Abstract
Bacterial Cellulose (BC) offers a wide range of applications across various industries, including food, biomedical, and textiles, owing to its distinctive properties. Its unique 3D reticulated network of cellulose nanofibers, imparts excellent mechanical qualities, a high water-holding capacity, and thermal stability. Additionally, it possesses remarkable biocompatibility, biodegradability, high crystallinity, and purity. These attributes have offered significant interest in BC within both academic and industrial sectors. However, BC production is associated with high costs due to the use of expensive growth media and low yields. The study reports the potential of our indigenous isolate, Komagataeibacter saccharivorans BC-G1, as BC producer. Statistical optimization of BC production was carried out using Placket-Burman design and Central composite design, by selecting different parameters. Eight significant factors such as temperature, pH, glucose, yeast, peptone, acetic acid, incubation time and % inoculum were studies using ANOVA-based response surface methodology. Results showed that BC yield (8.5 g/L) with 1.8-fold after optimization of parameters. Maximum cellulose production (8.5 ± 1.8 g/L) was obtained using 2% glucose, 0.3% yeast extract, 0.3% peptone, 0.75% (v/v) acetic acid at pH 7.0 for 10 days of incubation with 4% inoculum at 25 °C under static culture. Main effect graph showed incubation time and acetic acid concentration as the most significant parameters affecting BC production in our study. The physicochemical characterization of produced BC was done using FTIR, XRD and SEM techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose is the considered as most abundantly available natural biopolymer which is predominantly produced from plants. However, several research studies have revealed that this renewable and biodegradable polymer can also be extracted or obtained from various other sources specially microorganisms. In this regard, bacterial cellulose (BC) is also biodegradable polymer, containing glucose units joined via β-(1–4)-glycosidic bonds forming glucan chains [(C6H10O5)n] [1]. The distinctive bonding found in bacterial cellulose renders it remarkably superior to plant cellulose, exhibiting enhanced purity, mechanical and tensile strength, porosity, crystallinity, biodegradability, and biocompatibility. These attributes make it a unique biopolymer suitable for diverse applications including the food industry, medical and pharmaceutical sectors, textiles industry, and more. Several researchers have explored the application of bacterial cellulose as a scaffold for 3D cell culture in tissue engineering, contributing to the research on the potential uses of bacterial cellulose [2]. While several genera of bacteria are capable of synthesizing BC, Komagataeibacter species have garnered significant attention from researchers due to their ability to produce a substantial amount of BC. Compared to other bacterial strains, Acetobacter species have demonstrated the capacity to generate BC in quantities that make it more practical for commercial and industrial applications. However, it is crucial to note that achieving economic feasibility for Bacterial Cellulose (BC) across multiple domains relies on the effective and cost-efficient methods of its production [3]. Hence, it is considered that optimization of the growth media and culture conditions is crucial for achieving high productivity, as it can help reduce the overall cost of BC production. This, in turn, allows for wider utilization and application of BC in various industries. To assess the potential of a specific strain, such as Komagataeibacter, in BC synthesis for biotechnological applications, it becomes necessary to study and understand the combined effect of several other parameters that play a significant role in regulating the rate of production [4, 5]. These parameters may include factors such as temperature, pH, nutrient concentrations, incubation time, and other variables that influence BC synthesis. By systematically evaluating the cumulative effect of these parameters, researchers can identify the optimal conditions that maximize BC production by the Komagataeibacter strain [6]. This process of optimization helps alleviate the strain on the cost of BC production, making it more economically viable for large-scale applications. Additionally, by understanding the interplay between various parameters, researchers can fine-tune the production process, leading to improved yields and potentially expanding the range of applications for BC [7]. In the domain of bioprocess technologies, the need for appropriate problem-solving methods arises due to the involvement of multiple parameters which requires time-to-time adjustments. Response Surface Methodology (RSM) has been proven as a superior and more effective alternative approach that involves the utilization of classical one-at-a-time approaches or mathematical methods [8]. This is attributed to RSM’s capability to simultaneously study numerous variables with a minimal number of experiments, thereby saving resources, time as well as costs. Its successful application extends to optimizing the compositions of the fermentation medium, showcasing its efficiency in achieving optimal outcomes with resource efficiency [9, 10]. This study focuses on optimizing the conditions for bacterial cellulose production by Komagataeibacter saccharivorans BC-G1 using Response Surface Methodology (RSM). Initially, a modified version of Tang jia medium, commonly used for bacterial cellulose production, was developed by adjusting its components (unpublished data). The influence of various factors on BC production, including carbon, nitrogen, phosphate, and mineral sources, was investigated using a Plackett-Burman design (PBD) in order to select the most significant factors for further analysis (unpublished data). In the second phase of the study, a Central Composite Design (CCD) was employed to optimize the concentrations of the selected medium components and maximize BC production under shaking conditions. This experimental design allows for more comprehensive and efficient exploration of the parameter space, minimizing the drawbacks associated with conventional one-factor-at-a-time experiments. By utilizing RSM and the PBD and CCD experimental designs, the study aims to overcome the limitations of traditional approaches and provide a statistically robust evaluation of the factors influencing BC production. The ultimate goal is to identify the optimal conditions for Bacterial Cellulose production, leading to improved efficiency and productivity in the process.
Materials and methods
Microorganism and culture conditions
The Komagataeibacter saccharivorans BC-G1 strain utilized in this study was obtained from spoiled grapes. The identification of the isolate was conducted using standard microbiological techniques [11]. To further confirm the identification, the 16 S rRNA gene sequencing technique was performed (details provided in supplementary data). The resulting nucleotide sequence was compared to closely related strains available in databases using the BLAST program provided by the National Center for Biotechnology Information (NCBI) at http://www.ncbi.nlm.nih.gov/. The sequence was subsequently deposited in the GenBank database under the accession number Komagataeibacter saccharivorans BC-G1 (ON514605.1) [12]. For culture maintenance, Tang jia medium, as described by Tang jia, Jia and Yang 2010 was used to prepare slants. These slants were stored at 4 °C, and subculturing was performed at regular intervals of 2 weeks to ensure the viability and availability of the strain for further experiments.
Culture medium
The culture medium selected for BC production was done using Tang Jia media with composition (%w/v): glucose 2.5, peptone 1.0, yeast extract 1.0, acetic acid 1.0, di-sodium hydrogen phosphate 1.0, pH 6.5 under static culture condition at 30 °C [13].
Production and recovery of BC
Tang jia medium was used as the basal media for the optimization study in a static condition [13]. The growth and formation of bacterial cellulose (BC) films were observed in flasks. The BC films typically formed after an incubation period of 5–6 days. To prepare the BC films for further analysis, the extracted films were washed off using distilled water to remove any excess media or unwanted dead cells. Subsequently, the films were given alkaline treatment using 0.1 M NaOH at 90 °C for 30 min to lyse any other attached bacterial cells. Following the lysis step, the BC films were boiled and then purified again through extensive and rigorous washing with distilled water at ambient temperature. The washing process was continued till the pH of the water reached a neutral level. BC quantification relied on the determination of its dry weight following the purification process. The purified cellulose was subjected to drying in an oven at 50 °C for 8 h to achieve the dry weight measurement. This step further ensured the removal of any remaining moisture and provided a reliable basis for BC quantification [14].
Experimental design
For determining the optimal culture conditions required for the production of bacterial cellulose (BC) by the strain K. saccharivorans BC-G1, a two-phase approach was followed. In the initial phase, various culture conditions were examined using one-factor-at-a-time experiments (unpublished data). This allowed for the identification of influential factors that significantly impact BC production. Based on the results obtained, selected media ingredients were further optimized in the subsequent phase using statistical designs. In the first phase, a Plackett-Burman design (PBD) was employed to study the effects of different factors on BC production. This design allowed for the identification of key variables that have a maximum impact on BC production, as shown in Table 1. In the second phase, response surface methodology (RSM) utilizing a central composite design (CCD) was employed. The objective of this phase was to study and analyze the effects of selected variables on BC production and optimize their concentrations to maximize BC yield. The CCD allowed for the exploration of the response surface and determination of the optimal levels of the variables for achieving maximum BC production [15–16]. By employing these statistical designs and analyses, the study aimed to identify the most influential factors and optimize their concentrations to enhance the production of BC by K. saccharivorans BC-G1.
Central composite design
To maximize the production of bacterial cellulose (BC), Response Surface Methodology (RSM) was employed. Based on the results obtained from the Plackett-Burman design (PBD), the factors that demonstrated a significant effect on BC production were identified. These factors were acetic acid concentration and incubation time. To determine the optimal settings for these factors and maximize BC production, RSM was applied using the Central Composite Design (CCD) [17]. 12 experiments were meticulously designed, encompassing various combinations of acetic acid concentration and incubation time. The optimization of the response function, representing BC production, was carried out using a second-order model, which was earlier derived from the experimental data. The response surface model further provided the predicted value for the optimum BC production and the corresponding culture conditions. To assess the model’s accuracy, a comparison was made between the experimental BC production achieved under the optimized conditions and the predicted value derived from the model. This validation step aimed to confirm the reliability and predictive capability of the established model. This comparison served as a verification of the response surface model’s ability to accurately predict the BC production under optimal conditions [18].
Validation of BC production of K. saccharivorans BCG1
To confirm and validate the statistical findings, the BC production yield of K. saccharivorans BC-G1 in the optimized medium was compared with the predicted BC yield from the optimization plot. Furthermore, a comparative analysis was conducted between the BC production yield in the optimized medium and the BC yield in the standard Tang Jia medium (% w/v) to provide additional insights into the performance and efficiency of the optimized conditions.
Physicochemical characterization
Fourier transform infrared spectroscopy (FTIR)
The samples were subjected to characterization using FTIR spectroscopy. The comparison was made with commercial cellulose obtained from Himedia. The FTIR analysis was conducted at the material characterization laboratory of Jaypee Institute of Information Technology, India, using a Spectrum BX-II Spectrophotometer. For the FTIR analysis, the transmission mode was selected, which allows the collection of spectra over a wide range of wavenumbers. The spectra were collected in the range of 4000–400 cm− 1 with a resolution of 4 cm− 1. This range covers various functional groups and vibrations present in the samples, providing valuable information about the chemical composition and structure of the BC and commercial cellulose samples.
X-ray diffraction (XRD).
X-ray data were acquired utilizing a Shimadzu 6000 X-ray diffractometer (XRD) from Japan, employing Cu Kα radiation (voltage = 40 kV). Diffraction patterns were captured within the 2 θ range of 5° to 30°, maintaining a speed of 0.5°min-1 and a step size of 0.02°. The crystallinity (Cr.I) and crystallite size (Cr S) were determined through X-ray diffraction measurements. The crystallinity was evaluated utilizing Eq. 1 [19, 20].
The crystallite size of particles was determined using Eq. 2 [21].
where τ = crystal size, λ = 1.5418 Å (Cu Kα1); β (the full width at half maximum intensity of the peak (FWHM) (in Rad)), θ = 2θ/2) diffraction angle and constant K (0.89).
Scanning electron microscopy (SEM) analysis.
Morphological analysis of produced BC was done using Scanning electron microscopy. The samples were dried with air, placed on mounts, coated with gold, and then observed under SEM Zeiss EVO®50 series, Germany, at the Central Research Facility (CIF), IIT Delhi, India, using an accelerating voltage of 5 kV.
Statistical analysis
The generation and analysis of statistical experimental designs were conducted using Minitab 19(Minitab Inc., USA).
Results and discussion
Screening of the independent variables
The Plackett-Burman design (PBD) with two levels and 12 experimental runs was used to analyze the effect of eight individual variables on bacterial cellulose (BC) production by K. saccharivorans BC-G1. The variables considered (temperature, pH, glucose concentration, yeast extract concentration, peptone concentration, acetic acid concentration, incubation time, and media volume) on BC production by K. saccharivorans BC-G1.The experimental and predicted data of BC production for each run are given in Table 2. The highest yield of BC, 7.7 g/L, was obtained in run six, where the following conditions were applied: temperature 25 °C, pH 7, glucose concentration 4%, yeast extract concentration 0.8%, peptone concentration 0.3%, acetic acid concentration 1.5%, incubation time 10 days, and inoculate volume 2%.
On the other hand, the lowest yield of BC, 1.3 g/L, was observed in run twelve, where the conditions were as follows: temperature 25 °C, pH 5, glucose concentration 2%, yeast extract concentration 0.3%, peptone concentration 0.3%, acetic acid concentration 0.0%, incubation time 5 days, and inoculate 2%.
Table 3 represents the outcomes of the variance analysis and determination of parameters for BC production. It is stated that BC production showed a negative correlation with increasing temperature, glucose concentration, peptone concentration, and media volume. On the other hand, the other factors had a positive effect on BC production.
By applying regression analysis to the experimental data, the equation for BC production can be derived. The equation represents the relationship between the independent variables (factors) and the dependent variable (BC production). The equation for BC production is as follows:
In this equation, each coefficient represents the effect of the corresponding factor on BC production. The negative coefficients (e.g., Temperature, Glucose, Peptone, and Media Volume) indicate that an increase in these factors leads to a decrease in BC production. Conversely, the positive coefficients (e.g., pH, Yeast Extract, Acetic acid, Incubation, and Inoculate %) suggest that an increase in these factors increases BC production. The coefficients obtained from regression analysis quantify the impact of each factor while holding other factors constant. This equation can be used to predict BC production based on the levels of the independent variables. It provides a mathematical model that can be utilized for optimization or further analysis in the context of BC production [22].
This regression equation has a coefficient (R2) of 99%, indicating that the data in PBD could be explained by the model. Also, the values of, R2adj and R2pred were 96.33% and 84%, respectively.R2 value suggests a strong relationship between the independent variables (temperature, pH, glucose concentration, yeast extract concentration, peptone concentration, acetic acid concentration, incubation time, and inoculate volume) and the dependent variable (BC production). The regression equation provides an accurate representation of how changes in these factors influence BC production, adjusted R-squared (R2adj) is reported to be 96.33%. This value considers the number of independent variables in the model, ensuring that the inclusion of variables is justified and prevents overfitting. With a high R2adj value, the model effectively captures the relationship between the factors and BC production while considering the complexity of the model. The predicted R-squared (R2pred) is 84%, indicating that the regression model has a strong predictive capability. This means that the model can reliably estimate BC production for new or unseen data points based on the given factors. The 84% value suggests that the model is likely to generalize well and perform accurately in predicting BC production in similar experimental conditions [23].
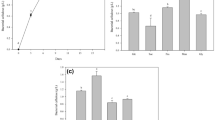
Based on the Pareto chart of standardized effects, it has been observed that the acetic acid concentration and incubation time have significant effects on BC production compared to the other tested factors, namely temperature, pH, glucose concentration, yeast extract concentration, peptone concentration, and inoculate volume.
In this case, the Pareto chart revealed that the bars representing the standardized effects of acetic acid concentration and incubation time were relatively larger compared to the bars of the other factors(Fig. 1). This indicates that these two factors have a more substantial impact on BC production. The significant effects of acetic acid concentration and incubation time suggest that manipulating these variables can lead to notable changes in BC production. Increasing or decreasing the acetic acid concentration or adjusting the duration of the incubation time can potentially enhance or reduce BC production, respectively.
Based on the higher significance and impact of acetic acid concentration and incubation time on BC production, these two factors were selected for further investigation in the second phase of experimentation. The other factors, namely temperature, pH, glucose concentration, yeast extract concentration, peptone concentration, and inoculate volume, were fixed at the levels where BC production was maximized, as determined by a mean effect plot (Fig. 2). By fixing the remaining factors at the levels that yielded the maximum BC production, it allows for isolating and focusing on the effects of acetic acid concentration and incubation time in the second phase of the experiment. This approach helps in gaining a deeper understanding of the specific contributions and interactions of these two factors without the confounding effects of the other variables. The mean effect plot provides valuable insights into the average effect of each factor at different levels. By identifying the levels that result in the highest BC production, it serves as a guide for setting the fixed values of the remaining factors. (Fig. 2).
Identifying the best culture conditions for BC production using central composite design (CCD)
The crucial variables influencing BC (Bacterial Cellu2.
lose) production of K. saccharivorans BC-G1 are specifically, acetic acid concentration and incubation time, which were subjected to further optimization using the Central Composite Design (CCD) method. This approach allows for a systematic and efficient exploration of the experimental space, facilitating the identification of optimal conditions for maximizing BC production by considering both main effects and interactions between the variables. The aim was to determine the optimum levels of these variables that could maximize BC production.
For this experiment, a total of thirteen runs were performed, including four cube points, five center points, and four axial points. The values of the other parameters, such as temperature, pH, glucose concentration, yeast extract concentration, peptone concentration, and media volume, were kept constant at specific levels (temperature: 25 °C, pH: 7, glucose concentration: 2%, yeast extract concentration: 0.8%, peptone concentration: 0.3%, media volume: 2%).Table 4 presents the experimental and predicted values of BC production for each run. Among the thirteen runs, it was observed that run eight, with an acetic acid concentration of 0.75% and an incubation time of 10 days, resulted in the highest BC production of 8.5 g/L. On the other hand, run number eleven, with an acetic acid concentration of 0% and an incubation time of five days, yielded the lowest BC production of 5.0 g/L (Table 4).
By analyzing the experimental and predicted values of BC production for different combinations of acetic acid concentration and incubation time, the optimal conditions for maximizing BC production can be identified. The CCD allows for a systematic exploration of the variable space to determine the optimum levels of the significant factors.
These results provide valuable insights for process optimization and control in BC production. By adjusting the acetic acid concentration and incubation time to the identified optimal levels, it is possible to achieve higher BC yields. The experimental and predicted values serve as a guide for selecting the most favorable operating conditions in future production processes [24].
The efficiency of the model was assessed using multiple regression analyses on the data. This analysis specifically focused on the individual effects of the variables, their squared terms, and the interactions among them on BC production by K. saccharivorans BC-G1. Multiple regression analysis allows for a comprehensive examination of how each variable and their interactions contribute to the variability in the observed BC production, providing insights into the model’s ability to capture the complex relationships within the system. The results in Table 5 indicate the significance of these effects. Firstly, the “Lack of Fit F-value” of 11.15 was obtained, indicating that the Lack of Fit is insignificant. This means that the model adequately fits the data, and any discrepancies or variation between the model predictions and the actual data are not statistically significant. An insignificant Lack of Fit value is a positive indicator for the model’s reliability. Furthermore, upon analyzing the regression coefficients of the model, it was found that there is an important interaction existing between acetic acid concentration and incubation time (P-value = 0.016). This suggests that the combined influence of these two variables has a notable impact on BC production. The interaction term represents a synergistic or antagonistic effect that is distinct from the individual effects of the variables. Based on the significant terms (P < 0.05), the regression equation for BC synthesis (Y) can be formulated as follows:
Experimental = -6.36 + 0.75 Acetic acid + 3.208 Incubation time − 3.720 Acetic acid2 − 0.2148 Incubation time2 + 0.693 Acetic acid * Incubation time.
By considering the significant terms in the regression equation, one can gain insights into the optimal levels of acetic acid concentration and incubation time for maximizing BC synthesis by K. saccharivorans BC-G1. This information can be utilized to fine-tune the process conditions and improve the efficiency of BC production.
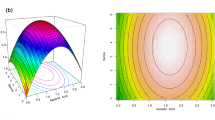
The analysis of interactions between acetic acid concentration and incubation time on BC production involved the use of response surface plots and contour plots. These graphical tools provided a visual representation of how changes in these two factors influence the yield of BC. In a response surface plot, the levels of acetic acid concentration and incubation time were deliberately varied within specific ranges (0-1.5% for acetic acid concentration and 5–10 days for incubation time). By observing the plot, it can be visualized that how different combinations of these factors affect BC production. Contour plots complement the response surface plots by displaying lines (contours) that connect points of equal BC yield. These lines help visualize the relationships and interactions between the factors, highlighting areas where BC production is likely to be higher or lower. Together, these plots offer a comprehensive and intuitive understanding of how acetic acid concentration and incubation time jointly impact BC production, aiding in the identification of optimal conditions for maximizing yield. The plot shows how BC yield changes as these two factors are adjusted. From the plot, it can be observed that increasing the incubation time generally leads to an increase in BC production. Indeed, the analysis indicates that as the acetic acid concentration increases from 0 to 1.0%, there is a corresponding increase in BC (Bacterial Cellulose) production. The contour plots serve as a valuable complement to the response surface plot by offering a more perspective manner. In contour plots, varying colors represent different regions of BC yield, providing a visual representation of how changes in acetic acid concentration and incubation time collectively affect the outcome. The curved lines observed in the contour plots are a result of the model’s consideration of interactions between the factors [25]. These interactions can lead to non-linear and more complex relationships between acetic acid concentration, incubation time, and BC production. The curvature in the contour lines suggests that the impact of one factor is influenced by the level of the other, reinforcing the importance of considering their joint effects for accurate predictions. In the response surface plot, the height of the surface serves as a visual representation of the value of BC production. On the other hand, the contour plots contribute by delineating regions with varying BC yields. Within these contour plots, the dark shaded region specifically indicates the area of maximum yield, surpassing 8 g/100 mL. This implies that this dark shaded region is where optimal conditions for BC production are achieved. Based on these findings, the recommendation is to focus on optimizing the levels of acetic acid concentration and incubation time within the identified region of higher yield, as highlighted by the dark shaded area in the contour plots. Concentrating experiments in this optimized region has the potential to maximize BC production by K. saccharivorans BC-G1, ensuring the conditions most conducive to achieving the highest yields of bacterial cellulose. Similarly study by Saleh et al. [26] employed statistical optimization techniques to enhance microbial bioprocesses, focusing on the production of bacterial cellulose (BC) by Komagataeibacter hansenii AS.5. The Plackett-Burman Design (PBD) identified MgSO4, ethanol, pH, and yeast extract as significant factors influencing BC production. Subsequently, a Box-Behnken Design (BBD) determined the optimal levels for these factors: 1.5% MgSO4, 0.718% ethanol, 1.3% yeast extract, and pH 5.5. Under these conditions, the BC yield reached 6.30 g/L.Another study conducted by Santoso and colleagues utilized Komactobacter intermedius (BCRC 910,677) for BC production using optimized modified culture media. The optimization was performed using the response surface methodology (RSM). In the RSM optimization study, a Box–Behnken design with three parameters was applied: fructose concentrations, peptone concentrations, and pH values. An increase in BC production of 3.82-fold (compared to the culture in HS medium) was achieved after a 6-day culture period (Fig. 3).
Validation of BC production by K. saccharivorans BC-G1
To validate the statistical findings, the fitted values of acetic acid concentration and incubation time were applied in the cultural medium. Subsequently, the resulting BC production was compared with the predicted values obtained from the optimization plot (Fig. 4). This comparison aimed to assess the reliability and accuracy of the model’s predictions under the specified conditions, providing confirmation of the statistical results.
The reported BC production by K. saccharivorans BC-G1 in the optimized medium was found to be 8.5 ± 1.8 g/L based on the experimental results being conducted. These findings closely matched the predicted values of 8.3 g/L, as indicated in the accompanying figure. This alignment between actual and predicted values suggests a reasonable degree of accuracy and validity for the predictive model employed. Additionally, when comparing the BC production in the optimized medium (8.5 ± 1.8 g/L) with that in the standard Tang Jia medium (4.6 ± 1.1 g/L), a notable improvement was observed in the optimized conditions. This increase in BC production further supports the effectiveness of the optimized medium, showcasing its potential for enhancing the yield of bacterial cellulose compared to the standard conditions [27].
FTIR spectra analysis
FTIR spectra obtained from BC samples were compared with the standard cellulose to determine the changes and similarities within the samples (Fig. 5). Upon analysis, results revealed the presence of multiple peaks in the case of BC produced in this study, as compared to the standard cellulose, hence revealing the presence of hydroxyl (3500 cm-1 to 3300 cm-1), alkane (3000 cm- to 2800 cm-1), alkene (3100 cm-1 to 3000 cm-1), alkyne (3300 cm-1) as well as other aromatic compounds. Initially, the major changes in the peaks were observed in the range of 3500 cm-1 to 3300 cm-1, which is attributed to the presence of stretching vibration of OH groups within the BC samples. Similarly, the peak changes observed around 2896 cm-1 signified the presence of stretching vibrations of CH2 as well as CH groups. The changes in the functional groups and fingerprint region within the cellulose samples were observed between 1700 cm and 1– 1600 cm-1 and 1200 cm-1– 1100 cm-1, specifically at 1100 cm-1 and 893 cm-1, hence indicating C-O stretching and C-O-C stretching of β– glycosidic linkages within the glucose monomeric units respectively. In addition to these, the peaks at 1455 cm-1 reveal the presence of CH2 deformation within the cellulose structure. Therefore, at specific wavenumbers, the presence of peaks associated with β-glycosidic linkages and CH2 deformation provides evidence of the preservation of key structural elements characteristic of cellulose in the BC samples produced in this current study. Our results are in compliance with the observations of Akintunde and others, who have reported the presence of similar peaks in the bacterial cellulose produced in their study using K. saccharivorans strains [28].
XRD analysis
Figure 6 presents a comparative analysis of XRD diffractogram patterns between bacterial cellulose (BC) and a reference sample of commercial cellulose (CC). In the diffractogram of the produced bacterial cellulose, distinctive peaks were observed around 2Ɵ=14.9° and 22.6°. Conversely, the commercial cellulose exhibited peaks at 15.2° and 22.3°, corresponding to crystallite plates 101 and 002, respectively, indicating the cellulose I structure [29].Table 6 provides a summary of the calculated crystallinity and crystallite size based on the raw data obtained from the XRD diffractograms. The crystallinity of the produced bacterial cellulose was found to be notably higher, with a value of 98%, compared to commercial cellulose, which displayed a crystallinity of 93%. Additionally, the average crystallite size for bacterial cellulose was measured at 4.79 nm, slightly larger than the commercial cellulose with a size of 4.08 nm. These findings underscore the superior crystalline structure and larger crystallite size of bacterial cellulose compared to commercial cellulose.
SEM analysis
The ultrastructure characteristics of the produced BC were analyzed using SEM, presented in Fig. 7 shows that BC pellicle structure consisted of undirected cellulose fibrils of multiple sizes, which formed bundles of different thicknesses and forms a fine and dene network, with a nano sized diameter [30].
Conclusion
This study demonstrates the efficiency of modern statistical approaches in determining the effective culture conditions for production of Bacterial Cellulose. After careful evaluation using response surface methodology approach, it was revealed that Tang Jia media, containing glucose, peptone, yeast extract, acetic acid and di-sodium hydrogen phosphate; provided the maximum BC under static conditions. The preference for static conditions further refines the practical application of the findings from this research study. FTIR analysis revealed distinct spectral signatures in bacterial cellulose (BC) samples compared to standard cellulose, indicating the presence of various functional groups characteristic of cellulose. XRD analysis demonstrated higher crystallinity and larger crystallite size in BC compared to commercial cellulose, highlighting its superior structural properties. SEM imaging illustrated the intricate network of cellulose fibrils forming BC pellicles, affirming its nanostructured architecture.By providing a comprehensive understanding of the correlation between culture conditions and BC production, this research offers valuable insights for industries seeking to maximize BC output efficiently. The insights gained from this study not only contribute to the advancement of Bacterial Cellulose production but also hold broader implications for the development of sustainable materials and applications across various industries.
References
Srivastava S, Mathur G(2022)bacterial cellulose: a multipurpose biomaterial for manmade world. Curr Appl Sci. https://doi.org/10.55003/cast.2022.03.23.014
Lin S, Singajaya S, Lo T, Santoso SP, Hsu H, Cheng K (2023) Evaluation of porous bacterial cellulose produced from foam templating with different additives and its application in 3D cell culture. Int J Biol Macromol 234:123680. https://doi.org/10.1016/j.ijbiomac.2023.123680
Lin S, Huang S, Ting Y, Hsu H, Cheng K (2022) Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int J Biol Macromol 204:136–143. https://doi.org/10.1016/j.ijbiomac.2022.01.186
Wahid F, Huang LH, Zhao XQ, Li WC, Wang YY, Jia SR, Zhong C (2021) Bacterial cellulose and its potential for biomedical applications. Biotechnol Adv 53:107856. https://doi.org/10.1016/j.biotechadv.2021.107856
Chen C, Ding W, Zhang H, Zhang L, Huang Y, Fan M, Yang J, Sun D (2022) Bacterial cellulose-based biomaterials: from fabrication to application. Carbohydr Polym 278:p118995. https://doi.org/10.1016/j.carbpol.2021.118995
Stumpf TR, Yang X, Zhang J, Cao X (2018) In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater Sci Eng 82:372–383. https://doi.org/10.1016/j.msec.2016.11.121
Carvalho T, Guedes G, Sousa FL, Freire CS, Santos HA (2019) Latest advances on bacterial cellulose-based materials for wound healing, delivery systems, and tissue engineering. Biotechnol J 14(12):p1900059. https://doi.org/10.1002/biot.201900059
El-Gendi H, Salama A, El-Fakharany EM, Saleh AK (2023) Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr Polym 302:120383. https://doi.org/10.1016/j.carbpol.2022.120383
Bae S, Shoda M (2005) Statistical optimization of culture conditions for bacterial cellulose production using Box-Behnken design. Biotechnol Bioeng 90(1):20–28. https://doi.org/10.1002/bit.20325
Rastogi A, Banerjee R (2020) Statistical optimization of bacterial cellulose production by Leifsonia soli and its physico-chemical characterization. Process Biochem 91:297–302. https://doi.org/10.1016/j.procbio.2019.12.021
Sievers M, Swings J (2005) Family II. Acetobacteraceae. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM, editors. Bergey’s manual® of systematic bacteriology, vol. 2, The proteobacteria. Part C, The alpha-, beta-, delta-, and epsilon proteobacteria. New York, NY, USA: Springer; 41–95
Srivastava Sand Mathur G (2022) Komagataeibacter saccharivorans strain BC-G1: an alternative strain for production of bacterial cellulose. Biologia 77:3657–3668. https://doi.org/10.1007/s11756-022-01222-4
Tang W, Jia S, Jia Y, Yang H (2010) The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J Microbiol Biotechnol 26 L:125–131. https://doi.org/10.1007/s11274-009-0151-y
Ul-Islam M, Khattak WA, Kang M, Kim SM, Khan T, Park JK (2013) Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose 20:253–263. https://doi.org/10.1007/s10570-012-9799-9
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 305–325. https://doi.org/10.2307/2332195
Box GE, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2(4):455–475
Yatsyshyn VY, Fedorovych DV, Sibirny AА (2010) Medium optimization for production of flavin mononucleotide by the recombinant strain of the yeast Candida famata using statistical designs. Biochem Eng J 49(1):52–60. https://doi.org/10.1016/j.bej.2009.11.010
Embuscado ME, Marks JS, BeMiller JN (1994) Bacterial cellulose. II. Optimization of cellulose production by Acetobacter xylinum through response surface methodology. Food Hydrocoll 8(5):419–430. https://doi.org/10.1016/S0268-005X(09)80085-4
Ruan C, Zhu Y, Zhou X, Abidi N,Catcmark JM (2016) Effect of cellulose crystallinity on bacterial cellulose assembly. Cellulose 23:3417–3427. https://doi.org/10.1007/s10570-016-1065-0
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Scherrer P (1918) Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse, 98–100. https://eudml.org/doc/59018
Zeng X, Small DP, Wan W (2011) Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter Xylinum BPR 2001 from maple syrup. Carbohydr Polym 85(3):506–513. https://doi.org/10.1016/j.carbpol.2011.02.034
Hegde S, Bhadri G, Narsapur K, Koppal S, Oswal P, Turmuri N, Jumnal V, Hungund B (2013) Statistical optimization of medium components by response surface methodology for enhanced production of bacterial cellulose by Gluconacetobacter Persimmonis. J Bioprocess Biotech 4(1):1–5. https://doi.org/10.4172/2155-9821.1000142
Mohite BV, Kamalja KK, Patil SV (2012) Statistical optimization of culture conditions for enhanced bacterial cellulose production by Gluconoacetobacter Hansenii NCIM 2529. Cellulose 19:1655–1666. https://doi.org/10.1007/s10570-012-9760-y
Anand Vera B (1993) Computational methods for the representation of contour lines. Computer-Aided Civil and Infrastructure Engineering 8. no. 2: 119–128. https://doi.org/10.1111/j.1467-8667.1993.tb00199.x
Saleh AK, Farrag AA, Soliman NA, Ibrahim MM, El-Shinnawy NA, Abdel Fattah YR (2019) Evaluation of culture requirements for cellulose production by Egyptian local isolate alongside reference strain Gluconacetobacter hansenii ATCC 23769. Pak J Biotechnol 16(2):69–80. https://doi.org/10.34016/pjbt.2019.16.1.12
Santoso SP, Chou CC, Lin S, Soetaredjo FE, Ismadji S, Hsieh C, Cheng K (2020) Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 27(5):2497–2509. https://doi.org/10.1007/s10570-019-02961-5
Akintunde MO, Adebayo-Tayo BC, Ishola MM, Zamani A, Horváth IS (2022) Bacterial cellulose production from agricultural residues by two Komagataeibacter Sp. Strains Bioengineered 13(4):10010–10025. https://doi.org/10.1080/21655979.2022.2062970
Ling Z, Chen S, Zhang X, Takabe K, Xu F (2017) Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Scientific 7(1). https://doi.org/10.1038/s41598-017-09885-9
Shao M, Shi Z, Zhai B, Zhang X, Li Z (2022) Preparation and performance analysis of bacterial cellulose-based Composite hydrogel based on scanning electron microscope. https://doi.org/10.1155/2022/8750394. Scanning,1–7
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Henrique Souza Guimaraes.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastava, S., Mathur, G. Statistical optimization of bioprocess parameters for enhanced production of bacterial cellulose from K. saccharivorans BC-G1. Braz J Microbiol 55, 2199–2210 (2024). https://doi.org/10.1007/s42770-024-01397-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01397-9