Abstract
Pseudomonas sp. 4B isolated from the effluent pond of a bovine abattoir was investigated as antifungal against toxigenic fungi. The complete genome of Pseudomonas 4B was sequenced using the Illumina MiSeq platform. Phylogenetic analysis and genome comparisons indicated that the strain belongs to the Pseudomonas aeruginosa group. In silico investigation revealed gene clusters associated with the biosynthesis of several antifungals, including pyocyanin, rhizomide, thanamycin, and pyochelin. This bacterium was investigated through antifungal assays, showing an inhibitory effect against all toxigenic fungi tested. Bacterial cells reduced the diameter of fungal colonies, colony growth rate, and sporulation of each indicator fungi in 10-day simultaneous growing tests. The co-incubation of bacterial suspension and fungal spores in yeast extract–sucrose broth for 48 h resulted in reduced spore germination. During simultaneous growth, decreased production of aflatoxin B1 and ochratoxin A by Aspergillus flavus and Aspergillus carbonarius, respectively, was observed. Genome analysis and in vitro studies showed the ability of P. aeruginosa 4B to reduce fungal growth parameters and mycotoxin levels, indicating the potential of this bacterium to control toxigenic fungi. The broad antifungal activity of this strain may represent a sustainable alternative for the exploration and subsequent use of its possible metabolites in order to control mycotoxin-producing fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are extremely toxic secondary metabolites produced by certain filamentous fungi and represent a huge impact for food safety since ingestion of products contaminated with these compounds may induce to acute and/or chronic diseases [1, 2]. Among more than 400 mycotoxins, identified up to now, aflatoxin B1 (AFB1) and ochratoxin A (OTA) have received particular attention due to their toxic properties [3, 4]. These toxins were classified by the International Agency for Research on Cancer according to carcinogenicity: AFB1 is recognized as carcinogen to humans (group 1) [5] and OTA belongs to group 2B as possible carcinogenic agent to humans [6].
Fungi-producing mycotoxins are widely distributed in nature, mainly representatives of the genera Aspergillus, Penicillium, and Fusarium [7]. Several studies report the presence of these fungi in various agricultural commodities, and when conditions are appropriate, mycotoxin production and subsequent contamination of food and feedstuffs may occur [8, 9]. These facts are worrying because most of these fungal compounds exert toxic effects at ng/mL concentration [10] and have cumulative effects on health [11]. Besides that, the presence of certain mycotoxins can also enhance the action of other ones, such as the synergism of OTA and the emerging mycotoxin citrinin [10, 12].
Several methods have been proposed to inactivate or detoxify the mycotoxins in food. However, the high cost, efficacy, and safety of many detoxification procedures are often questioned, as well as nutritional quality losses of the food [13, 14]. Biological control strategies have received great attention because of the possibility of replacing the use of fungicides, which present risks to health and environment, and may induce development of fungicide-resistant fungi and/or increase mycotoxins synthesis [15,16,17].
Many strains of the genus Pseudomonas produce a range of substances with antimicrobial activity. Suppressive effect against certain filamentous fungi by pseudomonads isolated from different environments has been investigated [18,19,20]. The ability of these bacteria to inhibit toxigenic fungi growth has also been explored [21, 22]. Some antifungal substances produced by Pseudomonas species have been reported as phenazines [23] and lipopeptides [24]. Therefore, research on new strains showing broad inhibitory spectrum can provide valuable data for biocontrol of phytopathogenic and toxigenic fungi.
In a previous study, a bacterial strain characterized as Pseudomonas sp. 4B was isolated from the effluent pond of a bovine abattoir, showing inhibitory effect against pathogenic and spoilage bacteria as well as a few yeasts [25]. However, this strain has not yet been investigated with regard the control of filamentous fungi, more specifically producers of mycotoxins. Furthermore, no detailed study on its genetic characteristics has been performed. Based on that, this study aimed to investigate the Pseudomonas sp. 4B genome by searching for gene clusters associated with production of antimicrobial substances, and evaluating the antagonistic activity of this strain against toxigenic fungi by addressing its effect on fungal growth parameters and mycotoxins production.
Materials and methods
Microorganisms
The strain Pseudomonas sp. 4B, belonging to the culture collection of the Laboratório de Bioquímica e Microbiologia Aplicada (Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil), was used in the study. This bacterium was isolated from the effluent pond of a bovine abattoir localized at southern Brazil [25]. Stock culture was maintained at − 20 °C in Brain Heart Infusion (BHI; Oxoid, Basingstoke, UK) broth containing 20% (v/v) glycerol, or stored at 4 °C in BHI agar plates. Before use, the bacterial cells were propagated twice in the same medium at 37 °C for 24 h.
Toxigenic fungi (Table S1) used as indicator microorganisms for antagonism assays were maintained on potato dextrose agar (PDA; Acumedia, Lansing, MI, USA) slants covered with 20% glycerol at 4 °C and routinely grown on PDA agar at 25 °C.
Genome sequencing and annotation
The total DNA of Pseudomonas sp. 4B was obtained using the standard phenol–chloroform procedure, followed by a purification step with a Genomic DNA Clean & Concentrator (Zymo Research, Irvine, CA, USA). DNA fragment libraries were further prepared with 1 ng of DNA using a Nextera XT DNA sample preparation kit and sequenced using an Illumina® MiSeq System (2 × 250 paired-end reads with the Illumina v2 reagent kit) (Illumina, San Diego, CA, USA). The FastQC tool was used to check the quality of generated sequences. The sequences with bases having a Phred quality score < 20 were trimmed with the aid of Geneious software (version 10.1.3). The paired-end sequence reads were assembled into contigs with SPAdes 3.9.0 [26], following confirmation by mapping reads of contigs generated by SPAdes using the Geneious software. NCBI Prokaryotic Genome Annotation Pipeline (PGAAP) was employed to identify coding sequences (CDS) based on the best-placed reference protein set. Similarly, to aid the gene prediction and annotation, the genome was analyzed by using Pathosystems Resource Integration Center (PATRIC) webservice through RASTtk. Genes of interest had their annotation refined manually. The genome sequence is available at the NCBI database under de accession number VCSJ00000000.
Phylogenetic analysis and genome comparisons
The multi locus sequence analysis (MLSA) and in silico genome-genome comparisons were carried for the Pseudomonas 4B species definition. The following genomes of Pseudomonas strains were selected for comparison considering their high complete genome similarity revealed by BLAST (GenBank accession numbers under parenthesis): P. aeruginosa DSM50071 (NC_002696), P. protegens CHA0 (NZ_CP007509), P. mendocina NCTC10897 (NC_016830), P. chlororaphis subsp. chlororaphis DSM50083 (NC_002947), P. chlororaphis subsp. aurantiaca DSM19603 (NC_008027), P. entomophila L48 (NC_007005), and P. oryzae KCTC32247 (NZ_LS999205). Moreover, genomes of some reference species such as P. aeruginosa PAO1 (NC_002516), P. putida KT2440 (NZ_CP013124), P. fluorescens F113 (CP_012001), P. stutzeri 19SMN4 (LR_134290), and P. syringae pv. syringae B728a (NZ_HG322950) were included in the genome comparison for a better understanding of the Pseudomonas subgroup containing our query species sequence. The sequence of Escherichia coli O157:H7 was included as an outgroup [27].
The MLSA was performed using 4 different housekeeping genes: 16S rDNA, gyrB (gyrase B subunit), rpoB (B subunit of RNA polymerase), and rpoD (D subunit of RNA polymerase), which are considered adequate for this analysis in the Pseudomonas group [28]. All the chosen gene sequences were retrieved from the NCBI database using PATRIC RASTtk-enabled Genome Annotation Service [29]. MUltiple Sequence Comparison by Log-Expectation (MUSCLE) was used for gene alignment. The phylogenetic tree was built employing MEGA 11 software with the Maximum Likelihood model using GTR + G + I substitution model [30].
The genome of Pseudomonas 4B and those of the above-mentioned strains were subjected to in silico DNA-DNA hybridization (DDH), using the Genome-to-Genome Distance Calculator (GGDC) version 2.1 online to calculate DDH values. DDH values ≤ 70% were considered an indication for differentiating species [31]. The Average Nucleotide Identity (ANI) was calculated from pairwise comparisons of all sequences shared between Pseudomonas 4B genome and each of the reference bacteria. ANI value above 95% includes genome allocated in the same bacterial species [32]. For an efficient genome comparison representation, the BLAST ring image generation (BRIG) software [33] and Circoletto tool with 1E − 10 e-value [34] were employed. Additionally, the virulence factor database (VFDB) was used to search gene sequences similar to those already reported as virulent [35].
Secondary metabolite gene clusters were identified by the online antibiotics and Secondary Metabolite Analysis Shell (antiSMASH) tool [36] using the complete nucleotide sequence of Pseudomonas 4B genome in order to compare with biosynthetic gene clusters from the gene sequence–based MIBiG database.
Antagonistic activity against toxigenic fungi
The antagonistic activity of Pseudomonas 4B against toxigenic fungi was evaluated as described previously [37] with minor modification. Pseudomonas 4B liquid culture and the cell-free culture supernatant were obtained from freshly grown colonies in BHI medium at 37 °C for 24 h followed by bacterial cultivation on BHI broth at 37 °C and 125 rpm for 48 h. Fifteen milliliters of sterile molten PDA at 45 °C containing 1 × 106 spores/mL of a fungal spore suspension was transferred to Petri dishes and allowed to solidify. The PDA plates were then inoculated with 20 μL spot of the bacterial liquid culture (1 × 107 CFU/mL) or the cell-free culture supernatant. Inoculation points were placed equidistantly on the medium surface. After incubation for 5 days at 25 °C, the antagonism was indicated by the formation of inhibition zones whose width was measured using a digital caliper.
Bacterial effect on fungal growth and sporulation
The effect on fungal growth, colony characteristics, and sporulation of two representative producers of AFB1, OTA, or citrinin was assessed as described previously [21, 38]. After incubation at 25 °C for 10 days, diameter of fungal colonies was measured and percentage inhibition of mycelial growth calculated according the following equation:
Inhibition of growth (%) = [(Dc – Dt) / Dc] × 100.
where Dc represents the diameter of fungal colony in control plate and Dt the diameter of fungal colony in the plate containing the antagonist bacterium.
After growth evaluation, samples were analyzed for bacterial influence on fungal spore production. The spores were washed by 15 mL Tween 80 solution (0.05%, v/v) and the assessment of conidial concentration (spores/cm2 of colony) was performed using a Neubauer chamber. The following formula was employed to calculate the percent of sporulation inhibition:
The number of fungal spores produced in control and treated plates containing the antagonist bacterium is represented by Nc and Nt, respectively.
Spore germination inhibition test
The inhibitory activity of Pseudomonas 4B on the fungal spore germination was evaluated by in vitro procedures [39]. Fungal spore suspensions (100 µL, 106 spores/mL) were transferred to sterile glass tubes containing 800 µL of YES broth (2 g/L yeast extract, 20 g/L sucrose, pH 6.5). Then, 100 µL of Pseudomonas 4B cells (107 CFU/mL) were added into the same tubes. Control treatment consisted of 100 µL sterile saline solution (8.5 g/L NaCl) instead of bacterial cells and used for comparison.
After 24 h at 25 °C, conidia germination was evaluated by using a light microscope. Spores were regarded to have germinated when the length of germination tube was equal or longer than the diameter of its own spore. Four counts of 100 conidia per repetition were performed and the percent spore germination was determined as follows:
Inhibition of spore germination (%) = [(Gc – Gt) / Gc] × 100.
In this equation, Gc represents the percentage of spore germinated in the control tube and Gt is the percentage of spore germinated in treated tube containing the test bacterium.
Mycotoxin production assays
The bacterial influence on the production of AFB1 and OTA was evaluated as described elsewhere [21]. PDA plates containing bacterial cells and test fungi were prepared as described above. The mycotoxins were extracted after 10 days of incubation by removing PDA agar pieces (1 × 1 cm), including microbial biomass, from the fungal colonies and transferred to a 2-mL tube. A 500-µL aliquot of chloroform was added in the same tube and then this mixture was shaken at 100 rpm for 30 min. The chloroform extract was filtered through 0.22-µm PTFE syringe filter (Millipore, Bedford, MA, USA) and poured into a glass vial to dry under nitrogen gas. The residue was dissolved in 250 μL of the mobile phase (acetonitrile/water/acetic acid, 99:99:2, v/v/v) for HPLC analysis. The extraction procedure was carried out twice for the same piece of agar medium.
Mycotoxin determination was performed using an HPLC instrument (model E2695, Waters Corporation, Milford, MA, USA) equipped with a fluorescence detector (Waters, model FL-2475). Standard methods were used for separation, identification, and quantification of AFB1 [40] and OTA [41]. Confirmation of AFB1 and OTA identity was performed using HPLC coupled to mass spectrometer with an electrospray ionization source (Bruker Daltonics, micrOTOF-Q III model, Bremen, Germany). Operational conditions are detailed in Table S2.
Curves were done under the same conditions with different levels of each mycotoxin standard (Sigma-Aldrich, Darmstadt, Germany), ranging from 0.5 to 20 μg/L (r2 > 0.99). The retention time of AFB1 and OTA was 3.9 and 6.5 min, respectively. Quantification was performed by correlating peak area of sample extracts and those of standard curves. Average recovery values were 98.9 ± 1.0 and 99.0 ± 1.2% for AFB1 and OTA, respectively. Limits of detection (LOD) and quantification (LOQ) were 60 and 180 ng/L for AFB1, and 50 and 80 ng/L to OTA, respectively.
Data analysis
All results were expressed as the means ± SD (standard deviation) of three biological replicates. Analysis of variance (ANOVA) was performed for the data obtained using SAS for Windows 9.0 (SAS Institute Inc., Cary, NC). Differences were reported at a significance level of 95% by the Tukey test.
Results
Genome properties and phylogenetic analysis
The draft genome sequence of Pseudomonas strain 4B comprises 6.3 Mb, with an overall G + C content of about 66.5%. The PATRIC webservice analysis predicted 5948 coding sequences. Pseudomonas 4B genome presented 242 genes associated with virulence, 98 associated with antibiotic resistance (Table S3).
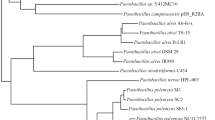
The phylogenetic tree (Fig. 1), made through MLSA analysis and employing the selected four housekeeping genes, allowed to include the Pseudomonas 4B inside the Pseudomonas aeruginosa group, specifically among P. aeruginosa PAO1 and P. aeruginosa DSM 50071. For confirmation, the virtually based DNA-DNA hybridization analysis was performed. This analysis resulted in DNA-DNA hybridization (DDH) values > 79% for the reference strains PAO1 and DSM 50071, revealing the correct inclusion of the Pseudomonas 4B strain into the P. aeruginosa group. In contrast, all the other genomes were considered as different bacterial groups, because of DDH < 70%. Moreover, the Pseudomonas 4B sequence comparison with P. aeruginosa PAO1 and P. aeruginosa DSM 50071 was the only showing ANI values over 95%. The detailed data from these analyses are provided in Table S4.
Phylogenetic analysis of Pseudomonas 4B based on the MLSA of the selected housekeeping genes. The General Time Reversible (GTR + G + I) model was used for the definition of the evolutionary history and the distances were computed with the Maximum Composite Likelihood approach. Next to the branches were allocated the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates). Branches are measured by the number of substitutions per site on the tree, which is drawn to scale. The evolutionary tree was based on the alignment of the four concatenated housekeeping genes
The level of BLAST genome similarities was graphically shown through the construction of the BLAST ring image generation (BRIG) (Fig. 2). The genomes considered for the phylogenetic analysis were incorporated from the most similar to the dissimilar one, which is represented by the external species group, in this case the gram-negative bacterium Escherichia coli. Notably, the absence and the lowest number of clear gaps in the figure rings indicate a high degree of sequence identity when comparing the Pseudomonas 4B genome with those belonging to the P. aeruginosa PAO1 and DSM 50071 strains, respectively. Moreover, the sequence alignment of P. aeruginosa reference strains (PAO1 and DSM 50071) had a higher level of similarity than their comparative analysis with the P. aeruginosa 4B genome (Fig. 3).
Genome sequence comparisons of Pseudomonas 4B and the other bacteria, respectively, from the internal to the external ring: P. aeruginosa DSM 50071, P. aeruginosa PAO1, P. oryzae KCTC32247, P. chlororaphis subsp. chlororaphis DSM50083, P. entomophila L48, P. mendocina NCTC10897, P. chlororaphis subsp. aurantiaca DSM 19603, P. protegens CHA0, P. putida KT2440, P. fluorescens F113, P. stutzeri 19SMN4, P. syringae pv. syringae B728a, E. coli O157H7. Pseudomonas 4B genome is represented by the innermost red circle, followed by its GC content (black circle)
Circos plots using the Circoletto program for visualization of sequence similarity between A Pseudomonas aeruginosa PAO1 and Pseudomonas aeruginosa DSM5007, B P. aeruginosa PAO1 and Pseudomonas 4B, and C P. aeruginosa DSM50071 and Pseudomonas 4B. Each band represents an individual genome. Local alignments produced by BLAST are presented using ribbons whose colors indicate the similarity percentages of the sequences, specifically blue ≤ 50%, green ≤ 75%, orange ≤ 99.9%, and red the maximum score of 100%. Red bands indicate the best alignment between the query sequence and the two reference strains (P. aeruginosa PAO1 and P. aeruginosa DSM5007)
Gene clusters for antimicrobial compounds
The presence of gene clusters related with biosynthesis of antimicrobial compounds was evaluated employing the antiSMASH online tool. It was possible to identify 15 gene clusters, and those with similarity at nucleotide level (> 20%) obtained by using the KnownClusterBlast algorithm with known compounds were emphasized (Table 1); these latter were found inside 11 different contigs of the genome. Some of the compounds were allocated inside other clusters; this occurs because the genes present on a contig occasionally do not match the antiSMASH criteria for gene cluster revelation [36]. Therefore, the presence of numerous gene clusters associated with biosynthesis of antifungal compounds encouraged to confirm the antagonistic capability of this strain against toxigenic fungi.
In vitro antagonism
The antagonistic activity was preliminarily evaluated against toxigenic fungi after 5 days on PDA plates. In general, strains of the genus Penicillium were more sensitive as compared to the other fungi (Fig. 4), although the formation of inhibition zones was observed against all fungal isolates tested (Table S1). The best results were observed against Penicillium citrinum ITAL197 and P. chrysogenum IFL2 since the strain 4B produced inhibition zones of 15.7 and 14.0 mm, respectively, during fungal growth (Fig. 4). The strains from genus Aspergillus were the most resistant, with inhibition zones ranging from 1.3 to 2.3 mm of diameter. However, the same fungi were not inhibited by the cell-free culture supernatant of strain 4B.
Growth and sporulation inhibition
The effect of Pseudomonas 4B on fungal growth parameters was evaluated, and results are detailed in Table S5. The bacterium caused a significant reduction (P < 0.05) of mycelial growth when compared with the diameter of fungal colonies in control treatments. Particularly, Pseudomonas 4B had greater influence on Aspergillus sp. UCO2A in which the fungal colony diameter was reduced by 86.1%. Besides that, the colony growth rate decreased from 3.2 to 0.25 mm/day when this fungus was co-inoculated with the strain 4B. Despite these results, the effect of bacteria on sporulation was not satisfactory, as the number of fungal spores per square centimeter produced was reduced to only about 32%.
Representative results were also observed on the growth of M. purpureus since the bacteria reduced the colony growth rate (0.06 mm/day) thereby influencing on the diameter of fungal colony, which decreased by 85.4%. Although there was a slight growth of the colony, sporulation was completely inhibited (Table S5) since it was not possible to view reproduction structures.
The same pattern was observed for A. parasiticus and P. citrinum: about 67% reduction in the colony diameter, a very slow mycelial growth when compared to control and 100% inhibition of conidia production (Table S5). Finally, A. flavus was the fungal isolate more resistant to Pseudomonas 4B, as only 43.3% reduction in growth was recorded. Even so, this bacterium was capable to decrease the colony growth rate (from 4.12 to 2.15 mm/day) and reduce the sporulation by almost 80%.
Inhibition of spore germination
Spores of toxigenic fungi were exposed to Pseudomonas 4B cells for assessing bacterial influence on their germination. A strong reduction in the number of germinated conidia was observed for all tested fungi after 24-h incubation (Fig. 5). The spore germination rate in the presence of antagonistic bacterium was significantly lower (P < 0.05) when compared with control (without bacterial cells), achieving values below 10%. Spores of P. citrinum were the most sensitive since the bacterium completely inhibited the conidial germination. Besides, spore inhibition rate for the other fungal isolates ranged from 84.1 to 98.5% and no abnormalities were observed in spores and germ tubes by microscope examination.
Effect on mycotoxin production
The influence of Pseudomonas 4B on mycotoxin production was also investigated. The maximum level of AFB1 produced by the A. flavus isolate growing under control conditions (without bacterial cells) was 27.7 ± 5.82 µg/g (Table 2). The AFB1 production reached values of only 1.58 ± 0.60 µg/g when the fungus was co-cultured with Pseudomonas cells, representing a drastic reduction of over 94%. Pseudomonas 4B also caused a significant effect on OTA synthesis by A. carbonarius since the OTA production was 13.7 ± 2.52 and 4.97 ± 0.78 µg/g in the control conditions and when co-cultivated with this bacterium, respectively (63.8% reduction). It could be observed that Pseudomonas 4B had a reducing effect on ratio of mycotoxins per colony diameter.
Discussion
The genome analysis allowed to classify the bacterium at species level. The multi locus sequence analysis (MLSA) using selected housekeeping genes included the strain 4B inside the P. aeruginosa cluster. This was confirmed by further comparative analyses with genomes of reference P. aeruginosa strains, as DNA-DNA hybridization (DDH) and average nucleotide identity (ANI) values were higher than 79% and 95%, respectively, which are proposed as criteria for defining bacterial strains of the same species [32].
The examination of P. aeruginosa 4B genome permitted to detect several clusters related with the biosynthesis of antifungal compounds. In this regard, the antibacterial activity of strain 4B was previously investigated [25], and the antifungal compounds pyoverdin and pyocyanin were confirmed for other P. aeruginosa strains [42]. P. aeruginosa typically produces pyocyanin, which was already observed for strain 4B [25]. This pigment is capable to arrest the electron transport chain of fungi [43] and therefore shows a broad spectrum of antifungal activity [44, 45]. Additionally, pyocyanin has been considered an alternative source in the manufacture of broad-spectrum eco-friendly agrochemicals and natural textile dyes with strong stability [46].
Moreover, many other antifungal compounds were predicted by genome-wide identification, annotation, and analysis through antiSMASH, and those with higher percentage of similarity include thanamycin [47], rhizomide [48], and pyochelin [49]. Other gene clusters were reported by the analysis, but showing reduced similarity and bioactivities other than antifungal. The presence of several gene clusters associated with biosynthesis of antifungal compounds indicated that strain 4B has great potential for synthesizing novel or structurally related metabolites for control of filamentous fungi. This fact was confirmed by the broad antagonistic activity against different strains of toxigenic fungi.
However, antifungal activity was not observed for the cell-free culture supernatant of strain 4B, suggesting that the cultivation condition used may not be suitable for the bacterium to produce antifungal compounds or at sufficient levels to inhibit the fungi under the assay conditions. Moreover, the inhibition of fungal growth was not observed in preliminary tests using lyophilized culture supernatants. Whereas this study focused on the P. aeruginosa 4B genome description, the various gene clusters found may direct more specific studies about the optimization of culture conditions for producing antifungal substances, as well as the development of suitable protocols for the extraction and identification of these compounds. In this regard, when different cultivation conditions of P. aeruginosa RS1 were evaluated to control the phytopathogen Phytophthora palmivora, the culture filtrate presented the highest inhibition (54.8%) activity when the strain RS1 was cultivated in Luria–Bertani (LB) broth, pH 7.0 at 37 °C compared to other culture media and conditions tested [50]. Another possibility for the observed results may be related to the antifungal molecules induction in response to the presence of fungi. Moussa et al. [51] found a clear enhancement of well-known antifungal metabolites of P. aeruginosa ATCC27853 (phenazine alkaloids, phenazine-1-carboxylic acid, and phenazine-1-carboxamide) obtained from a co-cultivation of this strain with a Fusarium tricinctum isolate. Therefore, further studies following this approach may contribute to the elucidation of the mechanisms involved in the production of antifungal metabolites by P. aeruginosa 4B.
Although the antagonistic activity of strain 4B was previously described against bacteria and some yeast like Candida utilis and Kluyveromyces marxianus [25], the results of the present study are very relevant because the ability to inhibit filamentous fungi, including mycotoxin producers, is demonstrated for the first time. Data obtained by in vitro co-inoculation (fungus-bacterium) showed a clear reduction of colony diameter for all fungi tested, ranging from 43 to 86%. Despite complete inhibition of fungal growth was not achieved, these values agree with other studies using P. aeruginosa strains [52,53,54]. A P. aeruginosa strain (NF011) isolated from wheat rhizosphere soil has been reported as promising biocontrol agent for reducing the mycelial growth of several phytopathogenic fungi, including Fusarium moniliforme (sin. F. verticillioides) (44.2%), Fusarium graminearum (64.5%), and Alternaria alternata (70.4%) [54].
P. aeruginosa 4B also influenced the spore formation in most of tested fungi. The complete sporulation inhibition for A. parasiticus, M. purpureus, and P. citrinum could be considered an advantage for the control of toxigenic fungi since mycotoxins production is usually associated with sporulation [55]. Few studies have reported significant mycotoxin production when there is no sporulation [56]. The inhibition results obtained for P. citrinum and M. purpureus are extremely important, since antagonism of Pseudomonas species against potential citrinin-producing fungi is rare [19]. Curiously, the strain 4B caused lower reduction in the colony diameter of A. flavus, but in contrast decreased significantly the number of spores. This result is promising, reducing the possibility of spreading spores in the atmosphere and surfaces susceptible to contamination.
Concerning the effect on spore germination, P. aeruginosa 4B also proved important inhibitory activity after 24-h contact with fungal spores. Previous studies reported conflicting data about inhibition of conidial germination by Pseudomonas species. P. fluorescens cells did not affect the spore germination of an aflatoxigenic A. flavus strain, but a reduction of 20% was observed when spores were inoculated with an extracellular chitinolytic enzyme extract of the bacterium [22]. The same authors suggest that different responses in spore germination assays could be related to species-specific targeting of each bacteria tested. In contrast, cells of Pseudomonas syringae strains isolated from herbaceous and woody plants inhibited spore germination of Penicillium digitatum [20]. After 24 h, a germination reduction ranging from 76.9 to 100% was observed, but the cell concentration (109 CFU/mL) and applied volume (250 µL) were greater than those used in our experiments.
The effect of co-culturing the bacterial strain with A. flavus or A. carbonarius exhibited influence on production of AFB1 and OTA, respectively. These strains were selected due to higher production of mycotoxins and because of the greatest number of reports about food contamination by these species, besides the risks associated with AFB1 and OTA [3, 57]. Our findings regarding the decrease of AFB1 production were consistent with those found for Pseudomonas solanacearum, which inhibited AFB1 formation by A. flavus and A. parasiticus strains, with reduction ranging from 56 to 100% [21]. Despite P. aeruginosa 4B caused a partial inhibition of OTA production, our data are critically important since the presence of microorganisms or chemicals that reduce the fungal growth is considered a stress condition, which in some cases might stimulate the synthesis of mycotoxins [56, 58].
Several factors may influence the production mycotoxins when fungi were co-cultivated with P. aeruginosa 4B. Competition for space and essential nutrients for the synthesis of aflatoxins as well as metabolites production by co-existing microorganisms could to play a certain role on aflatoxin formation, which may influence the expression of genes involved in mycotoxin synthesis [59]. The same considerations have been made for inhibition of OTA production [60]. Moreover, exposure of Fusarium graminearum to 25 mg/mL pyocyanin for 72 h significantly decreased the mycotoxins deoxynivalenol by 68.7% and nivalenol by 57.7% [61]. The capability of some bacteria, including Pseudomonas species, to reduce mycotoxins in foodstuffs, has been also associated with the degradation of mycotoxins [59].
The results of this study suggest that P. aeruginosa 4B could inhibit filamentous fungi due to the ability to reduce the fungal growth parameters. The decrease in the levels of mycotoxins (AFB1 and OTA) also indicates this bacterium as promising candidate for controlling toxigenic fungi. Similar to strain 4B, P. aeruginosa isolates from environmental origin have been investigated as biocontrol agents. Most of these studies have focused on the investigation of cell-free metabolites as an alternative approach for the safe use of this species in the fungal control [54, 62, 63]. Therefore, future studies using different cultivation conditions might be developed for optimizing the production and extraction of antifungal metabolite(s) from P. aeruginosa 4B. Despite the concern of P. aeruginosa be recognized as an opportunistic pathogen, successful studies on the evaluation of new strains revealed the production of valuable biomolecules, such as biosurfactants, antimicrobials, and polysaccharides [64, 65]. Recent reports showing the targeted development of hypovirulent P. aeruginosa strains [66, 67] reinforce the importance of genome and phylogenomics studies for assessing the full biotechnological potential of this species. Further experimentation will be necessary to elucidate the mode of action of the antagonistic bacterial strain in order to identify some antifungal compound responsible for this inhibition. This is the first report in which a Pseudomonas strain from aquatic environment was assessed about its potential antagonistic ability against toxigenic fungi. This encourages the searching for new biological control agents of toxigenic fungi, especially in the case of mycotoxin-producing fungi.
Data availability
Genomic data are available at the NCBI repository under the accession number VCSJ00000000. Data will be shared on reasonable request to the corresponding author.
References
Cinar A, Onbaşı E (2020) Mycotoxins: the hidden danger in foods. In: Sabucuoglu S (ed) Mycotoxins and Food Safety. IntechOpen, London
Nji QN, Babalola OO, Ekwomadu TI, Nleya N, Mwanza M (2022) Six main contributing factors to high levels of mycotoxin contamination in African foods. Toxins 14:318
De Ruyck K, De Boevre M, Huybrechts I, De Saeger S (2015) Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat Res 766:322–341
Alshannaq A, Yu JH (2017) Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health 14:632
IARC (1993) Ochratoxin A. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 56, Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. International Agency for Research on Cancer, Lyon, pp 489–521
IARC (2012) Aflatoxins. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 100F, Chemical agents and related occupations: A review of human carcinogens. International Agency for Research on Cancer, Lyon, p 225–248
Ismaiel AA, Papenbrock J (2015) Mycotoxins: producing fungi and mechanisms of phytotoxicity. Agriculture 5:492–537
Chiotta ML, Fumero MV, Cendoya E, Palazzini JM, Alaniz-Zanon MS, Ramirez ML, Chulze SN (2020) Toxigenic fungal species and natural occurrence of mycotoxins in crops harvested in Argentina. Rev Argent Microbiol 52:339–347
Luo S, Du H, Kebede H, Liu Y, Xing F (2021) Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control 127:108120
Janik E, Niemcewicz M, Ceremuga M, Stela M, Saluk-Bijak J, Siadkowski A, Bijak M (2020) Molecular aspects of mycotoxins - a serious problem for human health. Int J Mol Sci 21:8187
Huang Q, Jiang K, Tang Z, Fan K, Meng J, Nie D et al (2021) Exposure assessment of multiple mycotoxins and cumulative health risk assessment: a biomonitoring-based study in the Yangtze River Delta. China Toxins 13:103
Gayathri L, Dhivya R, Dhanasekaran D, Periasamy VS, Alshatwi AA, Akbarsha MA (2015) Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: in vitro study in HepG2 cell. Food Chem Toxicol 83:151–163
Luo Y, Liu X, Li J (2018) Updating techniques on controlling mycotoxins - a review. Food Control 89:123–132
Mir SA, Dar BN, Shah MA, Sofi SA, Hamdani AM, Oliveira CAF et al (2021) Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food Chem Toxicol 148:111976
Sangmanee P, Hongpattarakere T (2014) Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control 40:224–233
Medina A, Mohale S, Samsudin NIP, Rodriguez-Sixtos A, Rodriguez A, Magan N (2017) Biocontrol of mycotoxins: dynamics and mechanisms of action. Curr Opin Food Sci 17:41–48
Palmieri D, Ianiri G, Del Grosso C, Barone G, De Curtis F, Castoria R, Lima G (2022) Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 8:577
Cordero P, Cavigliasso A, Príncipe A, Godino A, Jofré E, Mori G, Fischer S (2012) Genetic diversity and antifungal activity of native Pseudomonas isolated from maize plants grown in a central region of Argentina. Syst Appl Microbiol 35:342–351
Gorantla JN, Kumar SN, Nisha GV, Sumandu AS, Dileep C, Sudaresan A et al (2014) Purification and characterization of antifungal phenazines from a fluorescent Pseudomonas strain FPO4 against medically important fungi. J Mycol Med 24:185–192
Panebianco S, Vitale A, Polizzi G, Scala F, Cirvilleri G (2015) Enhanced control of postharvest citrus fruit decay by means of the combined use of compatible biocontrol agents. Biol Control 84:19–27
Nesci AV, Bluma RV, Etcheverry MG (2005) In vitro selection of maize rhizobacteria to study potential biological control of Aspergillus section Flavi and aflatoxin production. Eur J Plant Pathol 113:159–171
Akocak PB, Churey JJ, Worobo RW (2015) Antagonistic effect of chitinolytic Pseudomonas and Bacillus on growth of fungal hyphae and spores of aflatoxigenic Aspergillus flavus. Food Biosci 10:48–58
Chen Y, Shen X, Peng H, Hu H, Wang W, Zhang X (2015) Comparative genomic analysis and phenazine production of Pseudomonas chlororaphis, a plant growth-promoting rhizobacterium. Genom Data 4:33–42
Reder-Christ K, Schmidt Y, Dörr M, Sahl HG, Josten M, Raaijmakers JM et al (2012) Model membrane studies for characterization of different antibiotic activities of lipopeptides from Pseudomonas. Biochim Biophys Acta 1818:566–573
Fontoura R, Spada JC, Silveira ST, Tsai SM, Brandelli A (2009) Purification and characterization of an antimicrobial peptide produced by Pseudomonas sp. strain 4B. World J Microbiol Biotechnol 25:205–213
Bankevich A, Nurk S, Antipov D et al (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477
Vásquez-Ponce F, Higuera-Llantén S, Pavlov MS, Marshall SH, Olivares-Pacheco J (2018) Phylogenetic MLSA and phenotypic analysis identification of three probable novel Pseudomonas species isolated on King George Island, South Shetland, Antarctica. Braz J Microbiol 49:695–702
Lalucat J, Mulet M, Gomila M, Garcia-Valdés E (2020) Genomics in bacterial taxonomy: impact on the genus Pseudomonas. Genes 11:139
Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ et al (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for annotating batches of genomes. Sci Rep 5:8365
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027
Meier-Kolthoff JP, Hahnke RL, Petersen J, Scheuner C, Michael V, Fiebig A et al (2014) Complete genome sequence of DSM 30083 T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand Genom Sci 9:2
Richter M, Rosselló-Móra R, Glöckner FO, Peplies J (2016) JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32:929–931
Alikhan N, Petty NK, Ben Zakour NL, Beatson SA (2011) BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402
Darzentas N (2010) Circoletto: visualizing sequence similarity with Circos. Bioinformatics 26:2620–2621
Liu B, Zheng D, Jin Q, Chen L, Yang J (2019) FDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res 47:D687–D692
Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E, Weber T (2013) AntiSMASH 2.0 - a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res 41:204–212
Benitez LB, Velho RV, Lisboa MP, Medina LFC, Brandelli A (2010) Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J Microbiol 48:791–797
Gandomi H, Misaghis A, Bastis AA, Bokaei S, Khosravi A, Abbasifar A, Javan AJ (2009) Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem Toxicol 47:2397–2400
Gemeda N, Woldeamanuel Y, Asrat D, Debella A (2014) Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: a potential source of botanical food preservative. Asian Pacific J Trop Biomed 4:S373–S381
Xu D, Wang H, Zhang Y, Yang Z, Xiulan S (2013) Inhibition of non-toxigenic Aspergillus niger FS10 isolated from Chinese fermented soybean on growth and aflatoxin B1 production by Aspergillus flavus. Food Control 32:359–365
Visconti A, Pascale M, Centonze G (2001) Determination of ochratoxin A in wine and beer by immunoaffinity column cleanup and liquid chromatographic analysis with fluorometric detection: collaborative study. J AOAC Int 84:1818–1827
Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV et al (2018) Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J Bacteriol 200:e00345-e417
Kerr R, Taylor GW, Rutman A, Cole PJ, Wilson R (1999) Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol 52:385–387
Hamad MNF, Marrez DA, El-Sherbieny SMR (2020) Toxicity evaluation and antimicrobial activity of purified pyocyanin from Pseudomonas aeruginosa. Biointerface Res Appl Chem 10:6974–6990
Sass G, Nazik H, Chatterjee P, Stevens DA (2021) Under nonlimiting iron conditions pyocyanin is a major antifungal molecule, and differences between prototypic Pseudomonas aeruginosa strains. Med Mycol 59:453–464
DeBrito S, Gajbar TD, Satapute P, Sundaram L, Lakshmikantha RY, Jogaiah S, Ito S-I (2020) Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci Rep 10:1542
Van Der Voort M, Meijer HJG, Schimidt Y, Watrous J, Dekkers E, Mendes R et al (2015) Genome mining and metabolic profiling of the rhizosphere bacterium Pseudomonas sp. SH-C52 for antimicrobial compounds. Front Microbiol 6:693
Wang X, Zhou H, Chen H, Jing X, Zheng W, Li R et al (2018) Discovery of recombinases enables genome mining of cryptic biosynthetic gene clusters in Burkholderiales species. PNAS 115:4255–4263
Briard B, Mislin GLA, Latg J, Beauvais A (2019) Interactions between Aspergillus fumigatus and pulmonary bacteria: current state of the field, new data, and future perspective. J Fungi 5:48
Sowanpreecha R, Rerngsamran P (2018) Biocontrol of orchid-pathogenic mold, Phytophthora palmivora, by antifungal proteins from Pseudomonas aeruginosa RS1. Mycobiology 46:129–137
Moussa M, Ebrahim W, Kalscheuer R, Liu Z, Proksch P (2020) Co-culture of the bacterium Pseudomonas aeruginosa with the fungus Fusarium tricinctum induces bacterial antifungal and quorum sensing signaling molecules. Phytochemistry Lett 36:37–41
Sandani HBP, Ranathunge NP, Lakshman PLN, Weerakoon WMW (2019) Biocontrol potential of five Burkholderia and Pseudomonas strains against Colletotrichum truncatum infecting chilli pepper. Biocontrol Sci Technol 29:727–745
Lawrance S, Varghese S, Varghese EM, Asok AK, S JM, (2019) Quinoline derivatives producing Pseudomonas aeruginosa H6 as an efficient bioherbicide for weed management. Biocatalysis Agricult Biotechnol 18:101096
Sun X, Xu Y, Chen L, Jin X, Ni H (2021) The salt-tolerant phenazine-1-carboxamide-producing bacterium Pseudomonas aeruginosa NF011 isolated from wheat rhizosphere soil in dry farmland with antagonism against Fusarium graminearum. Microbiol Res 245:126673
Brodhagen M, Keller NP (2006) Signalling pathways connecting mycotoxin production and sporulation. Mol Plant Pathol 7:285–301
Mossini SAG, Arrotéia CC, Kemmelmeier C (2009) Effect of neem leaf extract and neem oil on Penicillium growth, sporulation, morphology and ochratoxin A production. Toxins 1:3–13
Veras FF, Correa APF, Welke JE, Brandelli A (2016) Inhibition of mycotoxin-producing fungi by Bacillus strains isolated from fish intestines. Int J Food Microbiol 238:23–32
Ahmed H, Strub C, Hilaire F, Schorr-Galindo S (2015) First report: Penicillium adametzioides, a potential biocontrol agent for ochratoxin-producing fungus in grapes, resulting from natural product pre-harvest treatment. Food Control 51:23–30
Yang X, Zhang Q, Chen ZY, Liu H, Li O (2017) Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE 12:e0178810
Vankudoth KR, Boda A, Sivadevuni G, Solipuram MR (2016) Effect of indigenous fungi on ochratoxin A produced by two species of Penicillium. Anim Nutr 2:225–228
Houshaymi B, Awada R, Kedees M, Soayfane Z (2019) Pyocyanin, a metabolite of Pseudomonas aeruginosa, exhibits antifungal drug activity through inhibition of a pleiotropic drug resistance subfamily FgABC3. Drug Res 69:658–664
Lahkar J, Goswami D, Deka S, Ahmed G (2018) Novel approaches for application of biosurfactant produced by Pseudomonas aeruginosa for biocontrol of Colletotrichum capsici responsible for anthracnose disease in chilli. Eur J Plant Pathol 150:57–71
Zhang F, Yang C, Zhang X, Zhu H, Zhao D, Huang Y (2020) Isolation of an anti-entomopathogenic fungal protein secreted from Pseudomonas aeruginosa BGf-2: An intestinal bacterium of Blattella germanica (L.). J Invertebrate Pathol 173:107371
Sood U, Singh DN, Hira P, Lee J-K, Kalia VC, Lal R, Shakarad M (2020) Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. J Biotechnol 307:98–106
Valentine ME, Kirby BD, Withers TR, Johnson SL, Long TE, Hao Y, Lam JS, Niles RM, Yu HD (2020) Generation of highly attenuated strain of Pseudomonas aeruginosa for commercial production of alginate. Microb Biotechnol 13:162–175
Grosjean M, Guénard S, Giraud C, Muller C, Plésiat P, Juarez P (2021) Targeted genome reduction of Pseudomonas aeruginosa strain PAO1 led to the development of hypovirulent and hypersusceptible rDNA hosts. Front Bioeng Biotechnol 9:640450
Khatun MA, Hoque MA, Koffas M, Feng Y (2023) Reducing the virulence of Pseudomonas aeruginosa by using multiple quorum-quenching enzymes. J Ind Microbiol Biotechnol 50:kuad028
Acknowledgements
The authors thank the Instituto de Tecnologia de Alimentos (ITAL, Campinas, Brazil) for providing Aspergillus carbonarius and Penicillium citrinum strains.
Funding
This study was supported by Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) [grant 308880/2021–8] and Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) [grant 19/2551–0001855-0].
Author information
Authors and Affiliations
Contributions
FFV, AB, and JEW contributed to the study conception and design. Material preparation, data collection, data analysis, and statistical analysis were performed by FFV, PS, ACR, FMS, APMV, and FQM. The manuscript draft was written by FFV and critically revised by AB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study does not involve human or animal participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Eleni Gomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Veras, F.F., Stincone, P., Welke, J.E. et al. Genome analysis of Pseudomonas strain 4B with broad antagonistic activity against toxigenic fungi. Braz J Microbiol 55, 269–280 (2024). https://doi.org/10.1007/s42770-024-01253-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01253-w