Abstract
The mangrove ecosystem plays a crucial role in preserving the biodiversity of plants, animals, and microorganisms that are essential for materials cycles. However, the exploration of endophytic fungi isolated from mangroves, particulary in Santa Catarina (SC, Brazil), remains limited. Therefore, the purpose of this study was to assess the biodiversity of endophytic fungi found in Avicennia schaueriana, Laguncularia racemosa, Rhizophora mangle, and Spartina alterniflora from two mangroves on the Island of Santa Catarina: one impacted by anthropic action (Itacorubi mangrove) and the other environmentally preserved (Ratones mangrove). Samplings were carried out between January 2020 and May 2021. Fungi were isolated from leaves, stems, and roots, identified, and clustered into groups through morphological characteristics. Further, a representative strain of each group was identified through ITS1 sequencing. A total of 373 isolates were obtained from plant tissues, of which 96 and 277 isolates were obtained from Itacorubi and Ratones mangroves, respectively. Molecular identification showed that the endophytic fungal community comprised at least 19 genera. The data on fungal community diversity revealed comparable diversity indices for genera in both mangroves. However, we observed differences in the total frequency of fungal genera between impacted (27.38%) and non-impacted (72.62%) mangroves. These findings suggest that anthropic activities in and around the Santa Catarina mangroves have had negative impact on the frequency of endophytic fungi. This emphasizes the reinforcing the significance of preserving these environments to ensure the maintenance of fungal community diversity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mangrove is essential ecosystem in conserving plants, vertebrates, invertebrates, and microorganisms biodiversity and provides an ideal habitat for aquatic and terrestrial animals [1, 2]. This ecosystem microbial community can continuously transform nutrients from dead vegetation into sources of carbon, nitrogen, and phosphorus [3]. In mangrove environments, bacteria and fungi represent about 91% of the microbial biomass, while algae and protozoa represents 7 and 2%, respectively [4, 5]. It is estimated that about 625 fungal species come from mangroves, representing only about 0.62% of the fungal species described in the world [6].
The state of Santa Catarina (SC), currently has brazilians fourth smallest area of mangroves, with approximately 10.4 thousand hectares where three autochthonous plant species, Avicennia schaueriana, Laguncularia racemosa, and Rhizophora mangle, and associated species, such as the grass Spartina alterniflora can be found [7]. The microbial community can establish in an ecosystem through microorganisms-plant interaction [8]. Endophytic microorganisms colonize internal plant tissues without causing apparent harm and may have a neutral or beneficial relationship with their host [9].
Endophytic fungi can secrete biologically active compounds that protect plants from pathogen attacks an these secondary metabolites and enzymes have various biotechnological, pharmacological and industrial applications, such as phytohormone, bioremediation, biofertilization, biocontrol, immunosuppressive, antiparasitic, antimicrobial, antitumor, and antioxidant activity [10,11,12].
In Brazil, there are reports of mangrove endophytic fungi isolated from plants in Pernambuco [13], in São Paulo [14], in Ceará [15], and Bahia [16]. Although the endophytic fungal communities associated with mangrove plants have been studied in other parts in the world, in Santa Catarina Island, endophytic fungi isolated from mangroves have been poorly studied, which makes it imperative for the microorganisms prospection from this ecosystem [17]. Many different (epiphytic, endophytic, and pathogenic) fungi species with different mode of nutrition are associated with forest ecosystem. Endophytic fungal biodiversity is represented by a large number of species which can produce a high variety of compounds that are still unknown and may have essential biotechnological applications [18].
It is well known that the unplanned growth of cities and the diverse anthropic activities can cause significant negative impacts on the natural environment. Over the last three decades, conversions to aquaculture, industrial activities, urbanization development among others have destroyed more than 50,000 hectares (approximately 4%) of the Brazilian mangroves [19]. Although endophytic fungi communities associated to plants from mangrove ecosystem have been studied in other parts of the world, there is still limited knowledge about the fungal community in the mangroves of Santa Catarina. Therefore, the objective of this study was to isolate and compare the species diversity of the endophytic fungal community found in leaves, stems, and roots of Avicennia schaueriana, Laguncularia racemosa, Rhizophora mangle, and Spartina alterniflora from impacted (Itacorubi) and non-impacted (Ratones) mangroves in Santa Catarina Island, Santa Catarina state, Brazil.
Materials and methods
Location of sample collection
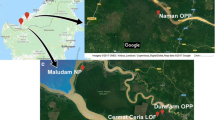
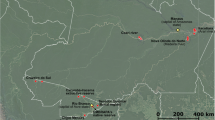
This study took place in two mangroves along Santa Catarina Island: Itacorubi mangrove (27°34′14″ S, 48°30′07″ W), and Ratones mangrove (27°44'50" S, 48°55'48" W). The field samplings were carried out between January 2020 and May 2021. Itacorubi mangrove covering an area of 150 hectares has been impacted since 1980s by uncontrolled urbanization. The lack of sufficient sewage collection network has led to the discharge of both domestic sewage and solid waste in the rivers and streams that form the Itacorubi hidrographic basin [20, 21]. On the other hand, the Ratones mangrove covers an area of 890 hectares is situated within the Carijós Ecological Reserve, which is a preserved area free from anthropic action. According to the Köppen classification, the climate region is Cfa, humid subtropical without a characteristic dry season, with a reduction in rainfall from April to September. The average annual temperature is approximately 21°C, with annual precipitation superior to 1500 mm, and characterized by strong winds blowing from south to north [22].
Plant material
Three sampling points were carried out in the Itacorubi mangrove (I), collection A1, A2, and A3, in two sampling points and two collections in the Ratones mangrove (R) collection B1 and B2, in five sampling points from January 2020 to May 2021 (Supplementary material fig. S1). The biological material of four plant species (A. schaueriana, L. racemosa, R. mangle, and S. alterniflora) was collected with the aid of a pruner, packed in polyethylene bags, and transported to the Laboratory of Microorganisms and Biotechnological Processes (LAMPB) at UFSC. The samples were divided into healthy and fresh leaves (Le), stems (St), and roots (Rt). All collections of biological material were authorized by the Biodiversity Authorization and Information System (SISBIO) number 73719-2.
Fungal culture media
To access fungal biodiversity and isolation of endophytic fungi, four culture media (Table 1) adapted from Ananda and Sridhar [23] were used. Potato dextrose agar (PDA) and Sabouraud agar (SA) media (Kasvi, Brazil) were sterilized by autoclaving (121°C and 20 min) and supplemented with Streptomycin (50 μg/mL) and Thiamphenicol (50 μg/mL) for bacterial growth inhibition.
Endophytic fungi isolation
Endophytic fungi were isolated from fresh leaves, stems, and roots. Plant tissues were subjected to washing in running water to remove dirt and subsequently, the surface disinfection steps was performed by a sequence of soaking in the following solutions: 70% ethanol (1 min), 2% sodium hypochlorite (4 min), 70% ethanol (30 s) and rinsed twice in sterile distilled water (1 min) [14]. After disinfection, the leaves, stems, and roots were cut into fragments (5 mm2) using a sterilized scalpel.
Five plant fragments from each plant part were randomly chosen and placed into Petri dishes containing the culture media (1, 2, 3, and 4) and maintained in a BDO incubator (25°C for 7–30 days). After fungal growth, colonies were repeatedly transferred to new culture media until pure colonies were obtained. To evaluate disinfection efficiency, 100 μl of the last wash water was inoculated in Petry dishes containing PDA and SA media and plates were incubated at 25°C for ten days.
Morphological fungi identification
Morphological identification of fungi strains was performed by observing the macroscopic and microscopic characteristics. Strains were cultivated in Petry dishes containing PDA medium (25°C, 7 days) through a punctual inoculation, and colonies characteristics were accessed. Microscopic structures were visualized by the slide microculture technique using PDA medium [24]. After incubation at 25°C for 7 to 14 days for fungal growth, each coverslip was removed, fixed with lactophenol with or without cotton blue, and mounted on a microscope slide. Microscopic structures were observed under an optical microscope at 400X and compared to the literature [25, 26].
Molecular fungi identification
The endophytic fungi DNA extraction, amplification, and sequencing procedures were carried out by the company Neoprospecta. An amount of 1 cm2 of each fungal colony growth on PDA (25°C, 7 days) was transferred to microtubes containing a buffer solution (Neosample X) and shipped to the company. DNA extraction was done with the DNA/RNA mini Kit QIAGEN® ID: 80004. The internal transcribed spacer region was amplified with primers ITS1 (GAACCWGCGGARGGATCA) and ITS2 (GCTGCGTTCTTCATCGATGC) [27] and amplification conditions were done according Neoprospecta Company protocol. The amplicons were sequenced using the MiSeq Sequencing system (Illumina Inc., USA) and the sequences quality were analyzed by Phred/Phrap (QP) and obtained through the Sentinel pipeline using the FastQC v.0.11.8 program. Taxonomic identification was performed through Blastn v.2.6.0+ using the NCBI database as a reference. The ITS1 region sequences obtained were compared with the ITS1 region sequences deposited in the GenBank Nucleotide Collection database.
Strains preservation
The endophytic fungi obtained in this study are storaged in Ultrafreezer (-80°C) in tubes with inclined PDA overlayed with mineral oil and cryopreserved in liquid nitrogen [25, 26] and deposited in the Culture Collection of Microorganisms at the Laboratory of Microorganisms and Biotechnological Processes, Biological Science Center, Federal University of Santa Catarina, Brazil.
Statistical analysis
Analysis of variance was performed considering plant tissue, plant species, and mangrove, from the endophytic fungal frequency data (Ffe), calculated by equation according [14].
The Ffe data were transformed (\(\sqrt{x+1}\) ) and normalized data were analyzed using the Shapiro-Wilk normality test. It was considered that Ffe data are non-parametric, and a generalized mixed model or mixed linear effects model was used, leaving repetition as a random effect, both with Poisson error distribution, since the number of repetitions (collections) was not the same, making it necessary to use the random effect. Therefore, the data that had p<0.01 was considered only as a significant difference for greater reliability. Analysis of variance was performed using the R version 4.2.1 program.
Fungal diversity
The diversity of the endophytic fungal community associated with mangrove plants was evaluated using frequency and diversity indices. The total and partial abundance (AbT and Ab) of each fungal genera was calculated (Eq. (2)) for isolation mangrove, plant species, tissue, and culture medium, where Ni= number of isolates of genus A and N= sum of all isolated genera.
AbT or Ab= (Ni/N) *100
To assess the diversity of endophytic fungal genera from different isolation sites, host species, tissue, and culture media, the Shannon index (H) was used (Eq. (4)) in PAST software, where Ni = number of isolates of genus A and N = sum of all isolated genera.
Results and discussion
Endophytic fungi isolation
A total of 373 isolates were obtained from plant tissue fragments, of which 96 and 277 strains were obtained from the Itacorubi (impacted) and Ratones (non-impacted) mangroves, respectively. Three hundred and sixty four strains were identified as endophytic fungi by microscopic caracteristics and nine were identified as endophytic bacteria, which are not the subject of the present study. After the plant tissue disinfection, inoculation of the last washing water (negative control) was performed and showed no microorganisms growth.
The variance analysis was performed based on endophytic fungal frequency data (Ffe) presented in Table 2. The following parameters were analyzed: plant part (leaf, stem, and root), sampling site (Itacorubi and Ratones), species (A. schaueriana, L. racemosa, R. mangle, and S. alterniflora), and culture media (PDA and SA with and without sea water).
The results showed that the variation factors (species and sampling site) had significant effects (p<0.01 and <0.05) on the number of endophytic fungi isolated from the two mangroves. Plant species significantly influenced (p= 0.0002) endophytic fungi frequency (Table 2). Although the fungal frequency between A. schaueriana and L. racemosa did not differ, it showed significant differences against R. mangle and S. alterniflora (Fig. 1A).
Endophytic fungal frequency of mangrove plant species from Santa Catarina Island. A Fungal frequency according to plant species. B Fungal frequency according to impacted and non-impacted mangrove. C Fungal frequency according to plant tissue. D Fungal frequency according to the culture medium. Different letters represent significant statistical differences. Data transformed by \(\sqrt{\mathrm{X}+1}\)
In terms of absolute number of isolates (Table S1), L. racemosa presented the highest number of isolates per species (140), followed by A. schaueriana (135), S. alterniflora (58), and R. mangle (40). In a study of the endophytic fungal community in mangroves plants in São Paulo state, L. racemosa was the was the most colonized plant species by endophytic fungi, whereas A. schaueriana hosting the smaller endophytic fungal population[14]. In the present study, the smallest endophytic fugal population was observed in R. mangle.
Regarding to fungal frequency between the non-impacted and impacted mangrove, the results showed a significant difference between them (p=0.0061). The non-impacted mangrove had a higher fungal frequency and a more considerable number of isolates (Fig. 1B). In the study of Sebastianes et al. [14], a significant lower endophytic frequency was observed in non-impacted mangrove. Considering the fungal frequency between plants part (leaf, stem, and root), the statistical analysis did not show significant differences (Fig. 1C). However, the highest number of isolates were obtained from leaves (184), followed by stems (107) and roots (82).
Culture media may play an important role in fungi isolation. The statistical analysis revealed significant differences between the culture media used (P= 0.0017). However, this difference only occurred between typical PDA and PDAS (Fig. 1D).
Morphological endophytic fungi identification
Based on macro and micromorphological characteristics, 373 isolates were identified as filamentous fungal strains (364 strains) and filamentous bacteria (9 strains), which were not the goal of this study. There was no isolation of yeast-like fungi in this study. Initially, the isolates were grouped into 62 morphogroups according to morphological characteristics (Supplementary material Fig. S1S2, S3, and S4). The 62 morpho groups are distributed among 16 different genera (Fig. 2). However, 109 isolates could not be identified since they did not produce reproductive structures at tested conditions.
Although morphological characteristics, such as reproductive and vegetative structures, are essential for describing new species or identifying described fungi, several endophytic fungi strains do not show these characteristics in culture condition [28]. Several factors as the carbon source, substrate concentration, light, pH, and temperature may influence morphological characteristics production [28].
Molecular fungi identification
Among 62 morphogroups of endophytic fungi previously clustered, 51 morphogroups were submitted to high-performance sequencing of the ITS1 region by Neoprospecta Microbiome Technologies company, Brazil. One representative strain of each morphogroup was molecularly identified (Table 3). The ITS1 sequences were obtained through the Sentinel pipeline using the FastQC v.0.11.8 program and compared with sequences deposited in the GenBank database (NCBI) (Table 3). The highest similarity (<97%) and bit-score values and the lowest e-value values were considered to determine the taxonomic classification of the evaluated fungi. In the present study, the 19 genera identified by ITS sequencing belong to the Ascomycota phylum (94,12%), which includes three classes: Eurotiomycetes, Sordariomycetes e Dothideomycetes and Basidiomycota phylum representing 5,88% with one class: Agaricomycetes. The predominance of Ascomycota phylum among endophytic fungi of mangrove plants also had been reported by Sebastines [14], which obtained 99,4% of ascomycetes from Eurotiomycetes, Sordariomycetes, Dothideomycetes, and Saccharomycetes. Even though only the sequences of two strains presented similarity lower than 97%, several strains were identified only at genus or section level, e.g., Aspergillus, Penicillium, Trichoderma, and Fusarium. This occurred because the identification of some fungal species required the use of multiple genetic markers and even a polyphasic approach [25].
Diversity of the endophytic fungal community
Fungal community diversity (frequency and diversity index) was calculated based on fungi identification at the genera or species level. The only exception was for the strain identified as the Xylariaceae family, which was also included, totaling 263 isolates. It was considered high fungi frequencies above 10% and minor frequencies smaller than 3%.
The total and partial frequency of endophytic fungal genera in mangroves ranged from 0.38 to 15.48% (Table 4). The genera with more isolates and highest frequencies were Fusarium (41), Trichoderma (37), and Colletotrichum (34). Fusarium genus represent 15.59% of the isolated fungal population. Strains from this group have been isolated as an endophyte from various hosts in subtropical and tropical regions, mainly in mangrove plants from Brazil, Nigeria, Malaysia, and Bangladesh [14, 16, 24, 29, 30]. In our study, the Fusarium community was not affected by the anthropic action since strains were obtained in similar frequency in impacted (16.67%) and non-impacted mangroves (15,18%). Similar results were reported for Fusarium in a study on impacted and non-impacted mangroves in São Paulo [14]. The authors did not observe statistical differences in the frequency of endophytes in impacted mangrove compared to non-impacted one. However, the Fusarium incarnatum-equiseti complex (Table 3) was isolated only from A. schaueriana collected from the non-impacted mangrove.
The genus Trichoderma was found to be the second most frequently isolated among mangrove plants accounting for approximately 14.07% of the isolates. Similar findings, these fungi have been reported in previous studies conducted in China and Brazil, where Trichoderma fungi were also isolated from mangrove plants [14, 16, 31]. In Brazil, endophytic strains of Trichoderma have been specifically identified in A. schaueriana, L. racemosa, and R. mangle within the Brazilian mangrove ecosystem [14]. Although Trichoderma spp. were isolated in both (Itacorubi and Ratones) mangroves, statistical analysis revealed significant differences in their frequency, with a higher occurrence observed in the non-impacted mangrove (Table 4). This suggests that anthropic action might have a impacted on the frequency of these fungi. In a study conducted in mangroves of São Paulo, Trichoderma was the fourth most abundant genus, with minor variations frequency observed between impacted and non-impacted mangroves [13, 14, 32].
Colletotrichum was the third most frequent genera in the evaluated community (12.93%). This group has already been isolated as an endophytic fungus in mangroves in Brazil, Bangladesh, Thailand, and Nigeria [14,15,16, 30, 33]. Similar to Fusarium and Trichoderma, Colletotrichum community was unaffected by anthropic action since strains were isolated in both mangroves. However, Colletotrichum spp. showed a higher difference in frequency and isolate numbers in Itacorubi mangrove (14.14%) than Ratones mangrove (9.72%). Unlike our result, Sebastines et al. [14] found a higher frequency (17.05%) in the impacted mangrove than the non-impacted mangrove (7.06%) in the São Paulo state.
The genera that showed a lower frequency and a lower number of isolates from the evaluated fungal population were Arthrinium, Diaporthe/Phomopsis, Pseudogymnoascus, Venturia, Stemphylium, Buergenerula, Neofusicoccum, Phlebia, and Scopulariopsis (Table 4). Strains from Buergenerula, Neofusicoccum, and Stemphylium genera were obtained only from mangrove areas impacted by anthropic action. However, in the Itacorubi mangrove Arthrinium, Diaporthe/Phomopsis, Scopulariopsis, and Venturia were not isolated from any plant, suggesting that pollution may affect the diversity of fungal communities.
Species of Neofusicoccum, Diaporthe/Phomopsis, Scopulariopsis, and Stemphylium have already been isolated as endophytes from Brazilian [13,14,15] and Asian mangroves [34,35,36,37,38]. However, other genera, such as Buergenerula, Arthrinium, Pseudogymnoascus, and Venturia, have not been yet reported as endophytes of mangrove plants. Therefore, this study documented for the first time the occurrence of endophytic fungi belonging to these genera in endemic mangrove plants.
Diversity index
The diversity of endophytic fungi was performed using the Shannon index (H'), and showed a similar index between non-impacted mangrove (H'= 2.38) and impacted mangrove (H'=2.33) (Table S2 Supplementary material). The very close values found may be related to genera number associated with impacted (14) and non-impacted (17) mangroves. However, the total frequency of isolates was 2.6 fold higher for the non-impacted mangrove. Similar results of diversity index were also reported between Bertioga (oil-affected) and Cananeia (unaffected) mangroves in the state of São Paulo [14].
Other sources of variation as plant species, plant part, and culture media also presented close Shannon index (Table S2). In a study conducted in São Paulo coast mangroves, a resembling Shannon index was reported between R. mangle, L. racemosa and A. nitida endophytes [14]. Even though our results showed similar genera diversity index, the total frequency of each fungal genus was different between impacted (27.38%) and non-impacted (72.62%) mangroves. These results may suggest that anthropic action in/around the Santa Catarina mangroves impacted negatively the endophytic fungi frequencies, reinforcing the importance of preserving these environments to maintain fungal community diversity.
Conclusion
The endemic plants of two mangroves (one impacted an other non-impacted) at Santa Catarina Island evaluated in this study are colonized by a diverse community of endophytic fungi belonging to at least 19 genera, such as Buergenerula, Arthrinium, Pseudogymnoascus, and Venturia, that have not been yet reported as endophytes of mangrove plants. The Shannon index showed similar fungal genera diversity between impacted and non-impacted mangrove. However, significant statistical differences in the endophytic fungal frequency between the plant species, plant tissues, and culture media were observed, suggesting the importance of preserving this ecosystem for maintenance of the fungal community present.
Data Availability
All data supporting the findings of this study are available within the article and in the Supplementary Information. All DNA sequences are deposited in the NCBI database (www.ncbi.nlm.nih.gov)
Abbreviations
- PDA:
-
Potato dextrose agar
- SA:
-
Sabouraud agar
- Ffe:
-
Fungal frequency data
- Ni:
-
Number of isolates of genus
- N:
-
Sum of all isolated genera
- AbT:
-
Total abundance
- Ab:
-
Partial abundance
- H:
-
Shannon index
- NT:
-
Total number of isolates of each fungal genera
- NiI:
-
Number of isolates of each genus from the Itacorubi mangrove
- NiR:
-
Number of isolates of each genus from the Ratones mangrove
- FpI:
-
Partial frequency of fungal genera from Itacorubi mangrove
- FpR:
-
Partial frequency of fungi genera from Ratones mangrove
- FT:
-
Total frequency of each genus
References
Abdel-Azeem AM, Salem FM, Abdel-Azeem MA, Nafady NA, Mohesien MT, Soliman EA (2016) Biodiversity of the genus Aspergillus in different habitats. In: Gupta VK (ed) New and future developments in microbial biotechnology and bioengineering. Elsevier, Amsterdam, pp 3–28
Rajpar MN, Zakaria M (2014) Mangrove fauna of Asia. In: Faridah-Hanum I, Latiff A, Hakeem KR, Ozturk M (eds) Mangrove ecosystems of Asia: Status, challenges and management strategies. Springer, New York, pp 153–197. https://doi.org/10.1007/978-1-4614-8582-7
Holguin G, Vazquez P, Bashan Y (2001) The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils 33:265–278. https://doi.org/10.1007/s003740000319
Li K, Chenn S, Pang X, Cai J, Zhang X, Liu Y (2022) Natural products from mangrove sediments-derived microbes: structural diversity, bioactivities, biosynthesis, and total synthesis. Eur J Med Chem 230:114117. https://doi.org/10.1016/j.ejmech.2022.114117
Thatoi H, Behera BC, Mishra RR, Dutta SK (2013) Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Ann Microbiol 63:1–19. https://doi.org/10.1007/s13213-012-0442-7
Palit K, Rath S, Chatterjee S, Das S (2022) Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: threats, vulnerability, and adaptations. Environ Sci Pollut Res 29:32467–32512. https://doi.org/10.1007/s11356-022-19048-7
Figueiroa AC, Brasil G, Pellin A, Scherer MEG (2016) Avaliação da efetividade da integração das Unidades de Conservação federais marinho-costeiras de Santa Catarina. Desenvolv Meio Ambiente 38:361–375. https://doi.org/10.5380/dma.v38i0.46974
Chen Q, Zhao Q, Li J, Ren H (2016) Mangrove succession enriches the sediment microbial community in South China. Sci Rep 6:27468. https://doi.org/10.1038/srep27468
Chapla VM, Biasetto CR, Araujo AR (2013) Fungos endofíticos: uma fonte inexplorada e sustentável de novos e bioativos produtos naturais. Rev Virtual Química 5:421–437
Chatterjee A, Abraham J (2020) Mangrove endophytes: a rich source of bioactive substances. In: Patra Jk, Mishra RR, Thatoi H, Biotechnological Utilization of Mangrove Resources. Elsevier, London, 27–47.
Robl D, Delabona PS, Mergel CM, Rojas JD, Costa PS, Pimentel IC, Vicente VA, Pradella JGC, Padilha G (2013) The capability of endophytic fungi for production of hemicellulases and related enzymes. BCM Biotech 13:94. https://doi.org/10.1186/1472-6750-13-94
Patel HK, Makampara RA, Kalaria RK, Joshi MP (2023) Endophytes: a novel tool for sustainable agriculture. In: Shah MP, Deka D (eds) Developments in Applied Microbiology and Biotechnology Endophytic Association: What, Why and How. Elsevier, New Delhi, India, pp 37–54
Costa IPM, Maia LC, Cavalcanti MA (2012) Diversity of leaf endophytic fungi in mangrove plants of northeast Brazil. Braz J Microbiol 43:1165–1173. https://doi.org/10.1590/S1517-83822012000300044
Sebastianes FLS, Romão-Dumaresq AS, Lacava PT, Harakava R, Azevedo JL, Melo IS, Pizzirani-Kleiner AA (2013) Species diversity of culturable endophytic fungi from Brazilian mangrove forests. Curr Genet 59:153–166. https://doi.org/10.1007/s00294-013-0396-8
Maia LKR, Alves DR, Jacinto-Junior SG, Morais SM, Freire FCO, Bordallo PN, Cardoso JE (2022) Identification and characterization of endophytic fungi found in plants from northeast Brazilian mangroves: a review. Res Soc Dev 11:e5111729459. https://doi.org/10.33448/rsd-v11i7.29459
Pinto RADFO (2019) Bioprospecção e caracterização de fungos endofíticos produtores de compostos bioativos isolados de Dalbergia ecastaphyllum L. Taub. Dissertação de Mestrado em Ciências Biológicas. Universidade Federal de Alfenas, pp 101
Dias LRL, Bastos DKL, Lima NS, Silva MRC, Miranda RCM (2017) Bioprospecção de Microorganismos de Interesse Biotecnológico Isolados em Ecossistema de Manguezal. Rev Investig Bioméd 9:24–30. https://doi.org/10.24863/rib.v9i1.84
Shu-Lei J, Zhe C, Guang-Lei L, Zhong H, Zhen-Ming C (2020) Fungi in mangrove ecosystems and their potential applications. Crit Rev Biotechnol 40:852–864. https://doi.org/10.1080/07388551.2020.1789063
Ferreira AC, Lacerda LD (2016) Degradation and conservation of Brazilian mangroves, status and perspectives. Ocean Coast Manag 125:38–46. https://doi.org/10.1016/j.ocecoaman.2016.03.011
Sovernigo MH (2009) Manguezal do Itacorubi (Florianópolis, SC): Uma revisão da disponibilidade de dados ecológicos visando o direcionamento de novos estudos. Oecologia Brasiliensis 13:575–595. https://doi.org/10.4257/oeco.2009.1304.03
Laurenti A, Fechine VY, de Vasconcelos AGC, dos Santos F, Cavalleri M, Reis M, Ayala Filho GGM (2018) UFSC Micro Basin—A Preliminary Study: A Stream to Call It Ours. In: Leal Filho W, Frankenberger F, Iglecias P, Mülfarth R (eds) Towards Green Campus Operations. World Sustainability Series. Springer, Cham, pp 635–652. https://doi.org/10.1007/978-3-319-76885-4_43
Pandolfo C, Braga HJ, da Silva Jr VP, Massignam AM, Pereira ES, Thomé VMR, Valci FV (2002) Atlas climatológico do Estado de Santa Catarina. Epagri. CD-Rom, Florianópolis
Ananda K, Sridhar KR (2002) Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can J Microbiol 48:871–878. https://doi.org/10.1139/W02-080
Riddell RW (1950) Permanent stained mycological preparations obtained by slide culture. Mycologia 42:265–270. https://doi.org/10.1080/00275514.1950.12017830
De Hoog GS, Guarro J, Gene J, Ahmed S, Al-Hatmi A, Figueras MJ, Vitale RG (2020) Atlas of Clinical Fungi. 4th edn, vol 1 and 2. Utrecht/Reus. Netherlands, pp 1600
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2019) Food and indoor fungi. Westerdijk Fungal Biodiversity Institute, Utrech, p 481p
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A Guide to Methods and Applications. Academic Press Inc, New York, pp 315–322
Senanayake IC, Rathnayaka AR, Marasinghe DS, Calabon MS, Gentekaki E, Lee HB, Hurdeal VG, Pem D, Dissanayake LS, Wijesinghe SN, Bundhun D, Nguyen TT, Goonasekara ID, Abeywickrama PD, Bhunjun CS, Jayawardena RS, Wanasinghe DN, Jeewon R, Bhat DJ, Xiang MM (2020) Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11:2678–2754. https://doi.org/10.5943/mycosphere/11/1/20
Akinduyite AE, Ariole CN (2018) Bioactive compounds and antibacterial activity of endophytic fungi isolated from Black Mangrove (Avicennia africana) leaves. Niger J Biotechnol 35:35–42. https://doi.org/10.4314/njb.v35i2.5
Nurunnabi TR, Sabrin F, Sharif DI, Nahar L, Sohrab MH, Sarker SD, Rahman SMM, Billah MM (2020) Antimicrobial activity of endophytic fungi isolated from the mangrove plant Sonneratia apetala (Buch.-Ham) from the Sundarbans mangrove forest. Adv Trad Med 20:419–425. https://doi.org/10.1007/s13596-019-00422-9
Zhang L, Niaz SI, Khan D, Wang Z, Zhu Y, Zhou H, Lin Y, Li J, Liu L (2017) Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungus Trichoderma sp. (strain 307) by co-cultivation with Acinetobacter johnsonii (strain B2). Mar Drugs 15:35. https://doi.org/10.3390/md15020035
Hamzah TNT, Lee SY, Hidayat A, Terhem R, Faridah-Hanum I, Mohamed R (2018) Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani. Front Microbiol 9:1707. https://doi.org/10.3389/fmicb.2018.01707
Chaeprasert S, Piapukiew J, Whalley AJS, Sihanonth P (2010) Endophytic fungi from mangrove plant species of Thailand: their antimicrobial and anticancer potentials. Bot Mar 53:555–564. https://doi.org/10.1515/BOT.2010.074
Li H, Li Z, Ruan G, Yu Y, Liu X (2016) Asymmetric reduction of acetophenone into R-(+)-1-phenylethanol by endophytic fungus Neofusicoccum parvum BYEF07 isolated from Illicium verum. Biochem Biophys Res Commun 473:874–878. https://doi.org/10.1016/j.bbrc.2016.03.142
Luo X, Lin X, Tao H, Wang J, Li J, Yang B, Zhou X, Liu Y (2018) Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J Nat Prod 81:934–941. https://doi.org/10.1021/acs.jnatprod.7b01053
Moussa M, Ebrahim W, El-Neketi M, Mándi A, Kurtán T, Hartmann R, Lin W, Liu Z, Proksch P (2016) Tetrahydroanthraquinone derivatives from the mangrove-derived endophytic fungus Stemphylium globuliferum. Tetrahedron Lett 57:4074–4078. https://doi.org/10.1016/j.tetlet.2016.07.091
Yu G, Zhou G, Zhu M, Wang W, Zhu T, Gu Q, Li D (2016) Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org Lett 18:244–247. https://doi.org/10.1021/acs.orglett.5b02964
Zhou XM, Zheng CJ, Song XP, Han CR, Chen WH, Chen GY (2014) Antibacterial α-pyrone derivatives from a mangrove-derived fungus Stemphylium sp. 33231 from the South China Sea. J Antibiot 67:401–403. https://doi.org/10.1038/ja.2014.6
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for financial support (Grant PRONEM N° 05/2019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Funding acquisition by Mario Steindel and Gislaine Fongaro. Material preparation, data collection and analysis were performed by Rafael Dorighello Cadamuro, Ana Claudia Oliveira de Freitas, and Mario Steindel. Writing of original draft, Isabela Maria Agustini da Silveira Bastos and Diogo Robl. Writing and review editing, data analysis, and discussion were carried out by Mario Steindel, Gislaine Fongaro, Diogo Robl, Izabella Thais da Silva, Helen Treichel, Louis Pergaud Sandjo, and Patricia Hermes Stoco. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Admir Giachini
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silveira Bastos, I.M.A., Cadamuro, R.D., de Freitas, A.C.O. et al. Diversity of fungal endophytes from mangrove plants of Santa Catarina Island, Brazil. Braz J Microbiol 55, 1477–1487 (2024). https://doi.org/10.1007/s42770-023-01234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01234-5