habitats and serve as food for insects and birds (Bilton et al. 2006; Scheffer, 2004), whereas rooted-submergent and free-submergent support aquatic communities (periphyton, zooplankton, and fish) also offering refuge, food, and shelter (Deosti et al. 2021; Quirino et al. 2021). Thus, macrophytes can have an even greater role in impacted streams, reducing the effects of land use and offering a more suitable environment for species to settle, grow, and develop (Thomaz, 2021). If macrophytes were not in those environments, the impacts of land use on biodiversity could be worse. Besides that, two other points need to be considered, first, natural riparian vegetation plays a great role in natural ecosystems supporting species adapted to shaded environments, providing resources to several organisms (de Paiva et al. 2021; Montag et al. 2019), and offering long-term services, such as water purification (Bunn & Arthington, 2002) and protection against invasive macrophyte species. Secondly, the pattern we observed is on a spatial scale, other studies with a temporal approach are needed to better identify the consequences of land use on macrophytes establishment, as the increase in the diversity of morphological forms may change with time and resistant forms became dominant leading to a more homogeneous community.
Conclusion
We found greater uniqueness of species composition and species richness in more altered streams than in preserved ones, due to canopy openness, and amphibious macrophytes had the greatest contribution to total beta diversity in streams. Therefore, in our study, the streams under greater disturbance were the ones that most contributed to beta diversity and should be considered when elaborating conservation strategies in the region, as they can support greater species richness and macrophytes diversity contributing to increase the regional diversity of macrophytes. However, to better understand these patterns, other biological groups should be studied in these environments to further support the decision-makers.
Our results bring an important contribution to understand the effect of land use on macrophytes distribution and the role played by different life forms. Nonetheless, we emphasize that although the environmental characteristics of altered streams favored the establishment of more macrophytes species, the species found could be generalist and the pattern for other types of environments is the opposite (Akasaka et al. 2010; Alahuhta et al. 2014). Also, long-term studies could show an inverse pattern with a more homogeneous community through the years. Finally, more environmental variables should be taken into account to better understand the pattern observed and studies focusing on temporal patterns will be important for this area to understand how the macrophyte community will stabilize.
References
Akasaka, M., Takamura, N., Mitsuhashi, H., & Kadono, Y. (2010). Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshwater Biology, 55, 909–922. https://doi.org/10.1111/j.1365-2427.2009.02334.x
Alahuhta, J., Johnson, L. B., Olker, J., & Heino, J. (2014). Species sorting determines variation in the community composition of common and rare macrophytes at various spatial extents. Ecological Complexity, 20, 61–68. https://doi.org/10.1016/j.ecocom.2014.08.003
Alahuhta, J., Kosten, S., Akasaka, M., et al. (2017). Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. Journal of Biogeography, 44, 1758–1769. https://doi.org/10.1111/jbi.12978
Alderton, E., Sayer, C. D., Davies, R., et al. (2017). Buried alive: Aquatic plants survive in ‘ghost ponds’ under agricultural fields. Biological Conservation, 212, 105–110. https://doi.org/10.1016/j.biocon.2017.06.004
Allan, J. D. (2004). Influence of land use and landscape setting on the ecological status of rivers. Limnetica, 23, 187–198. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Amaral, M. C. E., Bittrich, V., Faria, A. D., et al. (2008). Guia de Campo para Plantas Aquáticas e Palustres do Estado de São Paulo, 1st edn. Holos, Editora, Ribeirão Preto.
Anderson, M. J., Ellingsen, K. E., & McArdle, B. H. (2006). Multivariate dispersion as a measure of beta diversity. Ecology Letters, 9, 683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Asner, G. P., Llactayo, W., Tupayachi, R., & Luna, E. R. (2013). Elevated rates of gold mining in the Amazon revealed through high-resolution monitoring. Proceedings of the National Academy of Sciences of the United States of America, 110, 18454–18459. https://doi.org/10.1073/pnas.1318271110
Besemer, K. (2015). Biodiversity, community structure and function of biofilms in stream ecosystems. Research in Microbiology, 166, 774–781. https://doi.org/10.1016/j.resmic.2015.05.006
Bilton, D. T., Mcabendroth, L., Bedford, A., & Ramsay, P. M. (2006). How wide to cast the net? Cross-taxon congruence of species richness, community similarity and indicator taxa in ponds. Freshwater Biology, 51, 578–590. https://doi.org/10.1111/j.1365-2427.2006.01505.x
Bleich, M. E., Piedade, M. T. F., Mortati, A. F., & André, T. (2015). Autochthonous primary production in southern Amazon headwater streams: Novel indicators of altered environmental integrity. Ecological Indicators, 53, 154–161. https://doi.org/10.1016/j.ecolind.2015.01.040
Borcard, D., Legendre, P., (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices.
Brito, J. S., Michelan, T. S., & Juen, L. (2021). Aquatic macrophytes are important substrates for Libellulidae (Odonata) larvae and adults. Limnology (tokyo), 22, 139–149. https://doi.org/10.1007/s10201-020-00643-x
Bunn, S. E., & Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management, 30, 492–507.
Casotti, C. G., Kiffer, W. P., Costa, L. C., et al. (2015). Assessing the importance of riparian zones conservation for leaf decomposition in streams. Natureza e Conservacao, 13, 178–182. https://doi.org/10.1016/j.ncon.2015.11.011
Castello, L., & Macedo, M. N. (2016). Large-scale degradation of Amazonian freshwater ecosystems. Global Change Biology, 22, 990–1007. https://doi.org/10.1111/gcb.13173
Catian, G., da Silva, D. M., Súarez, Y. R., & Scremin-Dias, E. (2018). Effects of flood pulse dynamics on functional diversity of macrophyte communities in the Pantanal Wetland. Wetlands, 38, 975–991. https://doi.org/10.1007/s13157-018-1050-5
Cunha, E. J., Cruz, G. M., Faria, A. P. J., et al. (2022). Urban development and industrialization impacts on semiaquatic bugs diversity: A case study in eastern Amazonian streams. Water Biology and Security. https://doi.org/10.1016/j.watbs.2022.100061
de Esteves, F. A., (2011) Fundamentos de Limnologia, 3rd edn. Interciência, Rio de Janeiro.
de Paiva, C. K. S., Faria, A. P. J., Calvão, L. B., & Juen, L. (2021). The anthropic gradient determines the taxonomic diversity of aquatic insects in Amazonian streams. Hydrobiologia, 848, 1073–1085. https://doi.org/10.1007/s10750-021-04515-y
Deosti, S., de Fátima, B. F., Lansac-Tôha, F. M., et al. (2021). Zooplankton taxonomic and functional structure is determined by macrophytes and fish predation in a Neotropical river. Hydrobiologia, 848, 1475–1490. https://doi.org/10.1007/s10750-021-04527-8
Dray, S., Legendre, P., & Peres-Neto, P. R. (2006). Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Modell, 196, 483–493. https://doi.org/10.1016/j.ecolmodel.2006.02.015
Drucker, D. P., Costa, F. R. C., & Magnusson, W. E. (2008). How wide is the riparian zone of small streams in tropical forests? A test with terrestrial herbs. Journal of Tropical Ecology, 24, 65–74. https://doi.org/10.1017/S0266467407004701
Elo, M., Alahuhta, J., Kanninen, A., et al. (2018). Environmental characteristics and anthropogenic impact jointly modify aquatic macrophyte species diversity. Frontiers in Plant Science, 9, 1–15. https://doi.org/10.3389/fpls.2018.01001
Dray, S., Bauman, D., Blanchet, G., et al. (2022). Adespatial: Multivariate multiscale spatial analysis. R package version 0.3–16. https://cran.r-project.org/package=adespatial.
FAO (2011) The state of forests in the Amazon Basin, Congo Basin and Southeast Asia. Food and Agriculture Organization of the United Nations, Rome.
Fares, A. L. B., Calvão, L. B., Torres, N. R., et al. (2020). Environmental factors affect macrophyte diversity on Amazonian aquatic ecosystems inserted in an anthropogenic landscape. Ecological Indicators. https://doi.org/10.1016/j.ecolind.2020.106231
Felipe-Lucia, M. R., Soliveres, S., Penone, C., et al. (2020). Land-use intensity alters networks between biodiversity, ecosystem functions, and services. Proceedings of the National Academy of Sciences, 117, 28140–28149. https://doi.org/10.1073/pnas.2016210117
Finn, D. S., Bonada, N., Múrria, C., & Hughes, J. M. (2011). Small but mighty: Headwaters are vital to stream network biodiversity at two levels of organization. Journal of the North American Benthological Society, 30, 963–980. https://doi.org/10.1899/11-012.1
Gardner, T. A., Ferreira, J., Barlow, J., et al. (2013). A social and ecological assessment of tropical land uses at multiple scales: The Sustainable Amazon Network. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120166. https://doi.org/10.1098/rstb.2012.0166
Gomes, A. C. A. M., Gomes, L. F., Roitman, I., et al. (2020). Forest cover influences zooplanktonic communities in Amazonian streams. Aquatic Ecology, 54, 1067–1078. https://doi.org/10.1007/s10452-020-09794-6
Guida-Johnson, B., & Zuleta, G. A. (2013). Land-use land-cover change and ecosystem loss in the Espinal ecoregion, Argentina. Agriculture, Ecosystems & Environment, 181, 31–40. https://doi.org/10.1016/j.agee.2013.09.002
Hansen, M. C., Stehman, S. V., & Potapov, P. V. (2010). Quantification of global gross forest cover loss. Proceedings of the National Academy of Sciences, 107, 8650–8655. https://doi.org/10.1073/pnas.0912668107
Heartsill-Scalley, T., Aide, T. M., (2003). Riparian vegetation and stream condition in a tropical agriculture-secondary forest mosaic. Ecological Applications, 13, 225–234. https://doi.org/10.1890/1051-0761(2003)013[0225:RVASCI]2.0.CO;2
Heino, J. (2009). Species co-occurrence, nestedness and guild-environment relationships in stream macroinvertebrates. Freshwater Biology, 54, 1947–1959. https://doi.org/10.1111/j.1365-2427.2009.02250.x
Heino, J., Ilmonen, J., Kotanen, J., et al. (2009). Surveying biodiversity in protected and managed areas: Algae, macrophytes and macroinvertebrates in boreal forest streams. Ecological Indicators, 9, 1179–1187. https://doi.org/10.1016/j.ecolind.2009.02.003
Heino, J., Melo, A. S., & Bini, L. M. (2015). Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshwater Biology, 60, 223–235. https://doi.org/10.1111/fwb.12502
Hoyer, M., Frazer, T., & Notestein, S. (2004). Vegetative characteristics of three low-lying Florida coastal rivers in relation to flow, light, salinity and nutrients. Hydrobiologia, 528, 31–43. https://doi.org/10.1007/s10750-004-1658-8
Jiang, B., Xing, Y., Zhang, B., et al. (2018). Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environmental Science and Pollution Research, 25, 31272–31282. https://doi.org/10.1007/s11356-018-3069-9
Johnson, R. K., & Angeler, D. G. (2014). Effects of agricultural land use on stream assemblages: Taxon-specific responses of alpha and beta diversity. Ecological Indicators, 45, 386–393. https://doi.org/10.1016/j.ecolind.2014.04.028
Kolada, A. (2010). The use of aquatic vegetation in lake assessment: Testing the sensitivity of macrophyte metrics to anthropogenic pressures and water quality. Hydrobiologia, 656, 133–147. https://doi.org/10.1007/s10750-010-0428-z
Kuhar, U., Gregorc, T., Renčelj, M., et al. (2007). Distribution of macrophytes and condition of the physical environment of streams flowing through agricultural landscape in north-eastern Slovenia. Limnologica, 37, 146–154. https://doi.org/10.1016/j.limno.2006.11.003
Leão, H., Siqueira, T., Torres, N. R., & de AssisMontag, L. F. (2020). Ecological uniqueness of fish communities from streams in modified landscapes of Eastern Amazonia. Ecological Indicators, 111, 106039. https://doi.org/10.1016/j.ecolind.2019.106039
Legendre, P., & de Cáceres, M. (2013). Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecology Letters, 16, 951–963. https://doi.org/10.1111/ele.12141
Ligeiro, R., Hughes, R. M., Kaufmann, P. R., et al. (2013). Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecological Indicators, 25, 45–57. https://doi.org/10.1016/j.ecolind.2012.09.004
Lorenzi, H., (2008). Plantas daninhas do Brasil: terrestres, aquática, parasitas e toxicas, 4th edn. Instituto Plantarum, Nova Odessa.
Mackay, S. J., James, C. S., & Arthington, A. H. (2010). Macrophytes as indicators of stream condition in the wet tropics region, Northern Queensland, Australia. Ecological Indicators, 10, 330–340. https://doi.org/10.1016/j.ecolind.2009.06.017
Montag, L. F. A., Winemiller, K. O., Keppeler, F. W., et al. (2019). Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecology of Freshwater Fish, 28, 317–329. https://doi.org/10.1111/eff.12455
Morrison, B. M. L., Brosi, B. J., & Dirzo, R. (2020). Agricultural intensification drives changes in hybrid network robustness by modifying network structure. Ecology Letters, 23, 359–369. https://doi.org/10.1111/ele.13440
Naiman, R. J., Décamps, H., McClain, M. E., Likens, G. E. (2005). Catchments and the Physical Template. In: Riparia. Elsevier, pp 19–48
Nicolet, P., Biggs, J., Fox, G., et al. (2004). The wetland plant and macroinvertebrate assemblages of temporary ponds in England and Wales. Biological Conservation, 120, 261–278. https://doi.org/10.1016/j.biocon.2004.03.010
Nobre, C. A., Sampaio, G., Borma, L. S., et al. (2016). Land-use and climate change risks in the amazon and the need of a novel sustainable development paradigm. Proceedings of the National Academy Science USA, 113, 10759–10768. https://doi.org/10.1073/pnas.1605516113
Oksanen, J., Blanchet, F. G., Friendly, M., et al. (2019). Vegan: Community Ecology Package.
Paixão, E. C., da Noronha, J., NunesdaCunha, C., & Arruda, R. (2013). More than light: Distance-dependent variation on riparian fern community in Southern Amazonia. Brazilian Journal of Botany, 36, 25–30. https://doi.org/10.1007/s40415-013-0003-8
Peck, D., Herlihy, A., Hill, B., et al. (2006). Environmental monitoring and assessment program-surface waters western pilot study: Field operations manual for wadeable streams, 1st edn. U.S. Environmental Protection Agency, Washington, D.C.
Pinto, A., Amaral, P., Souza-Jr C., et al. (2009). Diagnóstico Socioeconômico e Florestal do Município de Paragominas. Belém-PA.
Pott, V. J., Pott, A., (2000). Plantas Aquáticas do Pantanal, 1st edn. Embrapa Comunicação para Transferência de Tecnologia, Brasília
Pozzobom, U. M., Heino, J., da Brito, M. T., & S, Landeiro VL,. (2020). Untangling the determinants of macrophyte beta diversity in tropical floodplain lakes: insights from ecological uniqueness and species contributions. Aquatic Science. https://doi.org/10.1007/s00027-020-00730-2
Quinn, L. D., Schooler, S. S., & van Klinken, R. D. (2011). Effects of land use and environment on alien and native macrophytes: Lessons from a large-scale survey of Australian rivers. Diversity and Distributions, 17, 132–143. https://doi.org/10.1111/j.1472-4642.2010.00726.x
Quirino, B. A., Teixeira De Mello, F., Deosti, S., et al. (2021). Interactions between a planktivorous fish and planktonic microcrustaceans mediated by the biomass of aquatic macrophytes. Journal of Plankton Research, 43, 46–60. https://doi.org/10.1093/plankt/fbaa061
R Core Team (2020). R: A Language and Environment for Statistical Computing.
Riis, T., Kelly-Quinn, M., Aguiar, F. C., et al. (2020). Global overview of ecosystem services provided by riparian vegetation. BioScience, 70, 501–514. https://doi.org/10.1093/biosci/biaa041
Rocha, J. C., Peterson, G., Bodin, Ö., & Levin, S. (2018). Cascading regime shifts within and across scales. Science 1979, 362, 1379–1383. https://doi.org/10.1126/science.aat7850
Scheffer, M. (2004). The story of some shallow lakes. Ecology of shallow lakes (1st ed., pp. 1–19). Dordrecht: Springer.
Schneck, F., Bini, L. M., Melo, A. S., et al. (2022). Catchment scale deforestation increases the uniqueness of subtropical stream communities. Oecologia, 199, 671–683. https://doi.org/10.1007/s00442-022-05215-7
Sonter, L. J., Ali, S. H., & Watson, J. E. M. (2018). Mining and biodiversity: Key issues and research needs in conservation science. Proceedings of the Royal Society B: Biological Sciences, 285, 20181926. https://doi.org/10.1098/rspb.2018.1926
Thomaz, S. M. (2021). Ecosystem services provided by freshwater macrophytes. Hydrobiologia. https://doi.org/10.1007/s10750-021-04739-y
Vitousek, P., Mooney, H. A., Lubchenco, J., & Mellilo, J. M. (1997). Human domination of earth. Science 1979, 227, 494–499.
Wang, H., Zhang, M., Wang, C., et al. (2022). Spatial and Temporal Changes of landscape patterns and their effects on ecosystem services in the Huaihe River Basin China. Land (basel). https://doi.org/10.3390/land11040513
Acknowledgements
We are thankful to Hydro Paragominas Company for supporting the research projects “Monitoring Aquatic Biota of Streams on Areas of Paragominas Mining SA, Pará, Brazil” and “Effects of soil use on diversity and ecophysiology on the riparian vegetation, aquatic macrophytes and plankton in streams and lagoons in mining areas of Paragominas, Pará, Brazil” through the Biodiversity Research Consortium Brazil-Norway (BRC). This paper is number 51 in the publication series of the BRC. We are also grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (processes: 433125/2018-7) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 for financial support and scholarships. We thank the Aquatic Biota field team (A.L. Andrade, C. Maia, C. Paiva, G. Salvador, L. Calvão, N. Raiol, L. Juen, and T. Barbosa) for helping with field sampling.
Funding
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (processes: 433125/2018-7), Coordination for the Improvement of Higher Education Personnel (CAPES—Financial Code 001) and Hydro through the Biodiversity Research Consortium Brazil-Norway (BRC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bomfim, F.F., Fares, A.L.B., Melo, D.G.L. et al. Land use increases macrophytes beta diversity in Amazon streams by favoring amphibious life forms species. COMMUNITY ECOLOGY 24, 159–170 (2023). https://doi.org/10.1007/s42974-023-00139-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-023-00139-5